Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Efficacy and Safety of Skin Radiance Collagen on Skin and Hair Matrix: A Placebo-Controlled Clinical Trial in Healthy Human Subjects
Authors Trehan A, Anand R , Chaudhary G, Garg H, Verma MK
Received 4 September 2023
Accepted for publication 16 January 2024
Published 11 March 2024 Volume 2024:17 Pages 581—591
DOI https://doi.org/10.2147/CCID.S438642
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Rungsima Wanitphakdeedecha
Anupam Trehan, Rachna Anand, Garima Chaudhary, Himanshi Garg, Manoj Kumar Verma
Bright Lifecare Pvt. Ltd, Gurugram, Haryana, India
Correspondence: Rachna Anand, Bright Lifecare Pvt. Ltd, The Presidency, Tower B, Second Floor, 46/4, M G Road, Sector-14, Opp. Girls College, Gurgaon, Haryana, 122001, India, Email [email protected]
Purpose: Collagen supplements are rising in the market as collagen has been demonstrated to be an important protein in the human aging process. Also, it is safe and easily absorbed in the body. Hence the aim of this study was to examine the effectiveness and safety of a collagen and antioxidant-rich treatment compared to a placebo in relation to various skin and hair indicators in healthy adult human subjects.
Patients and Methods: Forty healthy adult non-pregnant/non-lactating women (aged 38– 50 years) provided their informed consent in writing before their participation. Skin Radiance Collagen (SRC) treatment and a placebo were assessed for efficacy before application on Day 1, and post-application on Days 28 and 56, to measure changes in skin elasticity, hydration, brightness, pigmentation; texture, wrinkles, dryness, smoothness, fine lines, changes in the crow’s feet region; as well as hair strength and hair fall.
Results: It was observed after 56 days that therapy with SRC, compared to placebo, produced a substantial effect on reduction of wrinkle depth and fine lines by 48.11% and 39%, respectively, with p-value < 0.01 in the test group. There was a 15.69% improvement in skin hydration observed and 28% reduction in hair fall with p-value < 0.01.
Conclusion: SRC, a combination of collagen with hyaluronic acid (HA), biotin, and vitamins C and E, showed a significant improvement in skin and hair health, including improvements in skin elasticity, skin hydration, reduction in crow’s feet area wrinkles and fine lines, hair fall, and decrease in roughness, leading to improved skin texture. Vitamin C in the formulation also acts as a collagen builder for the body and helps in preventing oxidative stress in the body. The test treatment SRC was found to be efficacious and safe in healthy human adult subjects.
Keywords: skin hydration, skin roughness, hair fall reduction, crow’s feet wrinkles, skin elasticity
Introduction
To sustain healthy skin, collagen is vital, as it represents the most prevalent protein in the body. Its fibre-like structure contributes in the formation of connective tissue. As the name suggests, this particular tissue establishes connections between other tissues and serves as a significant constituent of bone, skin, muscles, tendons, and cartilage, playing a crucial role in upholding the structural integrity of tissues and organs. Additionally, it slows down both photoaging and intrinsic aging.1,2 Additionally, collagen preserves the skin’s pliability and tensile strength. Lack of collagen is evident as our skin begins to sag with age and develops fine lines and wrinkles.1 The aging process can be influenced by a variety of inherent processes, but it can also be accelerated by factors like pollution, lifestyle choices, sunlight exposure, smoking, and beverage intake.3,4 The majority of collagen supplements that specialists recommend contain amino acids like proline, glycine, and hydroxyproline, which are crucial parts of collagen.3 Furthermore, researchers have stated that collagen peptides are recognized for their ability to enhance fibroblast production and trigger various biochemical pathways, including an increase in hyaluronic acid (HA) production in dermal fibroblasts, leading to improved skin water content.5 When collagen decreases in skin, the skin becomes thin and loses its elasticity and flexibility.6 There is evidence suggesting that the decline in collagen synthesis in aging tissue is attributed to the aging of cellular fibroblasts and impaired mechanical stimulation. The decrease in fibrillar (types I and III) collagen is a distinctive characteristic of chronologically aged skin and photodamage.7 It was observed that the collagen peptides strengthen disulphide bonds in hair, thus reduce breakage and increase lustre over a short interval of time.8–11 Collagen supplements have been demonstrated to have a very high bioactivity, good safety profile, and great absorption in the human gastrointestinal barrier.10 The test treatment (SRC) contains biotin as one of the active ingredients. There are studies that have reported improvement in hair quality after usage of biotin supplementation where hair growth has been observed after 2 months of biotin supplementation.11 Various studies done in the past show a close link between vitamins C and E and anti-aging effects of skin. Vitamin E, an antioxidant, is known to regulate inflammatory responses and inhibition of melanogenesis.12 Vitamin C plays an integral role in reducing oxidative stress from ultraviolet ray exposure, inhibition of melanin production, and enhancing wound healing by stabilizing the collagen formation. The loss of skin moisture is also one of the reasons for skin aging. HA, a glycosaminoglycan (GAG), is one of the compounds known to have the capacity to bind and retain water molecules thus improving skin hydration.13
This placebo-controlled clinical study was done with an aim to assess effects of Skin Radiance Collagen (SRC) on skin and hair matrix in healthy adults. The study evaluated test treatment, formulated with marine collagen sourced from fish scales, an antioxidant blend of vitamin C and vitamin E, HA, and biotin, to determine its potential in reducing wrinkles and skin roughness, enhancing skin hydration, and minimizing hair fall.
Materials and Methods
Study Subjects and Criteria
Forty healthy adult females (aged 38–50 years), non-pregnant/non-lactating, provided written informed consent. As per inclusion and exclusion criteria, subject suitability was assessed. For this proof-of-concept study, 40 female adults (20 per group) were initially enrolled to ensure that 40 successfully completed the study. Three subjects withdrew; thus, 37 subjects completed the study (Figure 1).
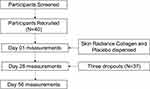 |
Figure 1 Study Flow Diagram. |
The subjects were eligible for enrolment if they had normal Fitzpatrick skin types III to VI (Human skin colour determination scale), achieved a minimum “degree of mild skin aging” according to the Physician Global Assessment (PGA) for skin using Griffith’s scale during the screening visit, and were determined to have Glogau skin age II or III by the dermatologist.
Subjects were not eligible for inclusion in the study if they had a prior history of allergy or sensitivity to the components of the test treatment or had a fish allergy, experienced prevalent skin conditions, had applied retinoids within the last 4 weeks, had a history of alcohol or addiction, or were pregnant/breastfeeding.
Subjects were provided with study restrictions/instructions on test treatment usage and were asked to follow these throughout the entire study duration. Subjects were informed about the next visit. Approval was granted by the local Independent Ethics Committee registered with the Central Drugs Standard Control Organization and the Office for Human Research Protections in the United States. The study complied with the guidelines of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use for Good Clinical Practice and adhered to the principles outlined in the Declaration of Helsinki regarding the treatment of human subjects. The clinical trial was formally registered with the Clinical Trial Registry of India (CTRI) under the registration number CTRI/2022/11/047566 on 23/11/2022.
Investigational Products
Treatment subjects were divided into group A (SRC) and group B (placebo) (powder without actives). SRC contains 5g fish collagen peptide, a blend of antioxidants (vitamins C and E), HA, and biotin. The powder was flavoured to make it palatable. Placebo was composed of a non-active ingredient (maltodextrin) flavoured similarly to the test treatment. Subjects were blinded to the content of their box, as boxes were provided within a non-descriptive pack. The subjects were instructed to take 8 g SRC and 6 g placebo in water (100–200 mL) daily as per the randomization schedule.
Study Design and Measurements
The study was done at NovoBliss Research Private Limited under a trained dermatologist wherein the screening department pre-screened the subjects’ characteristics and demographic details (Table 1). The subjects were instructed during the screening process (before enrolment) not to apply any facial make-up or use any hair products on the day of the study visit.
 |
Table 1 Subject Characteristics and Baseline Demographics |
Subjects reported at the clinical site on Day 28 + 2 days (2 indicates window period) and on Day 56 + 2 days (2 indicates window period) with few visit deviations. Subjects were acclimatized at room temperature for at least 15 minutes before every visit and before performing any clinical or instrumental assessments. Evaluations of efficacy parameters were conducted before the initiation of the test treatment on Day 1, and subsequent assessments were conducted after the application of the test treatment on Day 28 and Day 56, as detailed below.
Hair Pull Test
The hair pull test allows quick monitoring using non-invasive clinical examinations for various hair loss disorders.14 Herein, about 60 hair shafts were gathered between the index finger and thumb, held close to the surface of the scalp skin, and pulled tightly,15–17 away from the scalp with constant strength along the hair shaft up to the upper hair tip. Hairs that were epilated under this procedure were counted.
Hair Combing Test
To find out a range of shedded hairs, the 60-second hair count method was executed. Subjects were asked to perform 60 seconds of hair combing followed by counting of shedded hairs by the trained study staff.
Skin Elasticity
Derma Lab® Combo (Cortex Technology, Denmark) utilizes high-frequency ultrasound for immediate evaluation of the skin in conjunction with conventional skin assessment parameters such as moisture, sebum, elasticity, and trans-epidermal water loss (TEWL). The underlying principle involves stretch/strain through suction. It can assess three elasticity parameters: retraction time, Young’s modulus, and viscoelasticity. Derma Lab® Combo is now even more user-friendly and intuitive, featuring new application screens, a larger touch-screen, and it is equipped with dedicated LabView-based application software. The measurements were obtained from the right cheek of the participants to standardize the measurement area.
Skin Hydration
MoistureMeterEpiD (Delfin Technology Ltd, Finland) serves as a comprehensive measurement unit, consisting of an integrated probe, display, and an integrated contact force sensor. The liquid crystal display (LCD) displays the value measured non-invasively as a percentage (0–100%) of local tissue water available to the epidermis. The MoistureMeterEpiD emits high-frequency, low-power electromagnetic waves onto the skin. The reflected electromagnetic waves are analysed and the resulting value represents tissue permittivity, which is directly proportional to the water content at the measurement site. This TDC (tissue dielectric constant) figure is converted into moisture content and presented. This measurement rises with an increase in water intake. The assessment of skin hydration readings was conducted on the right cheek of the subjects.
Skin Brightness and Tone
The Skin Colorimeter CL 400 (C+K Electronic GmbH, Cologne) is designed specifically for skin colour measurement, and is also applicable for assessing hair parameters. Results are presented as coordinates in the L*a*b* colour space (or RGB). The probe is equipped with a large illumination area to ensure that ample light reaches the skin surface for measurement, while maintaining a small measuring area to exclusively capture surface colour without influencing the colour of deeper layers. Additionally, the individual typology angle (ITA), measuring skin tone, was evaluated. The parameters were measured on the right cheek of the study participants.
Crow’s Feet Region Around the Eyes: Fine Lines, Wrinkles, and Skin Roughness
The Visioscan® VC 20plus (C+K Electronic GmbH, Cologne) is a unique high-resolution ultraviolet A light video camera for direct inspection of the skin surface. This camera can be used not only for hair, but also for pigment spots and lesions. With its multifunctional software, the Visioscan® VC 20plus is a highly flexible system for simple, precise, and extremely economical characterization of skin surface conditions. 3D images were obtained using the instrument, and subject’s skin wrinkles and the fine lines, wrinkles, and skin roughness in the crow’s feet region around the eyes were assessed from the right cheek to maintain a similar area for every evaluation.
Physician Global Assessment for Skin Using Griffith’s Scale
On the scale of 0 to 9 points score, 0 indicated none, 1–3 indicated mild, 4–6 indicated moderate, and 7–9 indicated severe. The overall severity of photodamaged skin was graded for appearance of coarse and fine wrinkles, dyspigmentation (lentigines), elastosis, skin roughness, pore size, telangiectasia, sallowness, and skin laxity.18
Glogau Skin Type
The presence of wrinkles and the extent of photoaging can be categorized using the Glogau scale.19 The extent of photoaging and presence of wrinkles are assessed based on skin characteristics: type 1 – no wrinkles, early photoaging; type II – wrinkles in motion, early to moderate photoaging; type III – wrinkles at rest, advanced photoaging; type IV – only wrinkles, severe photoaging.19 The participants’ skin wrinkles were evaluated according to the Glogau Skin Age classification.
Subjective Product Perception Assessment
Subjective product perception assessment was measured to evaluate the test treatment’s effect on skin smoothness, wrinkles, radiance, effect on hair and skin firmness, and other beauty effects. A subjective questionnaire was completed by the study staff as per the subject’s answers. Subjective perception questionnaires on the sensorial evaluations of test treatment were asked by study staff after treatment usage and were evaluated on Day 28 (+2 Days) and Day 56 (+2 Day) compared to the placebo group.
Besides, digital facial photographs (right/centre/left) of the face before test treatment usage and after test treatment usage were also captured on Day 56 + 2 days.
On Day 28 and Day 56, the subject's diary card was reviewed and completed diary was collected. The subject participation was considered completed on Day 56.
Data Analysis and Statistics
Continuous variables were characterized using descriptive statistics (count, mean, SD, median, minimum value, and maximum value). Categorical variables and the comparison of treatments were presented through frequency and percentage, along with graphical representations when necessary. Paired t-tests and Wilcoxon signed rank tests (the latter being a non-parametric statistical test for evaluating significant differences between paired or matched samples) were employed to assess data for continuous variables, comparing from baseline to post-treatment.
During evaluation, the method best aligned with the study objective was selected. Statistical analysis was done utilizing R software (Version: 4.2.2) at a 5% significance level. Withdrawn subjects were excluded from analysis, and no multiplicity adjustments were made as given data were interpreted within the framework of an explorative analysis.
Results
The present study was conducted by trained research staff of NovoBliss Research Private Limited, Ahmedabad, Gujarat, India.
Effects on Hair Health
Treatment with SRC was significantly effective in reducing hair fall after 56 days of treatment usage with a p-value <0.01. Overall, 28% hair fall reduction was seen on Day 56 from baseline in SRC group (p-value <0.01) when compared to 7.24% reduction observed in subjects who received placebo as the treatment (Figure 2). Moreover, the test treatment improved hair strength as compared to baseline, but the change was not significant.
 |
Figure 2 Reduction of Hair Fall from Baseline to Post Baseline for Placebo (Powder) and Test Treatment Group (Skin Radiance Collagen). |
Effects on Skin Elasticity
There was a significant decrease in retraction time with p-value of 0.03 observed at Day 56 in subjects who received SRC when compared to those who received placebo (p=0.77). There was a significant increase in viscoelasticity initially at Day 28 by 63.87% and by 39.77% at Day 56 as compared to baseline in subjects who received SRC (Table 2). No statistical difference was observed in Young’s modulus values at Day 56. Thus, it was concluded that the test treatment was effective in improving the elasticity of the skin.
 |
Table 2 Descriptive Statistics of Visco Elasticity – Percentage Change from Baseline Using Wilcoxon Signed Rank Test |
Effects on Skin Hydration
Significant improvement in skin hydration was observed by 5.67% (p-value=0.01) at Day 28 and by 15.69% (p-value<0.01) at Day 56 as measured with MoistureMeterEpiD in subjects who received test treatment as compared to subjects who received placebo. From these results, it was inferred that the test treatment SRC improved skin moisturization (Table 3).
 |
Table 3 Descriptive Statistics of Skin Hydration – Percentage Change from Baseline Using Wilcoxon Signed Rank Test |
Effect on Skin Brightness and Skin Tone
Based on L*a*b* and ITA values at Day 56, skin tone showed improvement in subjects who received SRC, but the change was not significant. No improvement was observed in placebo group.
Effects on Fine Lines, Wrinkles, and Skin Roughness in the Crow’s Feet Region
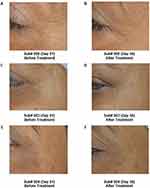
A notable significant decrease of 20.81% in crow’s feet wrinkles was observed at Day 28 and a substantial reduction of 48.11% at Day 56 (week 8) among SRC recipients, with a p-value <0.01. In contrast, subjects administered with placebo displayed no significant change (Table 4). Skin wrinkles in the crow’s feet region was determined whereby a decrease in the skin roughness parameters implies a reduction of skin wrinkles. 3D images showed the improvement in crow’s feet area wrinkles post administration of SRC (Figure 3). Furthermore, results showed statistically significant reduction in fine lines by 39% at Day 56 (Week 8) with p-value <0.01 in subjects who received SRC as compared to 26% reduction in subjects who received placebo as the treatment. There was a decrease in roughness of the skin in subjects who received test treatments as compared to no change in roughness of skin in subjects who received placebo (Figure 4).
 |
Table 4 Descriptive Statistics of Crow's Feet Area – Percentage Change from Baseline Using Wilcoxon Signed Rank Test |
The consumption of SRC led to a decrease in skin roughness parameters, implying an enhancement in coarse texture and reduction of fine wrinkles and improvement in skin smoothness (Figure 5). A substantial reduction in skin roughness was observed after 4 weeks (28 days) of intervention. This decrease was also observed in the placebo group but to a lesser extent.
 |
Figure 5 Improvement in Skin Roughness Pre and Post Consuming Placebo (Powder) and Test Treatment (Skin Radiance Collagen). |
Effect on Physician Global Assessment (PGA) Using Griffith’s Scale for Skin Appearance
The PGA score for wrinkles, lentigines, elastosis, skin roughness, pore size, telangiectasia, sallowness, and skin laxity was improved by 29.35% at Day 56 with p-value <0.01 in subjects who received SRC as their treatment as compared to no change of PGA score in subjects who received placebo as the treatment (Table 5). The treatment was found to be effective in improving the PGA score.
 |
Table 5 Descriptive Statistics of Physician Global Assessment for Skin Appearance – Percentage Change from Baseline Using Wilcoxon Signed Rank Test |
Effect on Glogau Skin Type
Change was observed in the Glogau skin type in SRC group. It was reported that 68% of subjects had change in Glogau skin type from type III to type II, while no change was observed in subjects who received placebo (Figure 6).
Subjective Product Perception Assessment
In response to the questionnaire, 100% of the participants reported feeling satisfied, compared to placebo group reporting 18.18% improvement. Additionally, all participants stated that SRC successfully enhanced various parameters including the improvement of fine lines, skin moisturization, skin texture, skin radiance, nail health, hair health, skin health, as well as in reducing skin pigmentation and wrinkles of the crow’s feet area.
Discussion
The study examined alterations in skin wrinkles, brightness, skin tone, texture improvement, roughness, hydration, elasticity, and self-perceived skin appearance after 56 days of collagen or placebo supplementation. Clinical and qualitative evaluations were performed at screening, Day 1, Day 28, and Day 56. At all visits, participants’ questionnaires were assessed for the effectiveness of therapies, adverse events, and usage of the study product, all of which were found 100% satisfactory. The enhancements in skin health noted in this study were consistent with other clinical studies evaluating collagen supplementation and related health benefits. The SRC supplement was linked to a higher level of subject satisfaction compared to the placebo. It was observed that 56 days of therapy with SRC in comparison to placebo produced substantial positive aesthetic effect on a crucial antiaging criterion, which is the reduction of wrinkle depth and fine lines in the facial area. After 28 days and 56 days, this favourable impact was already apparent, and notable enhancement of the fine and coarse structures resulted in an appealing change in the skin’s appearance. Indeed, the usage of SRC yielded a decrease in skin roughness, which was evident after both 28 and 56 days of usage. The reduction in retraction time at Day 28 and Day 56 indicates that SRC has likely improved collagen levels within the skin. This boost in collagen content enhances the skin’s structural integrity and strength, enabling the skin to regain its original form after being stretched. Skin hydration demonstrated improvement on both Day 28 and Day 56 following the usage of SRC. This positive enhancement in hydration aligns well with the concept that well-hydrated skin is closely connected to improved skin elasticity which was also improved post SRC usage.
After utilizing SRC for a duration of 56 days, a noticeable decline was seen in the amount of hair being shed. This reduction in hair fall is indicative of the positive influence that test treatment exerts on hair health.
There are certain limitations to this study. External factors and lifestyle modifications may have affected study results and comparisons of placebo and test treatment. Factors contributing to these differences are differing study collectives and designs, seasonal factors, differences in applied methodologies, dosage of nutrients, as well as normal physiological variations from person to person.
Conclusion
Currently, collagen is one of the most predominant ingredients in the nutrition and beauty industry. Also, it is suggested to promote healthy hair and skin. With age, collagen production decreases, affecting the radiance of the skin. The clinical study revealed that placebo had no effect on skin and hair health measures. SRC after continuous consumption for 28 days changed the skin roughness and crows’ feet wrinkles. The skin was smoother with decreased fine lines post therapy with SRC. Skin hydration and elasticity were also enhanced by 15.69% in 8 weeks (56 days) and 63.87% in 4 weeks (28 days), respectively, with a concomitant reduction in hair fall by up to 28% in 8 weeks. As per participants' perspective, SRC was effective in improving the skin radiance and skin glow after 8 weeks of usage. The study concluded that SRC not only improves skin elasticity, hydration, roughness, and wrinkles, but also rejuvenates skin and supports hair fall reduction.
Data Sharing Statement
Individual subject data are not publicly shared to uphold ethical standards and safeguard the confidentiality of the subjects.
Ethical Approval
Approval was secured from the local ethical committee named ACEAS Independent Ethics Committee.
Acknowledgment
Brightlife care is thankful to NovoBliss Research Private Limited for conducting this study.
Disclosure
The authors affirm that no associations have influenced the work reported in this paper. The authors report no conflicts of interest in this work.
References
1. Krutmann J, Bouloc A, Sore G, Bernard BA, Passeron T. The skin aging exposome. J Dermatol Sci. 2017;85(3):152–161. doi:10.1016/j.jdermsci.2016.09.015
2. Mora Huertas AC, Schmelzer CE, Hoehenwarter W, Heyroth F, Heinz A. Molecular-level insights into aging processes of skin elastin. Biochimie. 2016;128–129:163–173. doi:10.1016/j.biochi.2016.08.010
3. Cannarozzo G, Fazia G, Bennardo L, et al. A new 675 nm laser device in the treatment of facial aging: a prospective observational study. Photobiomodul Photomed Laser Surg. 2021;39(2):118–122. doi:10.1089/photob.2020.4908
4. Nistico SP, Silvestri M, Zingoni T, Tamburi F, Bennardo L, Cannarozzo G. Combination of fractional CO2 laser and rhodamine-intense, pulsed light in facial rejuvenation: a randomized controlled trial. Photobiomodul Photomed Laser Surg. 2021;39(2):113–117. doi:10.1089/photob.2020.4876
5. Felician FF, Xia C, Qi W, Xu H. Collagen from marine biological sources and medical applications. Chem Biodivers. 2018;15(5):e1700557. doi:10.1002/cbdv.201700557
6. McCabe MC, Hill RC, Calderone K. Alterations in extracellular matrix composition during aging and photoaging of the skin. Matrix Biol Plus. 2020;8:100041. doi:10.1016/j.mbplus.2020.100041
7. Varani J, Dame MK, Rittie L, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168(6):1861–1868. doi:10.2353/ajpath.2006.051302
8. Al-Atif H. Collagen Supplements for aging and wrinkles: a paradigm shift in the fields of dermatology and cosmetics. Dermatol Pract Concept. 2006;168(6):1861–1868.
9. Fernandes C, Medronho B, Alves L, Rasteiro MG. On hair care physicochemistry: from structure and degradation to novel biobased conditioning agents. Polymers. 2023;15(3):608. doi:10.3390/polym15030608
10. Yagoda M, Gans E. A nutritional supplement formulated with peptides, lipids, collagen and hyaluronic acid optimizes key aspects of physical appearance in nails, hair and skin. J Nutr Food Sci. 2014;5(2):S5. doi:10.4172/2155-9600.S5-002
11. Patel DP, Swink SM, Castelo-Soccio L. A review of the use of biotin for hair loss. Skin Appendage Disord. 2017;3(3):166–169. doi:10.1159/000462981
12. Casale F, Suh S, Yale K, Mesinkovska NA. Clinical role of oral vitamin C and E therapy in skin and hair disorders. Int J Cos Derma. 2021;1(1):12–20.
13. Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol. 2012;4(3):253–258. doi:10.4161/derm.21923
14. McDonald KA, Shelley AJ, Colantonio S, Beecker J. Hair pull test: evidence-based update and revision of guidelines. J Am Acad Dermatol. 2017;76(3):472–477. doi:10.1016/j.jaad.2016.10.002
15. Shrivastava SB. Diffuse hair loss in an adult female: approach to diagnosis and management. Indian J Dermatol Venereol Leprol. 2009;75(1):20–28. doi:10.4103/0378-6323.45215
16. Guarrera M, Semino MT, Rebora A. Quantitating hair loss in women: a critical approach. Dermatology. 1997;194(1):12–16. doi:10.1159/000246049
17. Gordon KA, Tosti A, Tosti A. Alopecia: evaluation and treatment. Clin Cosmet Invest Dermatol. 2011;4:101–106. doi:10.2147/CCID.S10182
18. Weinstein GD, Nigra TP, Pochi PE, et al. Topical tretinoin for treatment of photodamaged skin. A multicenter study. Arch Dermatol. 1991;127(5):659–665. doi:10.1001/archderm.1991.01680040067005
19. Oliveira R, Ferreira J, Azevedo LF, Almeida IF. An overview of methods to characterize skin type: focus on visual rating scales and self-report instruments. Cosmetics. 2023;10(1):14. doi:10.3390/cosmetics10010014
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.