Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 10 » Issue 1
Efficacy and safety of the long-acting β2-agonist olodaterol over 4 weeks in Japanese patients with chronic obstructive pulmonary disease
Authors Ichinose M, Takizawa A , Izumoto T, Tadayasu Y, Hamilton A, Kunz C, Fukuchi Y
Received 2 April 2015
Accepted for publication 2 June 2015
Published 20 August 2015 Volume 2015:10(1) Pages 1673—1683
DOI https://doi.org/10.2147/COPD.S86002
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Masakazu Ichinose,1 Ayako Takizawa,2 Toshiyasu Izumoto,2 Yusuke Tadayasu,2 Alan L Hamilton,3 Christina Kunz,4 Yoshinosuke Fukuchi5
1Department of Respiratory Medicine, Tohoku University Graduate School of Medicine, Sendai, Japan; 2Nippon Boehringer Ingelheim Co. Ltd, Tokyo, Japan; 3Boehringer Ingelheim, Burlington, Ontario, Canada; 4Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach an der Riß, Germany; 5Juntendo University School of Medicine, Tokyo, Japan
Background: Olodaterol is a novel long-acting β2-agonist with proven ≥24-hour duration of action in preclinical and clinical studies.
Objective: This randomized, double-blind, placebo-controlled, parallel-group study evaluated the dose response of once-daily (QD) olodaterol based on bronchodilator efficacy, safety, and pharmacokinetics over 4 weeks in Japanese patients with chronic obstructive pulmonary disease (COPD).
Methods: All eligible patients were randomized to receive 2 µg, 5 µg, or 10 µg of olodaterol or placebo for 4 weeks via the Respimat® Soft Mist™ inhaler. The primary end point was the change from baseline in trough forced expiratory volume in 1 second (FEV1) after 4 weeks of olodaterol treatment. Secondary end points included trough FEV1 after 1 week and 2 weeks of treatment, FEV1 area under the curve from 0 hour to 3 hours (AUC0–3), peak FEV1 from 0 hour to 3 hours (peak FEV1), and corresponding forced vital capacity (FVC) responses. Rescue medication use, COPD symptoms, physician global evaluation, pharmacokinetics, and safety were also assessed.
Results: A total of 328 patients with COPD were randomized to receive treatment. All olodaterol doses assessed in the study showed statistically significant increases in trough FEV1 compared to placebo at Day 29 (P<0.0001). Mean increases in peak FEV1 and FEV1 AUC0–3 compared to placebo were also significant (P<0.0001). A clear dose–response relationship was observed across all treatment groups. FVC responses (trough and FVC AUC0–3) supported FEV1 outcomes. All doses of olodaterol were well tolerated, and no safety concerns were identified.
Conclusion: QD olodaterol demonstrated 24-hour bronchodilator efficacy and was well tolerated in Japanese patients with COPD.
Trial registration: ClinicalTrials.gov: NCT00824382.
Keywords: trough FEV1, trough FVC, plasma concentration, pulmonary function, once-daily, dose-finding
Introduction
Chronic obstructive pulmonary disease (COPD), characterized by airflow limitation that is not fully reversible and progressive deterioration in lung function,1,2 is associated with chronic and progressive dyspnea, cough, and sputum production.3 The introduction of the long-acting β2-agonists salmeterol and formoterol allowed effective maintenance treatment of COPD in patients with symptoms inadequately controlled by short-acting agents.4,5 However, the 12-hour duration of action of these therapies necessitates twice-daily dosing.3 With the development of newer long-acting β2-agonists, such as indacaterol, with a 24-hour duration of action,6 there is an intriguing possibility of a once-daily (QD) posology, particularly in combination with other QD long-acting muscarinic antagonists such as tiotropium, which may improve treatment adherence.7
Olodaterol has high selectivity for the human β2-adrenoceptor, a potent, near full agonist response in vitro, and proven effective bronchoprotection over 24 hours in anesthetized guinea pigs and dogs in vivo.8,9
This is the first study evaluating the effect of olodaterol in Japanese patients with COPD, and given its similarity to a recent study conducted in a Caucasian population (Study 1222.5), the study presented here will help to evaluate the ethnic sensitivity of the efficacy and safety of this therapy.10
This study was designed to confirm the 24-hour bronchodilator efficacy, pharmacokinetics, and safety of olodaterol QD administered via the Respimat® Soft Mist™ inhaler (Boehringer Ingelheim, Ingelheim, Germany) in Japanese patients with COPD over 4 weeks of treatment and to determine the most appropriate dose for evaluation in Phase III study.
Methods
Study design
This was a 4-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study registered with ClinicalTrials.gov (NCT00824382). Following an initial screening, patients entered a 2- to 4-week screening period to determine clinical stability. All eligible patients were randomized to receive 2 μg, 5 μg, or 10 μg of olodaterol or placebo QD (all administered as two actuations) for 4 weeks via the Respimat® Soft Mist™ inhaler (Figure 1). All patients were evaluated for a further 2 weeks following completion of the 4-week treatment period or discontinuation. Patients were permitted to use inhaled corticosteroids and short-acting anticholinergics throughout the study, as needed, and the short-acting β-adrenergic salbutamol (100 μg) was allowed as rescue medication, if required.
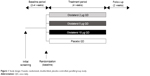  | Figure 1 Study design: 4-week, randomized, double-blind, placebo-controlled, parallel-group study. |
The study was approved by local ethics committees and was carried out according to the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice, and local regulations. Prior to study initiation, the protocol was approved by the local institutional review boards at each institution, Independent Ethics Committee, and the Competent Authority. All patients provided written, informed consent prior to the study commencing.
Patients
Patients were enrolled into the study if they met the following inclusion criteria: age ≥40 years, current or ex-smokers with a smoking history of >10 pack-years, diagnosis of COPD, post-bronchodilator forced expiratory volume in 1 second (FEV1) of ≥30% predicted normal and <80% predicted normal, and a post-bronchodilator FEV1/forced vital capacity (FVC) <70%. Key exclusion criteria were as follows: a significant disease other than COPD (defined by the investigator as a disease that may put the patient at risk by participating in the study, influence study outcomes, or cause concern with regard to the patient’s ability to participate in the study), history of asthma or a total blood eosinophil count of ≥600/mm3, history of myocardial infarction within the past year, unstable or life-threatening cardiac arrhythmia or hospitalization for heart failure within the past year, marked prolongation of QT/QTc interval (QTc interval >450 ms), and experience of any respiratory infection or COPD exacerbation 6 weeks prior to the baseline assessment.
Study outcomes
The primary end point was change from baseline in trough FEV1 after 4 weeks of treatment with olodaterol. Trough FEV1 was defined as the mean of two FEV1 values (60 minutes and 10 minutes prior to study medication inhalation) at the end of the dosing interval 24 hours after administration of olodaterol. Trough FEV1 response was defined as the change from baseline in trough FEV1. Baseline FEV1 was the mean of two pre-treatment FEV1 values (60 minutes and 10 minutes prior to first study medication inhalation). Secondary efficacy variables included trough FEV1 after 1 week and 2 weeks of treatment, FEV1 area under the curve from 0 hour to 3 hours (AUC0–3) responses, peak FEV1 from 0 hour to 3 hours (peak FEV1), FVC, FVC AUC0–3, and peak FVC from 0 hour to 3 hours (peak FVC) responses. Other observations included individual FEV1 and FVC responses at each time point, weekly mean peak expiratory flow rate in the morning (predose) and evening, weekly rescue medication use, COPD symptom scores, and physician global evaluation (PGE).
Safety end points, including adverse events (AEs) and vital signs, were assessed in all treated patients receiving at least one dose of the study medication. Other safety assessments included routine blood chemistry (including potassium), hematology, and urinalysis. Potassium was carefully assessed.
Pharmacokinetic parameters evaluated after the first and last dose of study medication included the maximum measured concentration of the analyte in plasma (Cmax), time from dosing to the maximum measured concentration of the analyte in plasma (tmax), and area under the concentration–time curve.
Assessments
Efficacy
The primary criterion for evaluation in this study was FEV1. All qualifying pulmonary function tests (FEV1 and FVC) and reversibility testing using salbutamol were conducted at the screening visit. FEV1 and FVC pulmonary function tests were performed at Day 1, Week 1, Week 2, and Week 4 at 60 minutes and 10 minutes prior to study medication inhalation and at 30 minutes, 60 minutes, 120 minutes, and 180 minutes post-study medication inhalation. All pulmonary function testing was performed according to American Thoracic Society and European Respiratory Society criteria.11
Rescue medication use was recorded in a patient diary, in which the patient recorded salbutamol use each day and night throughout the study, including screening and treatment periods.
The severity of COPD symptoms, including wheezing, shortness of breath, coughing, and tightness of chest, was recorded at baseline, at Weeks 1, 2, and 4 of the treatment period, and at follow-up, using the following assessment scale: 0= none, 1= mild, 2= moderate, and 3= severe.
PGE was carried out at baseline, at Weeks 1, 2, and 4 of the treatment period, and at follow-up. The assessments reflected the physicians’ opinion of the patients’ overall clinical condition based on the need for concomitant medication, number and severity of exacerbations, severity of cough, ability to exercise, and other relevant clinical observations. All evaluations were recorded by indication of the appropriate score from 1 (“poor”) to 8 (“excellent”), as done in previous studies.12
Safety assessments
Safety measurements included laboratory tests, vital signs (blood pressure and pulse rate), and 12-lead electrocardiogram. All AEs, irrespective of causality (as judged by physicians), were monitored throughout the study by checking patients’ paper diaries and asking patients for any changes in their health.
Pharmacokinetic assessments
Pharmacokinetics of olodaterol were evaluated in the target patient population by determining drug plasma concentrations following the first and last administrations of olodaterol. Plasma concentrations of olodaterol were determined by validated high-performance liquid chromatography coupled to tandem mass spectrometry assay.
Statistical analysis
The planned sample size of 80 evaluable patients per group was needed to demonstrate efficacy of both 5 μg and 10 μg of olodaterol against placebo with 86% power at a one-sided 2.5% level of significance. The full analysis set (for efficacy and pharmacokinetic analyses) consisted of all randomized patients with baseline data and at least one adequate measurement for one or more end points after ≥5 days of study medication inhalation. The per-protocol set comprised patients in the full analysis set with no important protocol violations. All treated patients who received at least one dose of study medication were included in the safety evaluation.
The primary analysis of trough FEV1 was conducted using the analysis of covariance (ANCOVA) model, in which trough FEV1 was compared between each of the olodaterol doses and placebo in a closed testing procedure. Sensitivity analyses were performed to confirm robustness of primary efficacy data and to assess the contribution of various baseline characteristics to the primary analysis model.
All secondary end points were also summarized using the ANCOVA model with terms for baseline, center, and treatment. The baseline was used as a linear covariate, and a separate ANCOVA was performed for each time point. All safety end points are summarized descriptively.
Results
Patient population
Written informed consent was obtained from a total of 515 Japanese patients, of whom 328 were randomized to receive study medication (Figure 2). In total, 15 patients prematurely discontinued from the study, primarily due to AEs (10 patients). Baseline patient characteristics were comparable across treatment groups (Table 1).
  | Figure 2 CONSORT diagram illustrating participant flow. |
Efficacy
Lung function
All doses of olodaterol QD provided a significant increase in trough FEV1 response compared to placebo following 4 weeks of treatment (P<0.0001) (Table 2), confirming the 24-hour bronchodilator activity of olodaterol. A clear dose–response relationship was observed, with a greater increase in bronchodilation with 5 μg and 10 μg of olodaterol compared to 2 μg (0.091 L for 2 μg, 0.132 L for 5 μg, and 0.132 L for 10 μg) (Table 2).
  | Table 2 Adjusted mean trough FEV1 after 4 weeks of treatment |
Statistically significant increases in trough FEV1 responses were demonstrated with all doses of olodaterol compared to placebo at Weeks 1 and 2 (P<0.005). A clear dose response was observed (Figure 3A), with dose ordering across the three doses of olodaterol. The responses for the 5 μg and 10 μg doses of olodaterol were significantly greater than 2 μg of olodaterol (P<0.05). No statistically significant differences in trough FEV1 responses between 5 μg and 10 μg of olodaterol were seen.
All doses of olodaterol provided significant improvements in FEV1 AUC0–3 and peak FEV1 responses over placebo after the first dose (P<0.0001). The dose response was maintained throughout the 4 weeks of treatment, and all three doses of olodaterol remained significantly different compared to placebo at Day 29 (P<0.0001) (Table 3). FEV1 AUC0–3 and peak FEV1 responses were significantly higher with 5 μg of olodaterol compared to 2 μg (FEV1 AUC0–3, P<0.01; peak FEV1, P<0.05) and with 10 μg of olodaterol compared to 2 μg (both end points, P<0.05), but there were no significant differences between 5 μg and 10 μg of olodaterol.
Similarly, all doses of olodaterol were statistically superior to placebo for trough FVC at Weeks 1 and 2, peak FVC, and FVC AUC0–3 responses after 4 weeks of treatment (Table 4). Trough FVC outcomes were all improved versus placebo (Table 4; Figure 3B).
The FEV1 time profiles after treatment with 2 μg, 5 μg, and 10 μg of olodaterol are illustrated in Figure 4A and B, and show a significant increase in FEV1 versus placebo within 30 minutes of drug administration, which was maintained up to 3 hours post-dose (P<0.0001). This pattern of dose response was consistent over 4 weeks of treatment, although there was no apparent dose separation between 5 μg and 10 μg of olodaterol at Day 29 (Week 4). The FVC time profiles after olodaterol administration followed a similar pattern to those for FEV1, with all doses of olodaterol showing improvements versus placebo (Figure 4C and D).
Adjusted mean weekly morning (predose) and evening peak expiratory flow rate readings showed significant improvements compared to placebo for all doses of olodaterol (P<0.0001). The magnitude of difference from placebo in both morning and evening readings was similar, with a range of 18 to 37 L/min and 23 to 37 L/min, respectively.
Rescue medication use was statistically significantly decreased (P<0.05) following treatment with 5 μg and 10 μg of olodaterol compared to placebo over 4 weeks of treatment (except with 10 μg of olodaterol at Week 1).
During the course of the treatment period with each dose of olodaterol, COPD symptom scores averaged between “none” and “mild”. There was no significant improvement in coughing, tightness of chest, or wheezing over the treatment period. However, there was directional improvement in shortness of breath with olodaterol compared to placebo (P<0.05), although no dose response was observed between the olodaterol groups.
The adjusted mean values for PGE were in the “good” range with all doses of olodaterol at all time points. At Day 29, however, PGE scores associated with 2 μg and 10 μg of olodaterol were significantly greater than placebo (P<0.01) but not with 5 μg of olodaterol.
Pharmacokinetics
Plasma concentrations after treatment with 2 μg of olodaterol were mostly below the limit of quantification (2 pg/mL). The mean plasma concentration–time profiles for olodaterol after treatment with 5 μg or 10 μg are presented in Figure 5. Peak plasma concentrations following inhalation (Cmax and Cmax at steady-state [Cmax,ss]) were observed within 20 minutes (tmax 0.283 hours and 0.300 hours; tmax at steady-state 0.333 hours and 0.300 hours for 5 μg and 10 μg of olodaterol, respectively; Tables 5 and 6). The apparent clearance at steady-state was 798 mL/min and 1,040 mL/min following administration of 5 μg and 10 μg of olodaterol, respectively. The accumulation ratios based on Cmax and area under the curve from 0 hour to 1 hour (AUC0–1) were low (1.59 to 1.77; Table 6).
The distributions of dose-normalized systemic exposure parameters (Cmax,norm, AUC0–1,norm, Cmax,ss,norm, and AUC0–1,ss,norm) overlapped between the olodaterol 2 μg, 5 μg, and 10 μg groups; hence, systemic exposure was considered to increase proportionally within the dose range investigated (Cmax,ss,norm and AUC0–1,ss,norm in Figure 6).
Safety
All doses of olodaterol were well tolerated, with the majority of AEs reported as mild to moderate in intensity. The number of patients who experienced any AE under treatment was well balanced across groups (Table 7). The most commonly reported AEs were nasopharyngitis, COPD exacerbation, and myalgia (Table 7). A total of seven patients receiving 2 μg, 5 μg, and 10 μg of olodaterol experienced at least one serious AE during the study that necessitated hospitalization (Table 7). Serious AEs were evenly distributed across all treatment groups and system organ classes; there was no indication that these were related to study medication. No deaths occurred during the study.
No clinically relevant changes in laboratory parameters and vital signs were reported. Outcomes from the 12-lead electrocardiogram showed no dose-dependent effects of olodaterol on conduction abnormalities, heart rate, or QT interval (including QT interval corrected by Bazett’s formula and Fridericia’s formula).
Discussion
This 4-week, multidose study provides evidence for the 24-hour bronchodilator efficacy and tolerability of QD administration of olodaterol in Japanese patients with COPD.
Statistically significant improvements in trough FEV1, peak FEV1, and FEV1 AUC0–3 responses were observed following all doses of olodaterol compared to placebo after 4 weeks of treatment. Trough FEV1 response was chosen as the primary end point for this study, as this measure gives a good indication of potential for 24-hour bronchodilator activity. Thus, the consistent improvement in trough FEV1 responses across all time points compared to placebo supports the potential for QD dosing. Findings from this study replicate those from previous single-dose-administration studies13 and add to the increasing bank of evidence that treatment with olodaterol QD provides effective 24-hour bronchodilation in patients with COPD regardless of ethnicity.
In all, 2 μg, 5 μg, and 10 μg of olodaterol QD provided a significant increase in trough FEV1 response compared to placebo after 4 weeks of treatment; FVC responses supported those observed for FEV1. Furthermore, a clear dose–response relationship was observed with respect to pulmonary function in this patient population, with 2 μg of olodaterol reported to lie on the steep portion of the dose–response curve. However, the efficacy of 5 μg and 10 μg of olodaterol was quite similar, suggesting that both doses of the study medication lie on or close to the plateau of the dose–response curve. These data have helped to provide the necessary information for selection of doses within an appropriate therapeutic range. Therefore, in light of these findings, 5 μg or 10 μg of olodaterol QD may be considered the optimal dose for 4-week administration in Japanese patients with COPD.
Although inhalation is the preferred administration route for treating COPD for a topical, rather than a systemic, bronchodilator effect, systemic pharmacokinetic data may provide a clue to evaluate safety. This trial was, therefore, conducted to evaluate lung function and pharmacokinetic parameters in Japanese patients and to assess appropriate doses of olodaterol in these patients. The findings from this study are broadly similar to another Phase II olodaterol dose-finding study (1222.5; NCT00452400) in non-Japanese patients with COPD, which provided evidence of the bronchodilator efficacy of 2 μg, 5 μg, 10 μg, and 20 μg of olodaterol QD over 24 hours.10 Regarding the comparison of the systemic exposure of olodaterol, systemic exposure tended to be higher in the current study than in the non-Japanese study (ie, geometric mean Cmax,ss 5.92 pg/mL versus 4.02 pg/mL [5 μg], 13.1 pg/mL versus 7.13 pg/mL [10 μg], and AUC0–1,ss 4.85 pg·h/mL versus 3.38 pg·h/mL [5 μg], 10.8 pg·h/mL versus 5.76 pg·h/mL [10 μg]). The main metabolic pathway of olodaterol clearance is related to uridine 5′-diphospho-glucuronosyltransferase (UGT), for which racial difference in activity has not been reported. The deposition pattern of (and, therefore, systemic exposure to) inhaled medications results from the interplay of device, formulation, and inhalation technique.14 The device and formula used in the two studies were identical, and it is unlikely that variation in technique was the reason for the difference between the Japanese and non-Japanese patients included in the two studies. Furthermore, to date there has been no reported evidence of any general ethnic difference in systemic exposure to inhaled medication, including β-agonists. Although the reason for the difference in systemic exposure between the studies with Japanese and non-Japanese patients is unclear, all dosages of olodaterol investigated in the study were well tolerated in Japanese patients with COPD across a broad range of patient characteristics. Occurrences of AEs were low, equally distributed across all treatment groups, and mild to moderate in intensity.
With regard to efficacy, in the study with non-Japanese patients, the change from baseline in trough FEV1 was 0.046, 0.082, and 0.109 with 2 μg, 5 μg, and 10 μg of olodaterol.10
Interestingly, efficacy outcomes in non-Japanese patients showed a clear distinction between 2 μg of olodaterol and 10 μg and 20 μg of olodaterol; however, in Study 1222.5, the positioning of the 5 μg dose with respect to 2 μg and 10 μg of olodaterol was variable, indicating that further evaluation of the doses is warranted. However, taking into account the results from both studies, we can conclude that the FEV1 dose response in Japanese patients is similar to that in non-Japanese patients.
In addition to evidence for the 24-hour bronchodilator efficacy in Japanese patients with COPD being provided, pharmacokinetic analysis indicated that exposure to olodaterol increased dose proportionally within the dose range investigated and was not affected by age, sex, weight, or renal, liver, or lung function. Encouragingly, there was little effect on exposure for patients taking cytochrome p450, UGT, or P-glycoprotein inhibitors, although the number of patients taking these co-medications was small.
Outcomes from this study indicate that the optimal dose of olodaterol inhalation solution is 5 μg or 10 μg for a 4-week administration in Japanese patients with COPD. In light of the 24-hour bronchodilatory efficacy observed with 5 μg and 10 μg of olodaterol and together with outcomes from Study 1222.5 and the international Phase III program,15–18 a QD posology is likely to be a viable strategy for Japanese patients with COPD to improve treatment adherence, particularly as outcomes from a recent study have demonstrated that adherence levels are strongly associated with daily dosing frequency. COPD drugs with QD dosing had the highest adherence levels, with adherence declining consistently as the frequency of dosing increased.7,19,20
Conclusion
QD administration of 2 μg, 5 μg, and 10 μg of olodaterol demonstrated effective 24-hour bronchodilation after 4 weeks in a dose-dependent manner in Japanese patients with COPD. In particular, treatment with 5 μg and 10 μg of olodaterol QD demonstrated an increase in lung function compared to 2 μg of olodaterol QD. No safety or tolerability concerns were identified, and systemic exposure to olodaterol was not affected by age, sex, weight, or renal, liver, or lung function. Data from this Phase II study were consistent with outcomes from other Phase II studies and together provided the rationale to further investigate 5 μg and 10 μg of olodaterol in a Phase III clinical program in COPD.
Acknowledgments
The authors received no compensation related to the development of the manuscript. This work was supported by Boehringer Ingelheim Pharma GmbH & Co. KG. Medical writing assistance was provided by Tanya Chaudry, PhD, of Complete HealthVizion, which was contracted and compensated by Boehringer Ingelheim Pharma GmbH & Co. KG.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
MI has received lecture fees from AstraZeneca, GlaxoSmithKline, Nippon Boehringer Ingelheim, and Novartis Pharma. YF has received honoraria for consultancy from Eisai, Otsuka Pharmaceuticals, and Nippon Boehringer Ingelheim. AT, TI, YT, ALH, and CK are all employees of Boehringer Ingelheim.
References
Guerra S. Asthma and chronic obstructive pulmonary disease. Curr Opin Allergy Clin Immunol. 2009;9(5):409–416. | ||
Pauwels RA, Buist AS, Calverley PMA, Jenkins CR, Hurd SS; GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop Summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. | ||
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2013. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Accessed February 17, 2015. | ||
Boyd G, Morice AH, Pounsford JC, Siebert M, Peslis N, Crawford C. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). Eur Respir J. 1997;10(4):815–821. | ||
Tashkin DP, Fabbri LM. Long-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agents. Respir Res. 2010;11:149. | ||
Rodrigo GJ, Neffen H. Comparison of indacaterol with tiotropium or twice-daily long-acting β-agonists for stable COPD: a systematic review. Chest. 2012;142(5):1104–1110. | ||
Toy EL, Beaulieu NU, McHale JM, et al. Treatment of COPD: relationships between daily dosing frequency, adherence, resource use, and costs. Respir Med. 2011;105(3):435–441. | ||
Bouyssou T, Hoenke C, Rudolf K, et al. Discovery of olodaterol, a novel inhaled β2-adrenoceptor agonist with a 24 h bronchodilatory efficacy. Bioorg Med Chem Lett. 2010;20(4):1410–1414. | ||
Cazzola M, Matera MG, Lötvall J. Ultra long-acting β2-agonists in development for asthma and chronic obstructive pulmonary disease. Expert Opin Investig Drugs. 2005;14(7):775–783. | ||
Maleki-Yazdi MR, Beck E, Hamilton AL, Korducki L, Koker P, Fogarty C. A randomised, placebo-controlled, Phase II, dose-ranging trial of once-daily treatment with olodaterol, a novel long-acting β2-agonist, for 4 weeks in patients with chronic obstructive pulmonary disease. Respir Med. 2015;109(5):596–605. | ||
Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. | ||
Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19(2):217–224. | ||
van Noord JA, Smeets JJ, Drenth BM, et al. 24-hour bronchodilation following a single dose of the novel β2-agonist olodaterol in COPD. Pulm Pharmacol Ther. 2011;24(6):666–672. | ||
Derendorf H, Nave R, Drollmann A, Cerasoli F, Wurst W. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma. Eur Respir J. 2006;28(5):1042–1050. | ||
Koch A, Pizzichini E, Hamilton A, et al. Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat® versus placebo and formoterol twice daily in patients with GOLD 2–4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:697–714. | ||
Ferguson GT, Feldman GJ, Hofbauer P, et al. Efficacy and safety of olodaterol once daily delivered via Respimat® in patients with GOLD 2–4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014;9:629–645. | ||
Feldman GJ, Bernstein JA, Hamilton A, Nivens MC, Korducki L, LaForce C. The 24-h FEV1 time profile of olodaterol once daily via Respimat® and formoterol twice daily via Aerolizer® in patients with GOLD 2–4 COPD: results from two 6-week crossover studies. Springerplus. 2014;3:419. | ||
Lange P, Aumann J-L, Hamilton A, Tetzlaff K, Ting N, Derom E. The 24 hour lung function time profile of olodaterol once daily versus placebo and tiotropium in patients with moderate to very severe chronic obstructive pulmonary disease. J Pulm Respir Med. 2014;4:196. | ||
Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310. | ||
Breekveldt-Postma NS, Koerselman J, Erkens JA, Lammers J-WJ, Herings RMC. Enhanced persistence with tiotropium compared with other respiratory drugs in COPD. Respir Med. 2007;101(7):1398–1405. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.










