Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Efficacy and Safety of TACE Combined with Lenvatinib and PD-(L)1 Inhibitor in the Treatment of Unresectable Hepatocellular Carcinoma: A Retrospective Study
Authors Yang H , Yang T , Qiu G , Liu J
Received 12 June 2023
Accepted for publication 30 August 2023
Published 5 September 2023 Volume 2023:10 Pages 1435—1443
DOI https://doi.org/10.2147/JHC.S423684
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Imam Waked
Hui Yang, Tiequan Yang, Guangpin Qiu, Jie Liu
Department of Interventional Therapy, Ningbo NO.2 Hospital, Ningbo, Zhejiang, People’s Republic of China
Correspondence: Hui Yang, Department of Interventional Therapy, Ningbo NO.2 Hospital, No. 41, Xibei Road, Ningbo, Zhejiang, People’s Republic of China, Tel +86 13567438474, Email [email protected]
Purpose: In the study, patients with unresectable hepatocellular carcinoma (uHCC) were treated with either transcatheter chemoembolization (TACE) combined with lenvatinib and PD-(L)1 inhibitor (TACE-L-P) or TACE combined with lenvatinib (TACE-L). We compared the efficacy and safety of TACE-L-P with TACE-L, and analyzed factors affecting prognosis.
Materials and Methods: A total of 122 patients were treated with either TACE-L-P (n = 64) or TACE-L (n = 58), and their data was collected and analyzed. We assessed tumor response, progression-free survival (PFS), prognostic factors for PFS and adverse events (AEs) to compare the efficacy and safety of TACE-L-P with TACE-L for patients with uHCC.
Results: TACE-L-P group’s patients had a better objective response rate (ORR) (57.8% vs 41.4%, P = 0.047) and a better disease control rate (93.7% vs 81%, P = 0.013), as long as a longer median progression-free survival (PFS) (8 months vs 4.6 months, HR: 0.461; 95% CI: [0.314– 0.675]; P = 0.001) than TACE-L group’s patients. According to multivariate analysis, independent prognostic factors for PFS included treatment option (TACE-L-P / TACE-L; RH = 0.461; 95% CI [0.314– 0.675]; P = 0.001), PVTT (Yes/No; RH =1 0.599;95% CI [1 0.095– 2 0.336]; P=0 0.017), extrahepatic metastasis (Yes/No; RH=1 0.847;95% CI [1 0.176 − 2 0.909]; P=0 0.008). AEs in TACE-L-P group was similar with TACE-L group.
Conclusion: TACE-L-P has more promising clinical outcomes in patients with uHCC than TACE-L, and their safety is similar.
Keywords: hepatocellular carcinoma, lenvatinib, transarterial chemoembolization, PD-(L)1 inhibitor
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide and the fourth leading cause of cancer death worldwide. Nearly half of HCC patients worldwide are from China.1,2 Although surgical resection and liver transplantation may cure HCC, about 80% of HCC patients are already in the middle and late stages at the time of diagnosis due to insidious onset, and they are not suitable for radical surgery, leading to poor prognosis.3–5 Transarterial chemoembolization (TACE) is commonly used to treat unresectable hepatocellular carcinoma (uHCC).6 Systemic therapy, including tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs), is the standard treatment for first-line treatment of uHCC. Sorafenib and Lenvatinib are recommended as first-line treatment drugs for uHCC. However, the single therapy efficacy of these drugs is limited.7–9 Although Phase III clinical trials KEYNOTE-240 and CheckMate 459 failed to demonstrate that immunotherapy is superior to traditional therapy, combination therapy with TKI and anti-PD-(L)1 drugs has showed its efficacy and safety for uHCC in multiple RCTs and is recommended for first-line treatment.10–14 Due to its local anti-cancer effect, TACE may promote anti-tumor immunity, but inevitably induces angiogenesis after TACE.15,16 In addition to anti-angiogenesis, Lenvatinib also has an immunomodulatory effect on the tumor microenvironment.17,18 Triple therapy of TACE, Lenvatinib and PD-(L)1 inhibitor (TACE-L-P) may improve synergistic anti-cancer activity in uHCC. However, the clinical experience of TACE-L-P in treating HCC patients is limited. Therefore, this retrospective study was conducted to compare the efficacy and safety of TACE-L-P and TACE combined with Lenvatinib (TACE-L) for uHCC.
Materials and Methods
Patient Criteria
Our study complied with the Declaration of Helsinki. The studies involving human participants were reviewed and approved by Ningbo NO.2 Hospital ethics committee. The study was retrospective; therefore, all requirements for informed consent were waived, and anonymous analyses of extracted data were performed. Data of uHCC patients receiving TACE-L-P or TACE-L treatment from 2019 to 2022 were collected. The inclusion criteria are as follows: (1) aged between 18 and 75 years with good surgical tolerance; (2) uHCC patients treated with TACE-L-P or TACE-L treatment; (3) according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST), patients must have at least one measurable and arterial-enhanced lesion. The exclusion criteria are as follows: (1) ECOG-PS score ≥2; (2) Child–Pugh C grade; (3) combined with other anti-cancer treatments; (4) other cancer history. (5) Incomplete information.
All laboratory tests were performed within 3 days before the first treatment, and contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) was performed within 7 days.
Treatment Process
All patients received Lenvatinib (8mg for weight < 60 kg or 12 mg for weight ≥ 60 kg) and PD-(L)1 inhibitor (sintilimab 200 mg, tislelizumab 200 mg, camrelizumab 200 mg, toripalimab 240 mg) intravenous injection every 3 weeks. Meanwhile, if enhanced CT or MRI showed that tumor had obvious arterial blood supply, TACE was performed every 1–2 months. Lenvatinib and PD-(L)1 inhibitor were discontinued for 3 days before and after TACE. In TACE, Seldinger method was used for femoral artery puncture, digital subtraction angiography was used to determine the feeding arteries of tumor, and then the catheter was inserted into the feeding arteries. Patients received drug-eluting bead TACE (D-TACE) or conventional TACE (C-TACE) according to their preferences. Drug-loaded microspheres or iodized oil containing doxorubicin was injected to embolize the tumor and feeding arteries. Evaluate the embolization situation by digital subtraction angiography. If the result is satisfactory, the operation ends.
Evaluation Criteria
The main results are objective response rate (ORR), progression-free survival (PFS), and prognostic factors affecting PFS, while the secondary results are adverse events (AEs). The mRECIST criteria was used to evaluate lesions and was classified as follows: complete response (CR): CT or MRI showed no enhancement of all target lesions in the arterial phase; partial response (PR): total diameter of target lesions decreased by 30% (arterial enhancement); stable disease (SD): total diameter of target lesions decreased (arterial enhancement) without reaching PR or increased without reaching PD; progressive disease (PD): total diameter of target lesions (arterial enhancement) increased by 20% or new lesions were detected.19 ORR is defined as the percentage of patients with CR and PR best tumor response rating. Disease control rate (DCR) is defined as the percentage of patients with CR, PR and SD best tumor response rating. Progression-free survival (PFS) is defined as the time from first TACE treatment to the first tumor progression or death or end of follow-up. Evaluate AEs and compare them with the standards of the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 4.0).
Follow-Up
After initial treatment, patients were followed up at intervals of 1–3 months. Medical history, physical examination, hematological and biochemical examination, and contrast-enhanced CT or MRI were included in each follow-up. If tumor progression or intolerable toxicity happened during follow-up, the original treatment plan was stopped. After discussion by a multidisciplinary team, these patients were treated with other suitable methods. The follow-up endpoint is April 1, 2023.
Statistical Analysis
Continuous variables were expressed as means ± standard deviation, compared by the Student’s t-test. Categorical variables were expressed as percentages, calculated by the chi-squared test. PFS was evaluated by the Kaplan-Meier method. Clinical characteristics was assessed by univariate analysis, and statistically significant variables were analyzed by multivariate Cox regression models to identify prognostic factors of PFS. The difference was considered statistically significant when P < 0.05. All analyses were performed with SPSS Statistics version 24.
Results
Patient Characteristics
From 2019 to 2022, 195 uHCC patients treated by TACE-L-P or TACE-L conformed to the inclusion criteria. Among them, 73 patients were excluded because of exclusion criteria (Figure 1). Finally, 122 patients were enrolled, including 64 in the TACE-L-P group and 58 in the TACE-L group. The types of PD-(L)1 inhibitors used in treatment were as follows: sintilimab for 12 (18.8%), tislelizumab for 7 (10.9%), toripalimab for 17 (26.6%), and camrelizumab for 28 (43.8%). All baseline characteristics of the two groups were similar (P>0.05), including sex, age, ECOG PS, Child–Pugh class, BCLC staging, AFP, number of tumors, largest tumor size, PVTT, hepatic vein invasion, extrahepatic metastasis, TACE technique and number of TACE (Table 1).
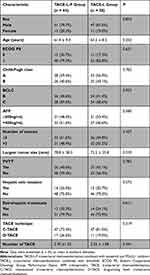 |
Table 1 Baseline Characteristics of the Patients |
Efficacy Outcomes
The two groups were compared, and the CRs were 17.2% and 5.2%, respectively; PRs were 40.06% and 41.4%, respectively; ORRs were 57.8% and 41.4% (P = 0.047); DCRs were 93.7% and 81.0% (P = 0.013). The overall tumor response in the TACE-L-P group was better than the TACE-L group (P = 0.022; Table 2). There was no statistically significant difference in tumor response between different PD-(L)1 inhibitor groups in the TACE-L-P group (Table 3).
 |
Table 2 Tumor Response |
 |
Table 3 Tumor Response of Different PD-(L)1 Inhibitor Groups |
The TACE-L-P group had a more prolonged PFS than the TACE-L group [8.0 months (95% CI 6.82–9.18) vs 4.6 months (95% CI 3.978–5.222); HR: 0.461; 95% CI: 0.314–0.675; P = 0.001; Figure 2].
Prognostic Factors Analysis for PFS
Based on the results of univariate and multivariate analysis based on Cox regression model (Table 4), Treatment option (TACE-L-P/TACE-L; RH = 0.461; 95% CI, 0.314–0.675; P = 0.001), PVTT (Yes/No; RH = 1.599; 95% CI, 1.095–2.336; P = 0.017), Extrahepatic metastasis (Yes/No; RH = 1.847; 95% CI, 1.176–2.909; P = 0.008) were identified as independent prognostic factors for PFS.
 |
Table 4 Univariate and Multivariate Analyses of Risk Factors for FPS |
Safety
AEs are summarized in Table 5. The incidence of adverse reactions exceeding 20% within each group included Hypertension (26.6% vs 22.4%), Fatigue (35.9% vs 32.8%), Anorexia and nausea (39.1% vs 36.2%), Rash (29.7% vs 34.5%), Oral ulcer (20.3% vs 22 0.4%). The incidence of all grades 3 AEs was 0–6.3%, and no grade 4–5 AEs occurred. All incidence and severity of AEs in two groups were similar, and symptomatic treatment and dose reduction cloud alleviate these AEs.
 |
Table 5 Treatment-Related Adverse Events in Two Groups |
Discussion
Our research showed that TACE-L-P therapy brought significant survival benefits to uHCC patients, compared with TACE-L. Patients in TACE-L-P group had better PFS, ORR, and DCR. Univariate and multivariate analyses also suggested that TACE-L-P is an independent predictor of prolonged PFS. At the same time, the AEs of TACE-L-P therapy are similar to those of TACE-L, and there is no statistical difference between the two. Compared with the ORIENT-32 trials and IMbrave 150 previously, the TACE-L-P group in this study had a longer median FPS (8.0 [95% CI 6.82–9.18] vs 4.6 [95% CI 4.1–5.7] vs 6.9 [95% CI 5.7–8.6]), and higher ORR (57.8% vs 24.3% vs 33.2%).13,14 In the ORIENT-32 trials and IMBRAVE 150, ≥3 grade AE were 61.6% and 56%, respectively.13,14 In our study, most of AEs were mild to moderate in severity and easy to control. Relevant studies also came to similar conclusions.20–23 These results suggest that compared with TACE-L, TACE-L-P may be a better therapy for uHCC patients.
The effect of TACE-L-P therapy may be related to the following reasons. PD-(L)1 inhibitor blockade hinders the signal of immune attack on tumors to promote an immune response against tumor cells.24 TACE can effectively reduce the blood supply of uHCC and activate the release of tumor-specific antigens, so the clinical efficacy of PD-(L)1 inhibitor is enhanced.25,26 The low oxygen microenvironment caused by TACE can lead to tumor angiogenesis, resulting in recurrence and metastasis. Lenvatinib can inhibit vascular endothelial growth factor 1–3, thereby inhibiting the original vascular generation and immune suppression effect of the tumor microenvironment and improving the clinical benefits of TACE and PD-(L)1 antibodies.27 The hepa1-6 HCC model shows that lenvatinib has immune regulatory activity, which can increase the number of CD8+ T cells by reducing the proportion of monocytes and macrophages and activating the interferon pathway when combined with PD-(L)1 inhibitor, thus showing excellent anti-tumor activity.17 The synergistic effect of TACE-L-P therapy brings better tumor response and survival benefits to patients with uHCC.
However, this study also has its limitations. Firstly, this study is a retrospective study, and the treatment plan is individualized according to the preferences of physician and patients, that inevitably leads to selection bias. Secondly, the sample size is limited, and the results of statistical analysis should be treated with caution. Finally, four different PD-(L)1 inhibitors were used in this study to treat patients in TACE-L-P group. Although our statistical results did not show statistical difference in tumor response among the various PD-(L)1 inhibitor groups, the potential impact of the efficacy and adverse reactions of these PD-(L)1 inhibitors still deserves attention.
Conclusion
In summary, TACE-L-P therapy showed significantly better PFS and ORR in uHCC patients with manageable toxicity, compared with TACE-L therapy. However, Large sample, prospective randomized controlled trials are needed to confirm these findings.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethic Statement
Our study complied with the Declaration of Helsinki. And the study involving human participants were reviewed and approved by Ningbo NO.2 Hospital ethics committee. The study was retrospective; therefore, all requirements for informed consent were waived in accordance with the national legislation and the institutional requirements.
Funding
This study received funds by Ningbo Clinical Research Center for Medical Imaging (No. 2021L003) and NINGBO Leading Medical&Health Discipline (No. 2022-S02).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer s tatistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi:10.3322/caac.21660
2. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–1462. doi:10.1056/NEJMra1713263
3. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9:682–720. doi:10.1159/000509424
4. Benson AB, Angelica MI, Abbott DE, et al. Hepatobiliary cancers, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:541–565. doi:10.6004/jnccn.2021.0022
5. European Association for the Study of the Liver. EASL. clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi:10.1016/j.jhep.2018.03.019
6. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. doi:10.1016/j.jhep.2021.11.018
7. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi:10.1016/S0140-6736(18)30207-1
8. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, doubleblind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34.
9. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi:10.1056/NEJMoa0708857
10. Finn RS, Ryoo B-Y, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38:193–202. doi:10.1200/JCO.19.01307
11. Yau T, Park J-W, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77–90. doi:10.1016/S1470-2045(21)00604-5
12. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib and pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38:2960–2970. doi:10.1200/JCO.20.00808
13. Finn RS, Qin S, Ikeda M, et al. Atezolizumab and bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi:10.1056/NEJMoa1915745
14. Ren Z, Xu J, Bai Y, et al. Sintilimab and a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, Phase 2–3 study. Lancet Oncol. 2021;22:977–990. doi:10.1016/S1470-2045(21)00252-7
15. Chang Y, Jeong SW, Young JJ, Jae KY. Recent updates of transarterial chemoembolilzation in hepatocellular carcinoma. Int J Mol Sci. 2020;21:8165. doi:10.3390/ijms21218165
16. Cheu JW, Wong CC. Mechanistic rationales guiding combination hepatocellular carcinoma therapies involving immune checkpoint inhibitors. Hepatology. 2021;74:2264–2276. doi:10.1002/hep.31840
17. Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109:3993–4002. doi:10.1111/cas.13806
18. Yi C, Chen L, Lin Z, et al. Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti–programmed cell death-1 in HCC. Hepatology. 2021;74:2544–2560. doi:10.1002/hep.31921
19. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi:10.1055/s-0030-1247132
20. Wu JY, Yin ZY, Bai YN, et al. Lenvatinib combined with anti-PD-(L)1 antibodies and transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2021;8:1233–1240. doi:10.2147/JHC.S332420
21. Cai M, Huang W, Huang J, et al. Transarterial chemoembolization combined with lenvatinib and PD-(L)1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study. Front Immunol. 2022;13:848387. doi:10.3389/fimmu.2022.848387
22. Zhu HD, Li HL, Huang MS, et al. Transarterial chemoembolization with PD-(L)1 inhibitors and molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. 2023;8:58. doi:10.1038/s41392-022-01235-0
23. Wang WJ, Liu ZH, Wang K, et al. Efficacy and safety of TACE combined with lenvatinib and PD-1 inhibitors for unresectable recurrent HCC: a multicenter, retrospective study. Cancer Med. 2023;12:11513–11524. doi:10.1002/cam4.5880
24. Elkhoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi:10.1016/S0140-6736(17)31046-2
25. Kudo M. A new treatment option for intermediate-stage hepatocellular carcinoma with high tumor burden: initial lenvatinib therapy with subsequent selective TACE. Liver Cancer. 2019;8(5):299–311. doi:10.1159/000502905
26. Hack SP, Zhu AX, Wang Y. Augmenting anticancer immunity through combined targeting of angiogenic and PD-(L)1/PD-L1 pathways: challenges and opportunities. Front Immunol. 2020;11:598877. doi:10.3389/fimmu.2020.598877
27. Yamamoto Y, Matsui J, Matsushima T, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18. doi:10.1186/2045-824X-6-18
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


