Back to Journals » Journal of Pain Research » Volume 16
Efficacy and Safety of SIKD1977 in Combination with Standard Treatment for Postherpetic Neuralgia: Study Protocol for a Double Blind, Placebo-Controlled, Randomized, Multicenter, Phase 2 Clinical Trial
Authors Jo HR , Kim YG, Sung WS , Park KS, Lee YJ , Cho SY , Seo BK , Kwon YE, Kim EJ
Received 9 December 2022
Accepted for publication 10 May 2023
Published 29 May 2023 Volume 2023:16 Pages 1755—1765
DOI https://doi.org/10.2147/JPR.S400682
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert B. Raffa
Hyo-Rim Jo,1 Yong-Gyun Kim,2 Won-Suk Sung,1 Kyoung Sun Park,3 Yoon Jae Lee,4 Sun Young Cho,5 Byung-Kwan Seo,6 Young-Ee Kwon,2 Eun-Jung Kim1
1Department of Acupuncture & Moxibustion, Dongguk University Bundang Oriental Hospital, Seongnam-si, Gyeonggi-do, Republic of Korea; 2Central Research Institute, Samik Pharmaceutical Company LTD., Incheon, Republic of Korea; 3Jaseng Hospital of Korean Medicine, Seoul, Republic of Korea; 4Jaseng Spine and Joint Research Institute, Jaseng Medical Foundation, Seoul, Korea; 5IntegroMedLab Company Ltd., Seoul, Republic of Korea; 6Department of Acupuncture & Moxibustion, Kyung Hee University Hospital at Gangdong, Seoul, Republic of Korea
Correspondence: Eun-Jung Kim, Department of Acupuncture & Moxibustion, Dongguk University Bundang Oriental Hospital, 268, Buljeong-ro, Bundang-gu, Seongnam-si, Gyeonggi-do, Republic of Korea, Tel +82-31-710-3719, Fax +82-31-710-3780, Email [email protected]
Purpose: Postherpetic neuralgia (PHN) is the most common chronic complication of herpes zoster, associated with poor quality of life and increased patient and healthcare resource expenditure. This randomized controlled trial aims to evaluate the efficacy and safety of SIKD1977 (Sogeonjungtang) in combination with standard treatment and estimate an effective dose for treating PHN.
Patients and Methods: This is a protocol for a randomized, placebo-controlled, double-blind, multicenter trial. A total of 90 eligible participants with PHN will be recruited from three hospitals and randomly allocated to high-dose group, low-dose group, or placebo group in a 1:1:1 ratio. The trial will involve a 6-week oral administration of SIKD1977/placebo, and a 1-week follow-up period. The primary outcome will be the weekly average change in average daily pain score (ADPS) from baseline to the end of treatment. The secondary outcomes will include the weekly average changes in ADPS from baseline to week 2, 4, and 7, differences in Short-Form McGill Pain Questionnaire, Visual analogue scale, 5-level EuroQol-5 dimensions, Patient Global Impression of Change, and consumption of rescue drugs. All adverse events will be assessed during the trial.
Conclusion: This study will provide evidence for the efficacy and safety of SIKD1977, and an effective dose for PHN.
Trial Registration: This protocol has been registered in the Clinical Research Information Service with the identification code KCT0007939.
Keywords: postherpetic neuralgia, herbal medicine, SIKD1977, Sogeonjungtang, randomized controlled trial, protocol
Introduction
Postherpetic neuralgia (PHN) is one of the intractable chronic pain syndromes, characterized by persistent pain for more than one month after the resolution of the herpes zoster rash.1 Patients with PHN experience several types of pain: persistent pain without a stimulus (often described as burning, aching, throbbing), intermittent pain without a stimulus (often described as stabbing, shooting, or electric shock-like), and pain caused by a stimulus but is disproportionate to the stimulus.2 In addition, it affects the quality of life by causing abnormal sensations (dysesthesias or paresthesias), depression, insomnia, and anorexia.3
Clinical guidelines for the management of PHN recommended tricyclic antidepressant (TCA), gabapentin, pregabalin, and topical lidocaine 5% patch as first-line therapies for patients with PHN. Although effective, TCAs have been associated with anticholinergic adverse events or cardiotoxicity, and calcium channels α2-δ ligands such as gabapentin and pregabalin have risks of dizziness, somnolence, peripheral edema, weight gain, asthenia, dry mouth, and vertigo.4 In addition, topical lidocaine 5% patch may increase the incidence of adverse effects, such as blisters, bruising, burning sensation, dermatitis on the application site, confusion, disorientation, headache, and nervousness.2
Herbal medicine treatment has been used to treat a range of pain-related conditions, and several reviews have demonstrated that herbal medicine can be an effective treatment for patients with PHN.5–7 Qua et al have found that herbal medicine groups significantly alleviated pain intensity compared to western medicine groups,5 and Liang et al further have shown that herbal medicine as an add-on therapy to pharmacotherapy had a better effect in reducing pain intensity and depression symptom severity compared to pharmacotherapy alone.6
Paeoniae Radix and Glycyrrhizae Radix, which are the main herbs of Sogeonjungtang (SGJT), have already been used as a sedative, pain reliever, and anti-spasmodic in East Asia through anticholinergic, prostaglandin-production-inhibiting, antioxidation, and anti-inflammation as Jakyakgamchotang (also known as Shakuyakukanzo-to, Shaoyaogancao-tang).8–11 In accordance with the study of Hidaka et al, Jakyakgamchotang could relieve pain in peripheral neuropathic pain-induced mice.12 Furthermore, SGJT, which adds four components to this herbal medicine, reduces the intensity, time, and frequency of neuropathic pain in a retrospective observational study.13 Thus, this clinical trial will assess the efficacy and safety of SIKD1977 (SGJT) in combination with standard treatment and confirm an effective dose for treating PHN.
Materials and Methods
Study Design and Setting
This study is a multicenter, randomized, placebo-controlled, double-blinded trial designed to evaluate the efficacy and safety of SIKD1977 combined with PHN standard treatment in patients with PHN. This trial will be performed at Dongguk University Bundang Oriental Hospital (Gyeonggi-do, Korea), Kyung hee University Hospital at Gangdong (Seoul, Korea), and Jaseng Hospital of Korean Medicine (Seoul, Korea). A total of 90 participants who meet the inclusion and exclusion criteria will be randomly divided into three groups in a 1:1:1 ratio: high-dose group, low-dose group, and placebo group. The trial will consist of a 6-week oral administration of SIKD1977/placebo and a 1-week follow-up period. The flow chart for this study is shown in Figure 1, and Table 1 shows the schedule for enrollment, intervention, and assessments.
 |
Table 1 Schedule of Enrolment, Treatments, and Assessments |
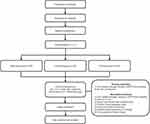 |
Figure 1 Study flow chart. Abbreviation: ADPS, Average daily pain score. |
Participants
Participants will be recruited through advertising posters approved by the Institutional Review Board (IRB). Recruitment posters will display inside and outside each hospital, such as on the subway and bus, and online.
Inclusion Criteria
The trial inclusion criteria are as follows:
- Age of 19 to 65 years old.
- Presence of persistent pain even after 1 month of PHN standard treatment (Pregabalin, Gabapentin).
- Average numeric rating scale (NRS) for the past week ≥ 4.
- Average daily pain score (ADPS) for the 1-week screening period ≥ 4.
- Able to decide the participation, read, and write the informed consent (Those who demonstrate no problems with communication, both visual and auditory (reading, writing, hearing, speaking, and seeing)).
- Voluntary willingness to participate and sign the informed consent agreement (Those who are not coerced or unduly influenced to engage in the research).
Exclusion Criteria
The exclusion criteria are as follows:
- Head and face affected by herpes zoster.
- The recurrence of herpes zoster or the requirement for treatment:
- Presence of severe nausea, vomiting, gastrointestinal diseases, or history of gastrointestinal surgery that may affect drug absorption.
- Diabetic patient (fasting blood sugar ≥ 128mg/dL or HbA1c ≥ 6.5%); history of diabetes diagnosis or taking diabetes medication.
- Presence or history of myopathy due to hypokalemia.
- Presence of severe uncontrolled hypertension.
- Presence or history of heart disease (coronary artery disease (eg cardiomyopathy, myocardial infarction, angina pectoris), congestive heart failure, asymptomatic cardiac dysfunction).
- Presence of other severe pain, neurological diseases, or red flag sign unrelated to postherpetic neuralgia.
- Presence of malignant cancer within the past five years, severe mental illness, or other systematic diseases.
- Presence of the following abnormalities in laboratory tests: serum potassium <3.5mmol/L, creatinine clearance <30mL/min, or HBsAg/HCV Ab (+); abnormal findings in electrocardiogram.
- Presence of hypersensitivity to components of SIKD1977; history of the allergic disease which needs to treat or drug allergy.
- Use of the following drugs for postherpetic neuralgia: NSAIDs, corticosteroid, local anesthetic, opioid analgesics, antidepressants, muscle relaxant, immunosuppressant, potassium/licorice-containing agents, glycyrrhizic acid, thiazide diuretics, loop diuretics (However, participation is possible after washing out for more than 7 days (up to 4 weeks) in accordance with a half-life of each drug).
- Those who have received or need the following therapy for treating postherpetic neuralgia: nerve block within the past 1 month, radiofrequency ablation, spinal cord stimulator insertion, implantation of a subarachnoidal drug administration system.
- Use of herbal medication within the past 14 days; use of an over-the-counter drug (including vitamin B1 or B12) within the past 10 days.
- Participation in other clinical trials within the past two months.
- Disagreements with the use of the following contraceptive methods during the study period: intrauterine device, barrier contraceptives with spermicide, vasectomy, tubectomy, tubal ligation, or hysterectomy.
- Females who are pregnant or lactating.
- Other disqualifications as determined by the researcher.
Drop-Out Criteria
Participants may request cessation or withdrawal of participation in the study at any point in time, or the investigators may withdraw participants if they meet any of the following criteria.
- Inclusion or exclusion criteria are violated.
- Serious adverse events which make participation difficult.
- Exacerbation of the disease requires to discontinue the study drug.
- Self-administration of medications which affects the trial.
- Participation in another clinical trial during this study.
- A patient requests to withdraw consent.
- A patient is pregnant.
- Protocol violation which significantly affects the trial.
- The researcher determines that further participation is inappropriate.
Randomization and Blinding
An independent statistician who is not involved in the clinical trial will perform randomization. It will be achieved with a computerized random number generator using the stratified block randomization method of SAS (SAS Institute, Inc., Cary, NC, USA). The random assignment will be done by the web-based service provided by the Interactive Web Response System (IWRS). A packer not related to this study will pack the study drug or placebo according to random codes of investigational products assigned through IWRS, and the pharmacist who does not participate in the study will provide the study drug or placebo with the same number as the random code.
The participants, investigators, clinical trial pharmacist, and other clinical trial associates will be blinded to the treatment assignment until the end of the study. Only when the study has been completed, the database query finished, and the study database locked will be the randomization schedule be made available for analysis.
If a serious adverse event cannot be managed without knowing what type of intervention has been received, the IWRS will be used to obtain treatment assignment information. The Medical Monitor must be notified before the study intervention is unblinded, preferably prior to unblinding a subject.
Intervention
Participants will receive high-dose SIKD1977, low-dose SIKD1977, or placebo three times per day according to group allocation. The duration of treatment in three groups is 6 weeks with a follow-up period of 1 week.
Samik Pharmaceutical Co. manufactures the SIKD1977 and placebo in compliance with Korea Good Manufacturing Practice standards. SIKD1977 (SGJT) used in this study is a sweet, dark brown, soft extract, and is permitted and regulated by the Ministry of Food and Drug Safety. It is composed of six components: Paeoniae Radix Rubra 0.5g, Cinnamomi Ramulus 0.33g, Jujubae Fructus 0.33g, Glycyrrhizae Radix 0.25g, Zingiberis Rhizoma 0.11g, Saccharum Granorum 1.67g (per 7.5g). The placebo has the appearance, shape, weight, taste, and color of the SIKD1977 being administered. SIKD1977 and placebo soft extract are sealed in aluminum bags and administered to subjects in doses of 7.5g. Participants will be instructed to take 30g of 4 packs per dose three times a day before meals. In the high-dose group and the placebo group, a total of 90g of SIKD1977 or placebo will be taken a day. In the low-dose group, two packs of SIKD1977 (15g) and two packs of placebo (15g) will be taken once together, and the daily dose was the same as 90g (SIKD1977 45g + placebo 45g) with the other two groups.
Concomitant Treatment
Pregabalin and gabapentin, which are PHN standard treatments, should be taken at least two weeks before trial and maintained under the same conditions during the clinical trial period.
If medications are needed to treat other diseases unrelated to pain, they can be taken during the trial period and should be taken at the same dosage and usage during the study.
Rescue Medication
If participants do not tolerate pain and want to take analgesics, acetaminophen will be permitted. Acetaminophen 500mg tablets should be taken once 1 ~ 2 tablets at intervals of 4 ~ 6 hours, and the maximum dose for 24 hours is limited to 4000mg. The participants will indicate the dosage, date of use in their diaries.
Outcome
Primary Outcome
The primary outcome measure will be the weekly average change in ADPS from baseline (visit 2) to the end of treatment (visit 5). The participants evaluated the average degree of pain within the previous 24 hours in the daily diary using NRS, which is defined as ADPS. The NRS ranges from 0 (no pain) to 10 (the worst imaginable pain) and is chosen to assess merely pain severity.
Secondary Outcome
The secondary outcomes of this trial are the weekly average changes in ADPS from baseline to week 2, week 4, and week 7, differences in Short-Form McGill Pain Questionnaire (SF-MPQ), Visual analogue scale (VAS), 5-level EuroQol-5 dimension (EQ-5D-5L), Patient Global Impression of Change (PGIC), and consumption of rescue drugs.
- The weekly average changes in ADPS from baseline to week 2, 4, and 7 Participants evaluated the average degree of pain over the past 24 hours in their diaries using NRS every day, and ADPS are checked and collected at each visit (Visit 2–6).
- Short-Form McGill Pain Questionnaire (SF-MPQ) SF-MPQ is a shortened version of MPQ, consisting of 15 items of multidimensional scales, VAS, and present pain intensity. The 15 items of multidimensional scales (11 sensory; 4 affective) are rated on an intensity scale as 0 (no pain) to 3 (severe pain) for each question. The pain intensity over the past week will be assessed using VAS where one end of a 100mm line indicates no pain while the other end refers to the worst imaginable pain. The present pain intensity will be rated between 0 (no pain) to 5 (excruciating).14,15 The participants will self-complete the SF-MPQ at visit 2–5.
- 100mm Visual analogue scale (VAS) VAS included in the second category of SF-MPQ will be collected for evaluating pain over the past week.
- 5-level EuroQol-5 dimension (EQ-5D-5L) EQ-5D-5L is a generic instrument for evaluating health-related quality of life. The descriptive system is composed of five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has five response categories corresponding to no problems, slight problems, moderate problems, severe problems, and extreme problems.16 The participants will be asked to indicate their health state for each of the five dimensions at visit 2–5.
- Patient Global Impression of Change (PGIC) PGIC, which has a consistent relationship with NRS changes regardless of study, disease type, age, sex, study result, or treatment group, is a self-reported tool for improvement levels using a 7-point scale (1: very much improved, 2: much improved, 3: minimally improved, 4: no change, 5: minimally worse. 6: much worse, 7: very much worse).17 The PGIC will be administered during visits 3, 4, and 5.
- Consumption of rescue drugs Whether or not the rescue drug is taken, the dosage and the duration of the drug are checked and collected through the participants’ diaries at each visit.
Safety Evaluation
For safety evaluation, vital sign, weight, physical examination, concomitant treatment will be measured at every visit, and hematology tests (white blood cell, red blood cell, hemoglobin, hematocrit, platelet, differential count), urinalysis (specific gravity, color, pH, protein, glucose, ketone, bilirubin, occult blood, urobilinogen, microscopic examination), ECG will be conducted before and after treatment for three groups to compare the incidence of adverse events.
At each visit, the participants will report adverse events, and the investigator will record details, including the specific symptom, onset, duration, severity, resolution, and possible association with treatment. When serious adverse events occur, the investigator will suspend participation, provide the treatment immediately, and report to the IRB within 24 hours from the time of recognition regardless of causality.
Quality Control
Investigators will undergo adequate and professional training before the study and follow guidelines for Good Clinical Practice and Korean Good Clinical Practice, to ensure the safety of the subjects, and adherence to the protocol. To protect the rights and welfare of the subjects and to sustain the quality of the study, its own quality assurance process and monitoring will be conducted. The clinical research associate will regularly visit three centers or access the data management system to confirm the source document and monitor whether the trial is performed according to the protocol and related guidelines.
The study data will be collected and recorded in the electronic case report form (e-CRF). The study-related documents including consent forms, the CRF, questionnaires, medical records, and other records will be kept in a locked space or on a password-protected computer in each hospital for three years after study completion.
Sample Size Estimation
No previous clinical trials have evaluated the effect of SIKD1977 on patients with PHN. This study is a phase 2 trial designed to assess whether a treatment has a sufficient signal of the activity or other meaningful benefit to warrant further investigation in a definitive Phase 3 trial. Typically, the sample size of the phase 2 trial is small or moderate, ranging from tens to hundreds.18 Teare et al suggested that pilot randomized controlled trials require a total of at least 70 for estimating a continuous outcome with good precision.19 In addition, previous studies assessing the efficacy of herbal medicine as an add-on therapy for PHN set sample size from 30 to 35 per group.20–24 Thus, considering the possibility of drop-outs and the other characteristics of this study, we have decided to recruit 30 participants per group.
Statistical Analysis
An independent statistician blinded to group allocation will perform both the full analysis set (FAS) and per-protocol (PP) analysis. FAS is performed on patients who take for study drug at least once, and missing data are replaced via the last observation carried forward method. PP analysis is used to evaluate data collected from the subjects, who complete all steps of the experimental protocol.
Data will be presented as the number of observations, mean, standard deviation, median, minimum, and maximum for continuous parameters, and the number, and percentage of subjects for categorical parameters. Comparison of the baseline characteristics will be analyzed by two-sample t-test or Wilcoxon’s rank sum test for continuous variables, and Pearson’s chi-square test or Fisher’s exact test for categorical variables. For the primary outcome measure, we will implement analysis of covariance (ANCOVA) in which the dose of SIKD1977 (stratified variable) is a factor with standard treatment (pregabalin, gabapentin) considered as a covariate. For the secondary outcome measure, we will conduct ANCOVA in which each group (stratified variable) is a factor with standard treatment considered as a covariate. To analyze the percentage of patients taking the rescue drugs, we will perform the Cochran-Mantel-Haenszel test with standard treatment as a covariate.
To assess safety, we will present the frequency and proportions of adverse events, and we will use Pearson’s chi-square test (or Fisher’s exact test) to analyze differences among the three groups. We will conduct paired t-tests (or Wilcoxon’s signed rank test) and McNemar’s test using the results of the vital sign, physical examination, weight, laboratory tests, ECG of the baseline tests, and end of intervention tests, and we will estimate differences among the groups. In addition, concomitant medicines are classified according to the latest version of the World Health Organization-Anatomical Therapeutic Chemical index, and concomitant therapies are standardized to System Organ Class and Preferred Term according to the latest version of Medical Dictionary for Regulatory Activities, and the frequency and proportions of concomitant medicine/therapy are presented.
Ethics
All procedures in this trial will be conducted in compliance with general ethical guidelines (the Declaration of Helsinki and Korean Good Clinical Practices). This study was approved by IRB of Dongguk University Bundang Oriental Hospital (2022-D11-N001) and has been registered at Clinical Research Information Service (KCT0007939, https://cris.nih.go.kr/cris/search/detailSearch.do/23099).
The Ministry of Food and Drug Safety approved this clinical trial under the Investigational New Drug number 8WDT-VB8G-OL0M-BQZG on 19 May 2022.
Before enrolment, all participants will sign informed consent forms including possible benefits and adverse events, and their responsibilities. All enrolled subjects will voluntarily enter the study, and their personal information will be strictly protected under IRB supervision.
Discussion
PHN is the most common complication following herpes zoster, causing intractable chronic pain syndrome.25 The first-line drugs, including calcium channel modulators, TCA, and lidocaine, are not suitable for long-term use and their efficacy is not completely reliable. Other treatments, such as opioid analgesics, tramadol, and topical capsaicin, have unclear long-term effects and safety.26 Therefore, effective pain relief for PHN is a challenge for clinicians.
SGJT, also known as Shokenchu-to, Xiaojianzhong-tang, is described in a medical book called “Sang-Han-Lun” written in the 3rd century.13 Previous studies have reported that SGJT has antioxidant, antidepressant, inhibitory of the immunoglobulin E dependent allergic reactions, anti-inflammatory, and immune response enhancement effects.27–29 This herbal medicine is composed of six kinds of herbs: Paeoniae Radix Rubra, Cinnamomi Ramulus, Jujubae Fructus, Glycyrrhizae Radix, Zingiberis Rhizoma, and Saccharum Granorum. Paeoniflorin, a chief active ingredient in the root of Paeoniae radix, is effective in alleviating chronic neuralgia and promoting the repair of the damaged nerves by reducing the levels of inflammatory factors and Schwann cell apoptosis in rats with chronic sciatica.30 Cinnamic acid, a standard compound from Cinnamomi Ramulus, has an effective analgesic action against oxaliplatin-induced neuropathic pain, especially in relieving cold and mechanical allodynia.31 Glycyrrhizin, as one of the main active components of Glycyrrhizae Radix, was indicated to have anti-hyperalgesic effect in mice with diabetic peripheral neuropathy.32 In addition, gingerol and shogaol had the effects of decreasing mechanical hypersensitivity and improving anxiety-like behavior in animal models, which showed that Zingiberis Rhizoma could have an important function in treating neuropathic pain.33 These experimental evidence support that SGJT could have important clinical functions in treating PHN.
Previous randomized controlled trials of SGJT have significantly affected digestive diseases such as chronic atrophic gastritis, peptic ulcer, constipation-predominant irritable bowel syndrome, and there are only observational studies in other fields, including nocturnal enuresis and heartburn.34–37 Previously, there have been severe studies for SGJT, but clinical trials using it for pain reduction have been scarce. Therefore, this study conducting a randomized controlled trial to assess the effectiveness and safety of SGJT for relieving neuropathic pain, will be highly meaningful.
Gabapentin and pregabalin, used as PHN standard treatments in this study, act on the α2-δ subunit of the calcium ion channel with an analgesic action on neuropathic pain.38 These are first-line drugs for treating PHN in the guidelines in Europe and the United States.39–42 Gabapentin and pregabalin have no serious complications compared with other anticonvulsants or antidepressants.43 In addition, these are considered attractive options for older patients who take several concomitant medications because they have a low propensity for drug interactions with co-administered drugs, not metabolized by cytochrome P450 system drug-metabolizing enzymes.2,44 Therefore, we designed these medications as suitable standard treatment for those who will be co-administered with study drugs.
In a review regarding herbal medicine for PNH, patients who received herbal medicine with western medicine for four weeks obtained a significant improvement in pain intensity relief than those who received less than four weeks. This suggests that a longer duration of treatment is required to attain meaningful pain relief in PHN treatment.6 Based on this result, this study will be conducted for six weeks.
To our knowledge, this is the first study to investigate the efficacy of SIKD1977 with standard treatments for PHN patients, as a randomized, placebo-controlled, double-blind, multicenter trial. The study will ascertain whether using SIKD1977 with standard treatment for PHN can relieve pain intensity, improve quality of life, and reduce the use of analgesic medicine. These findings will help clinicians to utilize SIKD1977 with standard treatment as a therapeutic option for patients with PHN.
Abbreviations
ADPS, average daily pain score; ANCOVA, analysis of covariance; e-CRF, electronic case report form; EQ-5D-5L, 5-level EuroQol-5 dimension; FAS, full analysis set; IRB, Institutional Review Board; IWRS, Interactive Web Response System; NRS, numeric rating scale; GIC, Patient Global Impression of Change; PHN, postherpetic neuralgia; PP, per-protocol; SF-MPQ, Short-Form McGill Pain Questionnaire; SGJT, Sogeonjungtang; TCA, tricyclic antidepressant; VAS, visual analogue scale.
Trial Status
The final protocol version is 2.2, dated 26 September 2022. The recruitment will begin on 1 February 2023. We anticipate that it would be take 2 years to complete this trial.
Data Sharing Statement
Data and material from this trial are available upon reasonable request and approved by the corresponding author.
Author Contributions
Hyo-Rim Jo is the first author. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; have agreed on the journal to which the article has been submitted; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. And they will also accept the responsibility for the study protocol.
Funding
This research is supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant number HF22C0071). The funding source has no role in the design of the study, collection, analysis, and interpretation of data, and in writing the manuscript.
Disclosure
Yong-Gyun Kim and Young-Ee Kwon are employees of Samik Pharmaceutical Company, the sponsor of this study. None of the other authors were financially compensated for their collaboration in this project or for the development of this paper. The authors report no other conflicts of interest in this work.
References
1. Jung BF, Johnson RW, Griffin DR, Dworkin RH. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004;62(9):1545–1551. doi:10.1212/01.WNL.0000123261.00004.29
2. Mallick-Searle T, Snodgrass B, Brant JM. Postherpetic neuralgia: epidemiology, pathophysiology, and pain management pharmacology. J Multidiscip Healthc. 2016;9:447–454. doi:10.2147/JMDH.S106340
3. Lukas K, Edte A, Bertrand I. The impact of herpes zoster and post-herpetic neuralgia on quality of life: patient-reported outcomes in six European countries. Z Gesundh Wiss. 2012;20(4):441–451. doi:10.1007/s10389-011-0481-8
4. Argoff CE. Review of current guidelines on the care of postherpetic neuralgia. Postgrad Med. 2011;123(5):134–142. doi:10.3810/pgm.2011.09.2469
5. Fu Q, Wang J, Tao GS, Lu S, Li L, Bao LL. Efficacy of traditional Chinese medicine on herpes zoster (meta analysis). J Dis Monit Control. 2014;8(3):133–135.
6. Liang H, Coyle ME, Wang K, et al. Oral Chinese herbal medicine for post-herpetic neuralgia: a systematic review and meta-analysis of randomized controlled trials. Eur J Integr Med. 2017;10:46–56. doi:10.1016/j.eujim.2017.01.005
7. Kim MJ, Cha HJ, Lee YR, et al. A review of Korean medicine treatment for postherpetic neuralgia. J Acupunct Res. 2021;38(4):245–256. doi:10.13045/jar.2021.00171
8. Shin YS, Lee SI, Review A. Study of researches on Jakyakgamcho-tang. Herbal Formula Sci. 2017;25(2):271–302. doi:10.14374/HFS.2017.25.2.271
9. Cao D, Zhang YM. Effect of adjuvant treatment with modified Shaoyao Gancao Decoction on pain mediators and inflammatory factors in patients with cancer pain. China Med Herald. 2020;17:143–146.
10. Chen Y, Huang W, Liu L, et al. Effectiveness and safety of traditional Chinese medicine Shaoyao Gancao Tang for the treatment of restless leg syndrome: a protocol for systematic review and meta-analysis. Medicine. 2020;99(40):e22401. doi:10.1097/MD.0000000000022401
11. Ota K, Fukui K, Nakamura E, et al. Effect of Shakuyaku-kanzo-to in patients with muscle cramps: a systematic literature review. J Gen Fam Med. 2020;21(3):56–62. doi:10.1002/jgf2.302
12. Hidaka T, Shima T, Nagira K, et al. Herbal medicine Shakuyaku-kanzo-to reduces paclitaxel-induced painful peripheral neuropathy in mice. Eur J Pain. 2009;13(1):22–27. doi:10.1016/j.ejpain.2008.03.003
13. Jo HR, Choi SK, Sung WS, et al. Clinical Study of Sogunjung-tang on neuropathic pain: a retrospective case series observational study. Herbal Formula Sci. 2021;29(4):229–237.
14. Park KS. Validation of the Korean Versions of the Short-Form McGill Pain Questionnaire (SF-MPQ) and Neuropathic Pain Scale (NPS) in Neuropathic Pain Patients (Masters thesis). Seoul, Korea: Seoul National University; 2015.
15. Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30(2):191–197. doi:10.1016/0304-3959(87)91074-8
16. Kim SH, Ahn J, Ock M, et al. The EQ-5D-5L valuation study in Korea. Qual Life Res. 2016;25(7):1845–1852. doi:10.1007/s11136-015-1205-2
17. Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole MR. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi:10.1016/S0304-3959(01)00349-9
18. Torres-Saavedra PA, Winter KA. An overview of phase 2 clinical trial designs. Int J Radiat Oncol Biol Phys. 2022;112(1):22–29. doi:10.1016/j.ijrobp.2021.07.1700
19. Teare MD, Dimairo M, Shephard N, Hayman A, Whitehead A, Walters SJ. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials. 2014;15:264. doi:10.1186/1745-6215-15-264
20. Zhang QH. Clinical observation of “Yiqi Huoxue Huatan Decoction” in treating postherpetic neuralgia. Shanghai J Trad Chin Med. 2011;12:61–62.
21. Cheng J. Quyuzhentong Decoction Jiajian Analgesic Treatment of Postherpetic Neuralgia Clinical Observation (Masters thesis). Hubei, China: Hubei University of Chinese Medicine; 2009.
22. Zha JD. The Clinical Observation of Shen Tong Zhu Yu Tang Combined with Doxepin on the Postherpetic Neuralgia (Masters thesis). Hubei, China: Hubei University of Chinese Medicine; 2013.
23. Sun CQ, Wen WW, Chen SL, Lou XH, Xu TH. Efficacy of traditional Chinese medicine combined with western medicine on treating postherpetic neuralgia. Chin J Dermato Venerol Integ Trad Med. 2012;11(2):98–99.
24. Zhang MX, Wang HT, Sun SH. Clinical observation of Xue Fu Zhu Yu Tang combined with gabapentin in treating on 34 cases of Postherpetic Neuralgia. Chin Med Modern Distance Educ China. 2014;12(4):47–48.
25. Kim YJ, Choi HH, Lee CG, Kim YC. Initiation of herpes zoster treatment and postherpetic neuralgia. Korean J Pain. 2008;21:93–105. doi:10.3344/kjp.2008.21.2.93
26. Wang H, Wan R, Chen S, et al. Comparison of efficacy of acupuncture-related therapy in the treatment of postherpetic neuralgia: a network meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2022;2022:3975389. doi:10.1155/2022/3975389
27. Park SY, Back JH, Seo JM. Effect of Gamisogunjung-tang on antioxidation activity in rats induced aging by D-galactose. J Korean Oriental Pediatr. 2005;19(1):153–171.
28. Bang JH. Anti-Depressive Effect of Sogeonjungtang (Masters thesis). Seoul, Korea: Kyunghee University; 2010.
29. Jung IH, Kim JY, Kam CW, Park DI. Inhibitory effects on the type I hypersensitivity and inflammatory reaction of Sogunjung-tang. J Physiol Pathol Korean Med. 2003;17(5):1188–1193.
30. Zhang D, Chang S, Li X, et al. Therapeutic effect of paeoniflorin on chronic constriction injury of the sciatic nerve via the inhibition of Schwann cell apoptosis. Phytother Res. 2022;36(6):2572–2582. doi:10.1002/ptr.7472
31. Chae HK, Kim W, Kim SK. Phytochemicals of cinnamomi cortex: cinnamic acid, but not cinnamaldehyde, attenuates oxaliplatin-induced cold and mechanical hypersensitivity in rats. Nutrients. 2019;11(2):432. doi:10.3390/nu11020432
32. Ciarlo L, Marzoli F, Minosi P, Matarrese P, Pieretti S. Ammonium glycyrrhizinate prevents apoptosis and mitochondrial dysfunction induced by high glucose in SH-SY5Y cell line and counteracts neuropathic pain in streptozotocin-induced diabetic mice. Biomedicines. 2021;9(6):608. doi:10.3390/biomedicines9060608
33. Shen CL, Wang R, Ji G, et al. Dietary supplementation of gingerols- and shogaols-enriched ginger root extract attenuate pain-associated behaviors while modulating gut microbiota and metabolites in rats with spinal nerve ligation. J Nutr Biochem. 2022;100:108904. doi:10.1016/j.jnutbio.2021.108904
34. He JM. XiaoJianZhongTang chronic atrophic gastritis of 65 cases. Gansu J TCM. 2010;23(7):15–16.
35. Weng SH. Clinical Research of Xiaojianzhongtang on Peptic Ulcer with Cool in Spleen and Stomach (Masters thesis). Guangzhou, China: Guangzhou University of Chinese Medicine; 2009.
36. Ogawa-Ochiai K, Ohama K. Efficiency of Japanese herbal medicine shokenchuto for nocturnal enuresis: an observational study. Medicine. 2022;101(32):e29220. doi:10.1097/MD.0000000000029220
37. Lee DE, Jang HY, Lee YL, Lee YS. Clinical study of sogunjung-tang granules in 30 cases of heartburn. J Int Korean Med. 2019;40(6):1193–1201. doi:10.22246/jikm.2019.40.6.1193
38. Ifuku M, Iseki M, Hidaka I, Morita Y, Komatus S, Inada E. Replacement of gabapentin with pregabalin in postherpetic neuralgia therapy. Pain Med. 2011;12(7):1112–1116. doi:10.1111/j.1526-4637.2011.01162.x
39. O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122:22–32. doi:10.1016/j.amjmed.2009.04.007
40. Attal N, Cruccu G, Haanpaa M, et al. EFNS guidelines on pharmacological treatment of neuropathic pain. EurJ Neurol. 2006;13:1153–1169. doi:10.1111/j.1468-1331.2006.01511.x
41. Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi:10.1016/j.pain.2007.08.033
42. Finnerup NB, Otto M, McQuay HJ, et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118:289–305. doi:10.1016/j.pain.2005.08.013
43. Yoon MH. Diagnosis and treatment of postherpetic neuralgia. J Korean Med Assoc. 2006;49:701–706. doi:10.5124/jkma.2006.49.8.701
44. Singh D, Kennedy DH. The use of gabapentin for the treatment of postherpetic neuralgia. Clin Ther. 2003;25(3):852–889. doi:10.1016/S0149-2918(03)80111-X
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
