Back to Journals » Journal of Pain Research » Volume 16
Efficacy and Safety of a Novel Plum Blossom Needling with Mild Moxibustion Device for Upper Limb Pain Disorder and Motor Dysfunction in Patients with Stage 1 Post-Stroke Shoulder-Hand Syndrome: Study Protocol for a Multi-Center, Single-Blind, Randomized Sham-Controlled Trial
Authors Meng X , Sun J, Liu Q, Huang Y, Qiu X, Seto DJ, Li Y, Wang L, Li C, Gao S, Yu H, Zhao J, Zhao B
Received 4 November 2022
Accepted for publication 19 January 2023
Published 13 February 2023 Volume 2023:16 Pages 407—420
DOI https://doi.org/10.2147/JPR.S396195
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Xiaonan Meng,1,2,* Jie Sun,3,* Qi Liu,4,* Yueping Huang,5 Xianwen Qiu,6 David Jung Seto,7,8 Ying Li,3 Liping Wang,2 Chunying Li,2 Sen Gao,9 Haikuo Yu,10 Jiping Zhao,1 Baixiao Zhao1
1Department of Acupuncture and Moxibustion, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 2Department of Acupuncture and Moxibustion, Beijing Huguosi TCM Hospital, Affiliated with Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 3Department of Integrated Chinese and Western Medicine Rehabilitation, Beijing Xiaotangshan Hospital, Beijing, People’s Republic of China; 4Department of General Internal Medicine, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, People’s Republic of China; 5School of Acupuncture-Moxibustion and Tuina, Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 6Department of Acupuncture and Moxibustion, Beijing Shichahai Community Healthcare Center, Beijing, People’s Republic of China; 7Division of Integrative Medicine, Department of Medicine, Veterans Affairs Greater Los Angeles Healthcare System, Los Angeles, CA, USA; 8Department of Medicine, David Geffen School of Medicine, University of California, Los Angeles, CA, USA; 9Department of Rehabilitation, Beijing Huguosi TCM Hospital, affiliated with Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 10Rehabilitation Department, Xuanwu Hospital Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiping Zhao; Baixiao Zhao, Email [email protected]; [email protected]
Background: Post-stroke shoulder-hand syndrome (PS-SHS), a common neurological comorbidity after stroke episodes, poses a grave threat on patients’ functional recovery. Preliminary trials have demonstrated that the acupuncture and moxibustion treatment, including a dermal acupuncture tapping method known as plum blossom needling (PBN) can improve pain and motor dysfunctions in patients with PS-SHS. However, there are few reports describing simultaneous moxibustion treatment in combination with PBN. Hence, a novel plum blossom needle device with mild moxibustion (PBNMM) was developed to evaluate its potential efficacy and safety in patients with stage 1 PS-SHS.
Materials and Methods: This multicenter, sham-controlled, randomized controlled trial (RCT) will recruit 102 eligible patients with stage 1 PS-SHS from three clinical centers, randomly allocated in a ratio of 1:1:1 to the PBNMM group, PBNMM with no moxa smoke (PBNMM-NMS) group and sham control group. Patients in each group will receive a 30-minute treatment once per day for 4 weeks, with 5 consecutive sessions per week, for a total of 20 sessions. The primary outcome measure will be defined as the decreased scores from baseline in the visual analog scale (VAS) assessment at week 4. Secondary outcome measures will include scores on the Fugl-Meyer Assessment of the Upper Extremity Scale (FMA-UE), the Modified Barthel Index (MBI), and the somatosensory evoked potential (SEP) records. All outcomes will be evaluated at baseline and weeks 4, 5, 6 and 10, and the intention-to-treat analysis will be applied.
Conclusion: This study aims to provide robust evidence for the efficacy and safety of the PBNMM for PS-SHS treatment, as well as the specific impact of moxibustion smoke itself in dealing with PS-SHS.
Clinical Trial Registration: Chinese Clinical Trial Registry No. ChiCTR2200062441. Registered on 7 August 2022.
Keywords: plum blossom needling, mild moxibustion, novel device, post-stroke shoulder-hand syndrome, protocol, randomized controlled trial
Introduction
Stroke, regarded as the top leading cause of death and disability, exerts heavy economic and social burdens worldwide.1–3 Post-stroke shoulder-hand syndrome (PS-SHS), also known as post-stroke complex regional pain syndrome, was first observed by Oscar Stejnbroker,4 and is experienced by up to 50% of stroke patients.5 Stage 1 PS-SHS is usually characterized by the regional pain, spasticity, sympathetic dysfunction and motor impairment.6–9 Of note, it is challenging for clinicians to prevent its progression from stage 1 to its end-stage presentation of irreversible joint degeneration and amyotrophy.10 The current interventions for PS-SHS treatment vary a lot, including physical rehabilitation, pharmaceutical therapy and neuromodulation. However, there is insufficient evidence or general expert-based consensus to support their efficacy and safety.11 Despite prospects for effective and feasible treatment for PS-SHS beginning to emerge, for example with a recent high quality clinical trial demonstrating that vagal nerve stimulation paired with rehabilitation may improve PS-SHS outcomes,12 this surgical approach will still be invasive and device-implanted, causing the potential extra risk and heavy economic burden. More therapeutic strategies for PS-SHS are still yet to be explored.13–15
Acupuncture and moxibustion, two essential components of Traditional Chinese Medicine (TCM), are considered equally potent therapeutic modalities that have historically been applied together in clinical treatments since ancient times.16 Acupuncture has been reported as an effective treatment method for various diseases, including PS-SHS.17–20 In contrast to some of the most commonly used insertional acupuncture needling techniques, the conventional plum blossom needling (PBN) is the one that provides superficial dermal stimulations simply by tapping along the pathway of meridians with its seven short filiform needles (1mm in diameter and 5mm in length) on the face of a hammer (15cm in length) (Figure 1A). Therefore, this technique is potentially more convenient for practitioners and more tolerable for patients because of its painless nature. Some recent clinical trials highlighted the use of PBN for the treatment of dermatologic and psychiatric issues.21–26 At the same time, other clinical results showed that PBN could also alleviate the upper limb post-stroke spasticity due to its potential effect of activating the regional blood circulation and restoring regional neurological impairment with the mild stimulation, which could possibly prevent from provoking the increase of spasticity.27,28
 |
Figure 1 (A) conventional plum blossom needling (PBN); (B) novel plum blossom needle with mild moxibustion (PBNMM). |
Moxibustion has been utilized for more than 2000 years in China as a form of preventive medicine, as well as for disease treatment. Presently, mild moxibustion (MM), one of the most popular forms of moxibustion application, is a technique that involves warming the skin and subdermal tissues using aged moxa floss that has been ignited and suspended over acupoints at a comfortable distance. MM, also known as indirect moxibustion, is a gentle and safe procedure with broad clinical applications, and it has also been explored as an adjunctive component in the rehabilitation of patients suffering from PS-SHS.8,29 Radiant heat, as well as chemical components liberated in volatile oils, other vaporized substances and moxibustion smoke (MS) generated through the combustion process, have been reported as being potential factors that drive the clinical effects of MM.30–33 Moxibustion utilized for PH-SHS rely on its favorable effects for regional skin congestion improvement, capillary expansion, activations of blood and lymph circulation, sedation and analgesic nature.34,35
Despite the improved policies of public health and ways of healthcare delivery, the burden of stroke has been increasing in China.36 Stroke patients have to spend hours on commuting and waiting before they finally receive treatments of PBN or MM once at a time, conducted by clinicians in hospitals, which is time-and-economy consuming. Considering the low cost of PBN and the convenience of MM, our team has developed a novel device with an authorized patent by the State Intellectual Property Office of China (No. ZL 202020221246.2) called “plum blossom needle with mild moxibustion (PBNMM)” (Figure 1B), which is able to efficiently deliver both of these treatment modalities simultaneously. What’s more, PBNMM could even be utilized in the community or family care through a comprehensive training. Therefore, this device is designed with the goal of alleviating pain and improving upper limb motor dysfunction for Phase 1 PS-SHS patients due to its characteristics of high-efficiency, low cost, easy acceptance and time-saving.
Given the considerable advantages and potential mechanisms of PBNMM in producing contributory effects in PS-SHS, a clinical trial will be conducted not only to evaluate its efficacy and safety of for upper limb pain disorder and motor dysfunction of stage 1 PS-SHS patients, but also to explore its feasibility in further widespread clinical practice.
Methods
Trial Design and Setting
This is a prospective, single-blind, sham-controlled, randomized controlled trial (RCT) that will be conducted at three medical centers in Beijing, China, including Beijing Huguosi TCM Hospital affiliated with Beijing University of Chinese Medicine (BUCM), Beijing Shichahai Community Healthcare Center, and Beijing Xiaotangshan Hospital, with assistance and guidance from Dongzhimen Hospital BUCM, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, and Xuanwu Hospital Capital Medical University. A total of 102 eligible patients with stage 1 PS-SHS from 3 clinical centers will be randomly recruited and allocated in a ratio of 1:1:1 to (A) the PBNMM group, (B) the PBNMM with no moxa smoke (PBNMM-NMS) group, and (C) the sham control group. Patients in each group will receive the 30-minute treatment once per day for 4 weeks, with 5 consecutive sessions per week, for a total of 20 sessions. The primary outcome will be defined as the decreased scores from baseline in the visual analog scale (VAS) assessment of the intensity of regionalized pain in the affected upper limb at week 4. Secondary outcome measures will include scores on the Fugl-Meyer Assessment of the Upper Extremity Scale (FMA-UE), the Modified Barthel Index (MBI), and the somatosensory evoked potential (SEP) records. All outcomes will be evaluated at baseline and week 4, 5, 6 and 10 post-randomization. This trial protocol follows the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline and the Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) guideline, the Standard Protocol Items: Recommendations for Intervention Trials (SPIRIT) 2013,37 and details of the intervention are described in accordance with the Template for Intervention Description and Replication (TIDieR) checklist for reporting placebo and sham controls.38 The study has been pre-registered (No. ChiCTR2200062441) before the first participant’s recruitment. The study’s flow chart and schedule chart are presented in Figure 2 and Table 1, respectively.
 |
Table 1 Study Schedule Chart |
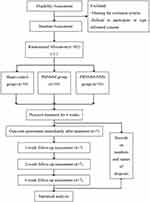 |
Figure 2 Study flow chart. |
Recruitment of Participants
Participants who meet the criteria and sign the informed consent documents will be eventually recruited in these three clinical centers through our official websites, social media and bulletin boards. The recruitment is expected to begin on December 1, 2022 and end in June 2024.
Inclusion Criteria
Participants who meet the following criteria will be included, and a minimal 1-week washout period will be enforced:
- Patients diagnosed by cerebral computed tomography (CT) or magnetic resonance imaging (MRI) and characterized by upper limb pain and motor dysfunction.39,40
- Within 90 days following the onset of stroke episodes.
- Patients suffering from stroke for no more than two times with the muscle strength greater than grade II and muscle tone less grade II on the affected side.
- Male and female patients between the ages of 35–76 years old, and no restrictions on any other demographic factors.
- Patients with the stable vital signs, (ie, heart rate of 60–105 beats per minute, blood pressure of 100–140/60-90 mmHg, pulse oximetry of 95–100% oxygen saturation on room air, and respiratory rate of 16–22 breaths per minute).
- Patients with a full understanding and total agreement with the purpose of this study, explained by researchers in details. And willingness to participate and cooperate, by signing the informed consent documents, with the reserved right to withdraw from the study anytime for any reason.
Exclusion Criteria
Participants who meet the following criteria will be excluded:
- Patients with diagnosis of any cerebrovascular diseases other than stroke.
- Patients with a diagnosis of any severe psychiatric disorder that would significantly impair the individual’s ability to participate in the clinical trial.
- Patients with an abnormal pain threshold caused by thalamic allodynia.
- Patients with MS intolerance, current pulmonary infection or any history of chronic respiratory diseases.
- Any pre-existing soft tissue injury of the shoulder, including dislocation or subluxation, that occurred prior to the onset of stroke.
- Any other treatments rather than this protocol.
Randomization
The randomization sequence, which employs block randomization method without stratification using the Matlab software (The MathWorks, Inc., USA), will be generated by an independent statistician who will not be involved in the intervention procedure and had no direct contact with
the study participants, and kept in an opaque sealed envelope. The block size will be also concealed rigorously. The envelopes with only randomization numbers will be unsealed by treatment providers who will be informed of the group allocation and detailed treatment protocol, after a feasible participant has signed the informed consent documentation. The subjects’ identification codes will be recorded on the case report forms (CRFs) and randomization envelopes.
Blinding
Due to the nature of the interventional RCT and PBNMM intervention, all treatment providers must be informed of the allocation and the detailed treatment procedure. However, all participants will be blinded throughout the whole course. The novel autonomously-designed PBNMM device (manufactured by the Beijing Zhongyan Taihe Medical Instrument Co., Ltd) that will be applied is constructed entirely of steel. Seven short filiform needles with dimensions of 1 mm in diameter by 5 mm in length are studded at the base of this device, 1 cm apart from each other, in an arrangement that is similar to the structure of a conventional plum blossom needle. A chamber of 20 mm diameter and 30 mm length was also innovatively designed within the head of the hammer, in which a lit moxa stick is placed inside. For air and smoke ventilation, four apertures of 3 mm diameter each have been placed at the base, as well as four apertures of 8 mm diameter each on the wall of the device.
For the sham control group, a modified PBNMM device will be used, in which the tips of the seven short filiform needles will be dull instead of sharp, in order to minimize needle stimulation on the dermal tissues. Additionally, charcoal moxa, which generates only small amounts of visible smoke, will be placed inside of the modified device instead of conventional moxa. Several insulation layers, consisting of aluminum foil combined with high silica glass fiber, will also be placed within the chamber at the base of the modified device, in order to limit the transmission of radiant heat (Figure 3B). These blinding measures are intended to ensure that the sham devices will all externally appear to be identical to the verum devices (Figure 3A). Additionally, all participants will wear eye masks throughout the treatment to further ensure the implementation of blinding. The rehabilitation assessors responsible for treatment outcomes, and the statisticians responsible for the outcome analysis, will also be blinded to the participants’ classification, which will not be revealed until the final data analysis report is completed.
 |
Figure 3 The exterior view (A) and the interior view (B) of PBNMM and sham-PBNMM devices. |
Interventions
Throughout this clinical trial, all participants’ vital signs will be closely monitored and standard-of-care medical treatment, including any necessary medications, will be provided to help maintain hemodynamic stability. Additionally, each participant will continue to participate in a standard-of-care physical and occupational therapy program to address their basic rehabilitation needs.
Each participant will be treated individually, alone in a treatment room with the treatment provider, without any ability to communicate with any other participants. The indoor temperature will be maintained within a range of 24 ± 3 °C and the humidity will be maintained within a range of 50 ± 5%, and these environmental parameters will be continuously monitored using the thermometer and hygrometer functions of a weather meter (Kestrel NK3000, USA). We have selected treatment facilities in which no interior remodeling has been performed over the past three years, in order to help minimize the presence of any volatile chemical substances in the air. Only one window in the treatment room will remain slightly ajar, in order to provide air and smoke ventilation and maintain a stable ambient concentration of particulate matter (PM) with an aerodynamic diameter of less than 10 mm (PM10). Each participant will lie down in a lateral decubitus position, with the healthy side down, and will face away from the treatment provider and wear eye masks during the session. All the tapping regions will be regularly disinfected. At the conclusion of each session, the participant and treatment provider will both vacate the room and all remaining MS will be removed through air ventilation. As noted above, all conventional moxa floss utilized in this study will have been aged for three years.
Sham Control Group
As described above, the novel PBNMM device will be modified by adding an insulation layer that fully occludes the four ventilation apertures located at its base. One smokeless charcoal moxa stick will be lit and sealed inside of each modified device to minimize smoke production. A few minutes later, the complete consumption of all oxygen within the base-sealed chamber will invisibly extinguish the charcoal moxa without any external indications that the fire has been quenched. The tips of the seven short filiform needles on the face of the modified PBNMM device will be dull, rather than sharp. The device will be held by the end of its handle, which measures 17 cm in length, and gently tapped along two sham meridian pathways, each being located 1 cun (a unit of measurement that is used in acupuncture) away from each verum meridian pathway, as listed in Supplementary File, where the most painful points were localized according to our team’s previous clinical results.41 Each sham meridian pathway will be tapped gently back and forth for 15 minutes at a frequency of 140 taps per minute, in a manner that leaves no marks or discoloration of the overlying skin in these regions.
PBNMM Group
The novel PBNMM device will be utilized in its conventional design, with seven short, sharp filiform needles studding its face, and with one conventional moxa stick lit inside. No thermal insulation barrier will be present. Instead, a metal mesh will cover the inside of the chamber’s base, in order to catch any falling ashes and prevent them from landing on the participants’ skin. After local skin disinfection using cotton balls with 75% ethanol, the verum device will be also held by the end of its handle, which also measures 17 cm in length, and will be gently tapped along the two verum meridian pathways as stated above, in such a manner that the overlying skin in these regions will become slightly reddish and warm by the conclusion of the treatment session.
PBNMM-NMS Group
One lit stick of smokeless charcoal moxa will be placed inside of an unmodified PBNMM device, and the rest of the intervention procedures will be identical to that of the PBNMM group.
For all groups, each treatment will last for 30 minutes and will be provided once per day, five days each week, over a course of four weeks. Given the fact that levels of moxibustion smoke concentration (MSC) may vary at different distances from the locus of combustion, once each week during the interventions in all groups, a portable microcomputer dust monitor (Model No. LD-5C [B], Beijing Lvlin Innovation Digital Technology Co., Ltd) will be utilized in order to record the MSC in PM10. This dust monitor will be placed at a distance of 5 cm from each participant’s nostril, where the measurements should closely reflect the MSC in the air that each individual is actually inhaling. Based on our pre-test results, the average PM10 concentrations in the three groups are projected to be (A) 0.03 ± 0.02 mg/m3, (B) 0.97 ± 0.16 mg/m3 and (C) 0.32 ± 0.12 mg/m3, respectively. In all groups, once each week, the dermal temperature in the region of intervention will be recorded at the conclusion of treatment using a non-contact thermometer (BOSCH GIS500, Germany).
Outcome Measurements
Primary Outcome
The change in the visual analog scale (VAS) pain assessment from baseline to week 4 has been designated as the primary outcome measure. Pain is the most common symptom of PS-SHS and usually disables upper limb motor function or greatly limits the normal range of motion, thus contributing to further exacerbation of regional muscle spasm and eventually irreversible amyotrophy.39,42,43 With the VAS scoring system, a horizontal line of 100 mm in length will be utilized, with its left extreme of “0” which representing completely no pain, and its right extreme of “10” representing intolerable pain. On a scale of 0 to 10, participants will each rate their pain accordingly by marking a point along the straight line, in order to categorize the degree of pain that they experienced over a most recent period of 3 days.
Secondary Outcome
The FMA-UE will be utilized to quantify motor function on the affected side, as it is widely recognized for its consistency and reliability.44 This scale includes a systemic evaluation of motion, range of motion, and coordination of the affected joints of the upper limbs, and is composed of 33 items, with up to 2 points being awarded for performance on each item, and with a total possible score of 66 points overall. Higher scores generally indicate a better prognosis for improvement of upper extremity motor function. The FMA-UE is reliable (Cronbach’s α = 0.94~0.98) and valid (R = 0.68).45,46
The MBI is a functional assessment for evaluating post-stroke patients’ capacity for independently performing activities of daily living,47–49 and evaluates ten common daily activities, with a total possible score of 100 points. Lower scores indicate more severe impairment in the ability to perform independent self-care. The MBI is also reliable (Cronbach’s α = 0.90) and valid (R = 0.946).48,50 The two patient-reported scales applied to evaluate the clinical outcomes are of sufficient adequate reliability and validity. The outcome measures used here are common in stroke rehabilitation trials and are valid, reliable, and sensitive to change.
The somatosensory evoked potential (SEP) is commonly adopted as an objective indicator of functional recovery for post-stroke patients in the rehabilitation process.51,52 In our study, a standard electromyography device (Model: Viking Quest, Nicolet, US) will be applied for measuring SEP. The operation will be conducted in a room with average ambient temperature of 24°C. In accordance with the International 10–20 System for scalp electrode placement, participants in the supine position will have electrodes affixed over the contralateral somatosensory zone known as Cc’ and the reference zone known as Fz.53,54 Constant current square wave pulses will be used to stimulate the median nerve at the affected wrist at a frequency of 2–3 Hz, with 0.1–0.2 ms duration, and 20–3000 Hz for overall band pass width with 200 responses and a 50 ms analysis timeframe. The potential and N20 amplitude as key indicators for functional recovery will finally be recorded.
Sample Size
The primary outcome in this clinical trial is the decreased VAS score as stated above. To the best of our knowledge, there are no any previously reports concerning PBN combined with MM for PS-SHS treatment. Based on our pre-test results, the decrease VAS scores (Mean ±Standard Deviation) among three groups were 3.39 ± 0.67, 2.99 ± 0.90 and 2.53 ± 0.81, respectively. The PASS 15.0 (Power Analysis and Sample Size) software was applied for sample size calculation. This study was designed with a difference test hypothesis. According to one-way analysis of variance F-Tests, a total of 87 participants were considered necessary to achieve a significance level of 0.017 at a power of 0.8 in terms of multiple comparisons, 29 participants in each group. Assuming a 10% dropout rate, the final required sample size was expanded to 34 in each group, a total of 102 participants.
Safety Evaluation and Adverse Events
Each participant’s vital signs will be closely monitored throughout the entire course of this clinical trial. Any potential adverse events (AEs), such as cough, reactive airway disease, infection, burns or dermatologic disorders, will be carefully monitored and recorded in case report forms (CRFs). Any severe AE that leads to instable vital signs and withdrawal from this trial will be treated with immediate standard-of-care medical intervention. At the conclusion of the study, each AE will be carefully analyzed, categorized and reported. Any dropouts occurring for any reason during the course of this trial will be documented in the CRFs as well.
Data Management
The qualified evaluators will collect all patient data and meticulously record them on the CRFs. An independent researcher will also be designated as being responsible for reviewing and ensuring the accuracy of the CRFs. After the completion of this trial, all collected data will be input into an electronic database by two independent researchers, and then will be encrypted for privacy and in order to prevent any modifications. All paper and electronic data containing participants’ protected health information (PHI) will be kept confidential and stored safely by the researchers under the surveillance by the Medical Ethics Committees of Beijing Huguosi TCM Hospital, affiliated with BUCM.
Statistical Analysis
An independent statisticians will perform data analysis by using SPSS 21.0 among intention-to-treat population, defined as all randomly assigned participants with baseline data. Missing data will be treated via the last-observation-carried-forward method. For sensitivity analysis, a per protocol analysis (wherein participants completed at least 13 sessions, 65% treatment protocol) will be performed. We will also conduct the post hoc subgroup analysis to assess the effects of stroke subtypes on change of VAS at week 4. The chi-squared or Fisher’s exact test will be applied for categorical variables shown as the number and percent, such as gender, occupation et al. Considering that PS-SHS patients may be affected by differences in variables such as age, level of severity and time of onset, these potential continuous variables will be set as a covariate. Analysis of covariance (ANCOVA) will be used for continuous variables, described as mean ± standard deviation (SD), with the confidence interval (CI) at 95%, and a significance level of 5% (p < 0.05). To control for multiple comparisons, we will apply the least-significant-difference approach, in which pairwise intervention comparisons are measured at a given time only if the overall omnibus P value is statistically significant at 0.05. Cohen’s d will also be evaluated to measure the effect size of the difference. The effect size is normally interpreted as follows: 0.2, small effect size; 0.5, medium effect size; and 0.8, large effect size.
Quality Control
For better execution, experts of neurology, acupuncture-moxibustion, rehabilitation and statistics were consulted prior to drafting the final version of this protocol. The multidisciplinary discussions will be held once a month during the study period once per month during the study period. Only qualified researchers who have completed detailed training regarding the trial protocol may participate in this study. Only senior acupuncturists with more than five years of clinical experience will be permitted to administer the treatments, and only senior rehabilitation physicians with more than five years of clinical experience will be permitted to evaluate outcomes.
All collected data will be monitored by the Medical Ethics Committees at each medical center, with these independent entities having declared no conflicts of interest. A quality supervision team consisting of clinical experts will be established and will conduct the regular quality control once every two months.
Trial Status
The initial enrollment has not yet begun. The recruitment of participant is expected to begin on December 2022 and complete on June 2024.
Discussion
PS-SHS greatly threatens the survivors’ health and quality of life because of its high morbidity and disability rate.1–3,55 To date, the clinical effectiveness of acupuncture and moxibustion as potential treatments for this condition have been demonstrated by a number of studies.8,56 The majority of these acupuncture studies focus the use of the filiform needle. However, PBN, which is another important method of treatment within the field of acupuncture, has been underutilized in both research and clinical practice. At the same time, the clinical safety and efficacy of MS, which is one of the factors that potentially drives the effects of moxibustion treatment, has also yet to be studied in depth as well.
PBN has thus far been mostly utilized as an alternative technique to conventional acupuncture needling, and is preferred in some situations because of its safety, painlessness and less invasive nature. Most published research on PBN to date focuses on dermatologic disorders, such as its use as an adjunctive treatment in Bowen’s Disease, with the rationale that the technique may enhance topical drug delivery through the activation of regional blood circulation.21 Previously published research has also reported that PBN may enhance the effect of photodynamic therapy on basal cell carcinoma through augmenting the effects of aminolevulinic acid-photodynamic therapy.24 A study result showed that recovery from wrist motor impairment following stroke was enhanced with the addition of PBN tapping along the affected meridians.27 Given the positive outcomes from an admittedly small number of studies addressing diseases in a variety of medical specialties, it is not hard to see how patients and the medical community could all benefit from an expansion of research regarding the clinical uses of PBN.
On the other hand, with rising concerns about the safety of MS in an era in which air pollution is rapidly becoming a global public health issue, it is urgent that clinicians and researchers thoroughly investigate both the efficacy and safety of MS, which has been associated with environmental air pollution as well. Some chemical compounds that are present in MS, including CO2, CO and formaldehyde (CH2O), are indeed hazardous to the health of human beings.33,57 Our team has conducted quite a few animal experiments. However, all results surprisingly demonstrated clinical efficacy and safety of MS under the carefully designed concentration, totally out of expectations.58–63 Therefore, our hypothesis is that the efficacy and safety of MS possibly should be affected by the level of MSC, moxa quality and duration of exposure in MS. Firstly, moxa aged more than three years is considered as quality guarantee for its characteristics, and the moxibustion duration usually lasts for 20–30 min for each session.64 Hence, moxa that has been aged for three years will be utilized in 30-minute treatment sessions in this trial.
Based on a noteworthy study, the PM10 utilized to evaluate the air quality during moxibustion,65 was measured to be 3.54 mg/m3 in a typical clinical moxibustion room in Beijing and Tianjin, China.66 Although there are very few published clinical trials that have examined the effects of various levels of MSC, a few animal studies have demonstrated how certain thresholds of different levels of MSC may be associated with beneficial biochemical effects. One murine study showed that exposure to MS within a MSC range of 10–15 mg/m3 could help reduce the symptoms of Alzheimer’s disease in APP/PS1 mice through regulating the tricarboxylic acid cycle.61 Another study reported that different levels of MSC also yielded positive anti-aging effects through modulating monoamine neurotransmitter activity, between the MSC levels of 5 mg/m3 and 95 mg/m3.67 A toxic threshold study conducted in rats found that pulmonary function was adversely affected when the level of MSC reached to 168.76 mg/m3, although pulmonary function remained unaffected at the MSC level of 27.45 mg/m3, with both thresholds being way above the typical MSC levels that are observed in actual clinical practice. A cross-sectional survey showed that long-term, regular exposure to MS in 803 acupuncturists who regularly practice moxibustion did not appear to have a significant causal relationship with respiratory symptoms or diseases.68
Because of safety concerns, significantly lower MSC levels will be cautiously employed in this trial as follows: 1.37 ± 0.28 mg/m3 (PBNMM group), 0.53 ± 0.14 mg/m3 (PBNMM-NMS group) and 0.03 ± 0.01 mg/m3 (sham control group), with even the highest MSC level in the PBNMM group being 61.3% lower than the average MSC of 3.54 mg/m3 that has been observed to occur during conventional moxibustion treatments.66
Patients with PS-SHS rely heavily on the public health resources for treatment, leading to huge socioeconomic burdens. PBN and MM are both important components of acupuncture-moxibustion, but if a novel treatment could effectively combine these two therapeutic methods with simultaneous delivery, better clinical effects could potentially be expected with higher efficiency and at a lower cost. Therefore, our novel device PBNMM was developed for its low cost, ease of administration and potential for cost savings. Also, the novel PBNMM is constructed by metal with a bigger size comparing with the conventional PBN made of plastic, which could effectively enlarge the tapping area with relatively stronger stimulations.
The strengths of our trials are, if medical research can demonstrate its safety and effectiveness, this trial will be the first endeavor to employ this novel device, which may eventually become popularized for widespread use in community health and outpatient treatment centers, or even for at-home care of stroke patients by family members themselves, and help decrease the social and economic burdens of this disease. Also, this trial will take MSCs into consideration for its potential clinical efficacy. However, we do recognize that this trial does carry some limitations. Out of an abundance of caution regarding the safety of MS, this study will initially utilize a significantly lower MSC than that which is observed in typical clinical settings in which moxibustion treatment is administered. Additionally, a relatively small sample size consisting of an ethnically homogenous Chinese patient population at three hospitals in China may limit the immediate generalizability and global recognition of any positive results that may be observed in this study. Only short-term follow-up has been designated for this trial initially as well, although the follow-up interval could also be expanded in the future.
Ethics Statement
This trial involving human participants will be conducted in accordance with the Declaration of Helsinki, and the protocol has been reviewed and approved by the Medical Ethics Committees of Beijing Huguosi TCM Hospital, affiliated with BUCM (No. 2022–04) shared with Beijing Shichahai Community Healthcare Center, and Beijing Xiaotangshan Hospital (No. 2022–71). All participants will have unhindered access to verbal and written explanations of the experimental protocol and encouraged to consult the details. Participants will be fully informed of this study and sign the informed consent forms.
Conclusion
In short, our trial will aim to establish a foundation of clinical evidence regarding the potential effects of MS and this novel PBNMM device in the treatment of patients suffering from PS-SHS, thus providing hope and an innovative new method of treatment for this difficult condition.
Abbreviations
PS-SHS, post-stroke shoulder-hand syndrome; PBN, plum blossom needling; PBNMM, plum blossom needle device with mild moxibustion; RCT, randomized controlled trial; PBNMM-NMS, plum blossom needle device with mild moxibustion with no moxa smoke; VAS, visual analog scale; FMA-UE, Fugl-Meyer Assessment of the Upper Extremity; MBI, Modified Barthel Index; SEP, somatosensory evoked potential; TCM, Traditional Chinese Medicine; MM, mild moxibustion; MS, moxibustion smoke; BUCM, Beijing University of Chinese Medicine; CONSORT, Consolidated Standards of Reporting Trials; STRICTA, Standards for Reporting Interventions in Clinical Trials of Acupuncture; SPIRIT, the Standard Protocol Items: Recommendations for Intervention Trials; TIDieR, Template for Intervention Description and Replication; CT, computed tomography; MRI, magnetic resonance imaging; PM, particulate matter; MSC, moxibustion smoke concentration; PASS, Power Analysis and Sample Size; AEs, adverse events; CRFs, case report forms; PHI, protected health information; ANCOVA, analysis of covariance; SD, standard deviation; CI, confidence interval.
Acknowledgments
The authors thank all the staff who contributed to this trial with their effort and support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Beijing Xicheng District Talents Funding Project (No.2021-XCRC-Xicheng Healthcare Commission), Capital’s Funds for Health Improvement and Research, CFH (No. 2022-3-7028) and Beijing Xicheng District Financing Technology Project (XCSTS-SD2022-11). All funders play no role in the trial design, clinical intervention, data management, or manuscript publication.
Disclosure
All authors declare that there is no any conflicts of interest in this study.
References
1. Catala-Lopez F. Global, regional, and national mortality among young people aged 10-24 years, 1950-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2021;398(10311):1593–1618. doi:10.1016/S0140-6736(21)01546-4
2. Feigin VL, Stark BA, Johnson CO. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021.
3. Lanas F, Seron P. Facing the stroke burden worldwide. Lancet Global Health. 2021;9(3):e235–e236. doi:10.1016/S2214-109X(20)30520-9
4. Steinbrocker O. The shoulder-hand syndrome: present perspective. Arch Phys Med Rehabil. 1968;49(7):388–395.
5. Kim J, Yoon S, Kim J, Jeong Y, Kim Y. Neural substrates for poststroke complex regional pain syndrome type I: a retrospective case-control study using voxel-based lesion symptom mapping analysis. Pain. 2020;161(6):1311–1320. doi:10.1097/j.pain.0000000000001816
6. Borchers AT, Gershwin ME. Complex regional pain syndrome: a comprehensive and critical review. Autoimmun Rev. 2014;13(3):242–265. doi:10.1016/j.autrev.2013.10.006
7. Hannan MA, Sabeka MM, Miah MBA. Shoulder hand syndrome in hemispheric stroke. J Neurol Sci. 2013;1:333.
8. Lee MS, Shin BC, Kim JI, Han CH, Ernst E. Moxibustion for stroke rehabilitation: systematic review. Stroke. 2010;41(4):817–820. doi:10.1161/STROKEAHA.109.566851
9. Maihöfner C, Handwerker H, Neundörfer B, Birklein F. Patterns of cortical reorganization in complex regional pain syndrome. Neurology. 2003;61(12):1707–1715. doi:10.1212/01.WNL.0000098939.02752.8E
10. Zyluk A, Zyluk B. Zespół ramię-ręka u pacjentów po udarze mózgu [Shoulder-hand syndrome in patients after stroke]. Neurol Neurochir Pol. 1999;33(1):131–142. Polish.
11. Forouzanfar T, Köke A, Van Kleef M, Weber W. Treatment of complex regional pain syndrome type I. Eur j Pain. 2002;6(2):105–122. doi:10.1053/eujp.2001.0304
12. Dawson J, Liu C, Francisco G, et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial. Lancet. 2021;397(10284):1545–1553. doi:10.1016/S0140-6736(21)00475-X
13. Kondo I, Hosokawa K, Soma M, Iwata M, Maltais D. Protocol to prevent shoulder-hand syndrome after stroke. Arch Phys Med Rehabil. 2001;82(11):1619–1623. doi:10.1053/apmr.2001.25975
14. Pandian J, Sebastian I. Integrated approach to stroke burden: are we doing enough? Lancet Neurol. 2021;20(10):774–775. doi:10.1016/S1474-4422(21)00287-8
15. Acerra N, Souvlis T, Moseley G. Stroke, complex regional pain syndrome and phantom limb pain: can commonalities direct future management? J Rehabilitation Med. 2007;39(2):109–114. doi:10.2340/16501977-0027
16. Huang C, Liang J, Han L, Liu J, Yu M, Zhao B. Moxibustion in Early Chinese Medicine and Its Relation to the Origin of Meridians: a Study on the Unearthed Literatures. Evid Based Complementary Alternative Med. 2017;2017:8242136. doi:10.1155/2017/8242136
17. Choi T, Jun J, Lee H, Yun J, Joo M, Lee M. Traditional Chinese Medicine Interventions in the Rehabilitation of Cognitive and Motor Function in Patients With Stroke: an Overview and Evidence Map. Front Neurol. 2022;13:885095. doi:10.3389/fneur.2022.885095
18. Liu S, Zhang C, Cai Y, et al. Acupuncture for Post-stroke Shoulder-Hand Syndrome: a Systematic Review and Meta-Analysis. Front Neurol. 2019;10:433. doi:10.3389/fneur.2019.00433
19. Zhang K, Tang Q, Zhao C. Traditional manual acupuncture combined with rehabilitation therapy for shoulder hand syndrome after stroke within the Chinese healthcare system. Clin Rehabil. 2019;33(10):1699–1700. doi:10.1177/0269215519877739
20. Zhan J, Ai Y, Zhan L, et al. Effect of abdominal acupuncture combined with routine rehabilitation training on shoulder-hand syndrome after stroke: a randomized controlled trial. Integrative Med Res. 2022;11(2):100805. doi:10.1016/j.imr.2021.100805
21. Wu Y, Wang P, Zhang L, Wang B, Wang X. Enhancement of Photodynamic Therapy for Bowen’s Disease Using Plum-Blossom Needling to Augment Drug Delivery. Dermatol Surg. 2018;44(12):1516–1524. doi:10.1097/DSS.0000000000001608
22. Wittfoth D, Beise J, Manuel J, Bohne M, Wittfoth M. Bifocal emotion regulation through acupoint tapping in fear of flying. Neuroimage Clin. 2022;34:102996. doi:10.1016/j.nicl.2022.102996
23. Wang X, Han Y, Jin J, et al. Plum-blossom needle assisted photodynamic therapy for the treatment of oral potentially malignant disorder in the elderly. Photodiagnosis Photodyn Ther. 2019;25:296–299. doi:10.1016/j.pdpdt.2019.01.011
24. Mu X, Wang L, Wang L, Ge R, Dang H, Mou K. Plum-blossom needling enhanced the effect of photodynamic therapy on basal cell carcinoma. Photodiagnosis Photodyn Ther. 2018;23:339–341. doi:10.1016/j.pdpdt.2018.08.001
25. Li Q, Xie Y, Zha X. The clinical effect of plum blossom needle acupuncture with qi-invigorating superficies-consolidating therapy on seborrheic alopecia. Ann Palliat Med. 2020;9(3):1030–1036. doi:10.21037/apm-20-909
26. Church D, Stapleton P, Yang A, Gallo F. Is Tapping on Acupuncture Points an Active Ingredient in Emotional Freedom Techniques? A Systematic Review and Meta-analysis of Comparative Studies. J Nerv Ment Dis. 2018;206(10):783–793. doi:10.1097/NMD.0000000000000878
27. Wang YY, He L, Ye JB, et al. 梅花针叩刺腕部三阴经治疗中风后腕关节拘挛临床观察 [Clinical effect of plum-blossom needle tapping at three yin meridians of wrist on wrist joint contracture after stroke]. Zhongguo Zhen Jiu. 2020;40(1):26–29. Chinese. doi:10.13703/j.0255-2930.20190107-0008
28. Chen S, He L, Gao X, Wang Y, Kang G, Feng L. 梅花针叩刺联合康复训练治疗中风后手拘挛临床观察 [Clinical observation on plum-blossom needle combined with rehabilitation training for hand spasm after stroke]. Zhongguo Zhen Jiu. 2018;38(8):799–802. Chinese. doi:10.13703/j.0255-2930.2018.08.001
29. Liu Y, Chen W, Tan Y, et al. Analysis of the Registration Information on Interventions of Acupuncture and Moxibustion Trials in the International Clinical Trials Registry Platform. Evid Based Complementary Alternative Med. 2018;2018:1054629. doi:10.1155/2018/1054629
30. Gadau M, Yeung W, Liu H, et al. Acupuncture and moxibustion for lateral elbow pain: a systematic review of randomized controlled trials. BMC Complement Altern Med. 2014;14:136. doi:10.1186/1472-6882-14-136
31. Deng H, Shen X. The mechanism of moxibustion: ancient theory and modern research. Evid Based Complement Alternat Med. 2013;2013:379291. doi:10.1155/2013/379291
32. Sun C, Li Y, Kuang J, Liang X, Wu J, Ji C. The thermal performance of biological tissue under moxibustion therapy. J Therm Biol. 2019;83:103–111. doi:10.1016/j.jtherbio.2019.05.018
33. Xu J, Deng H, Shen X. Safety of moxibustion: a systematic review of case reports. Evid Based Complement Alternat Med. 2014;2014:783704. doi:10.1155/2014/783704
34. Shu Q, Wang H, Litscher D, et al. Acupuncture and Moxibustion have Different Effects on Fatigue by Regulating the Autonomic Nervous System: a Pilot Controlled Clinical Trial. Sci Rep. 2016;6:37846. doi:10.1038/srep37846
35. Li ZY, Huang Y, Yang YT, et al. Moxibustion eases chronic inflammatory visceral pain through regulating MEK, ERK and CREB in rats. World J Gastroenterol. 2017;23(34):6220–6230. doi:10.3748/wjg.v23.i34.6220
36. Zhao WY, Hua X, Ren X, et al. Increasing burden of stroke in China: a systematic review and meta-analysis of prevalence, incidence, mortality, and case fatality. Int J Stroke;2022. 17474930221135983. doi:10.1177/17474930221135983
37. Chan A, Tetzlaff J, Gøtzsche P, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi:10.1136/bmj.e7586
38. Howick J, Webster R, Rees J, et al. TIDieR-Placebo: a guide and checklist for reporting placebo and sham controls. PLoS Med. 2020;17(9):e1003294. doi:10.1371/journal.pmed.1003294
39. Pertoldi S, Di Benedetto P. Shoulder-hand syndrome after stroke. A complex regional pain syndrome. Eura Medicophys. 2005;41(4):283–292.
40. Frank A. The latest national clinical guideline for stroke. Clin Med. 2017;17(5):478. doi:10.7861/clinmedicine.17-5-478
41. Gao S, Meng XN, Li CY, Sun J, Yu HK. 王居易经络诊察法联合Bobath康复训练治疗脑卒中后肩手综合征I临床观察 [WANG Ju-yi’s Meridian diagnosis method combined with Bobath rehabilitation training for post-stroke shoulder-hand syndrome type]. Zhongguo Zhen Jiu. 2022;42(1):28–32. Chinese. doi:10.13703/j.0255-2930.20210202-k0011
42. Peng L, Zhang C, Zhou L, Zuo HX, He XK, Niu YM. Traditional manual acupuncture combined with rehabilitation therapy for shoulder hand syndrome after stroke within the Chinese healthcare system: a systematic review and meta-analysis. Clin Rehabil. 2018;32(4):429–439. doi:10.1177/0269215517729528
43. Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019;160(1):19–27. doi:10.1097/j.pain.0000000000001384
44. Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16(3):232–240. doi:10.1177/154596802401105171
45. Lin J, Hsueh I, Sheu C, Hsieh C. Psychometric properties of the sensory scale of the Fugl-Meyer Assessment in stroke patients. Clin Rehabil. 2004;18(4):391–397. doi:10.1191/0269215504cr737oa
46. Fu T, Wu C, Lin K, et al. Psychometric comparison of the shortened Fugl-Meyer Assessment and the streamlined Wolf Motor Function Test in stroke rehabilitation. Clin Rehabil. 2012;26(11):1043–1047. doi:10.1177/0269215511431474
47. Mahoney FI, Barthel DW. FUNCTIONAL EVALUATION: THE BARTHEL INDEX. Md State Med J. 1965;14:61–65.
48. Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42(8):703–709. doi:10.1016/0895-4356(89)90065-6
49. Ohura T, Hase K, Nakajima Y, Nakayama T. Validity and reliability of a performance evaluation tool based on the modified Barthel Index for stroke patients. BMC Med Res Methodol. 2017;17(1):131. doi:10.1186/s12874-017-0409-2
50. Leung S, Chan C, Shah S. Development of a Chinese version of the Modified Barthel Index-- validity and reliability. Clin Rehabil. 2007;21(10):912–922. doi:10.1177/0269215507077286
51. Al-Rawi MA, Hamdan FB, Abdul-Muttalib AK. Somatosensory evoked potentials as a predictor for functional recovery of the upper limb in patients with stroke. J Stroke Cerebrovasc Dis. 2009;18(4):262–268. doi:10.1016/j.jstrokecerebrovasdis.2008.11.002
52. Han EY, Jung HY, Kim MO. Absent median somatosensory evoked potential is a predictor of type I complex regional pain syndrome after stroke. Disabil Rehabil. 2014;36(13):1080–1084. doi:10.3109/09638288.2013.829530
53. Jurcak V, Tsuzuki D, Dan I. 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. NeuroImage. 2007;34(4):1600–1611. doi:10.1016/j.neuroimage.2006.09.024
54. Klem G, Lüders H, Jasper H, Elger C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:3–6.
55. Berthelot JM. Current management of reflex sympathetic dystrophy syndrome (complex regional pain syndrome type I). Joint Bone Spine. 2006;73(5):495–499. doi:10.1016/j.jbspin.2005.11.022
56. Chau JPC, Lo SHS, Yu X, et al. Effects of Acupuncture on the Recovery Outcomes of Stroke Survivors with Shoulder Pain: a Systematic Review. Front Neurol. 2018;9:30. doi:10.3389/fneur.2018.00030
57. Lu CY, Kang SY, Liu SH, Mai CW, Tseng CH. Controlling Indoor Air Pollution from Moxibustion. Int J Environ Res Public Health. 2016;13(6). doi:10.3390/ijerph13060612
58. Liu Y, An Y, Xing G, et al. Effect of moxa smoke on sperm parameters and oxidative stress in rats with asthenozoospermia. Anatomical record. 2022;1:548.
59. Ouyang X, Duan H, Jin Q, et al. Moxibustion may delay the aging process of Wistar rats by regulating intestinal microbiota. Biomed Pharmacother. 2022;146:112147. doi:10.1016/j.biopha.2021.112147
60. Han L, Liu C, Sun W, et al. Twelve-week study of moxa smoke: occupational exposure in female rats. J Traditional Chine Med. 2019;39(2):207–212.
61. Ha L, Yu M, Yan Z, Rui Z, Zhao B. Effects of Moxibustion and Moxa Smoke on Behavior Changes and Energy Metabolism in APP/PS1 Mice. Evid Based Complement Alternat Med. 2019;2019:9419567. doi:10.1155/2019/9419567
62. He R, Han L, Liu P, et al. Lung Function Decline after 24 Weeks of Moxa Smoke Exposure in Rats. Evid Based Complement Alternat Med. 2019;2019:9236742. doi:10.1155/2019/9236742
63. Han L, Liu C, Yang J, et al. Repeated exposure to moxa-burning smoke: its acute and chronic toxicities in rats. J Traditional Chine Med. 2018;38(1):67–75.
64. Lim M, Zhang X, Huang J, et al. Study of Thermal Behavior of Moxa Floss Using Thermogravimetric and Pyrolysis-GC/MS Analyses. Evid Based Complement Alternat Med. 2021;2021:6298565. doi:10.1155/2021/6298565
65. Mo F, Chi C, Guo M, Chu X, Li Y, Shen X. Characteristics of selected indoor air pollutants from moxibustion. J Hazard Mater. 2014;270:53–60. doi:10.1016/j.jhazmat.2014.01.042
66. Huang C, Zhao B, Liu P, Shao L. Mass concentration and morphological characteristics of PM10 in moxibustion rooms in Beijing and Tianjin during summer. China J Traditional Chine Med Pharmacy. 2012;27(12):3104–3108.
67. Xu H, Zhao B, Cui Y, et al. Effects of Moxa Smoke on Monoamine Neurotransmitters in SAMP8 Mice. Evid Based Complement Alternat Med. 2013;2013:178067. doi:10.1155/2013/178067
68. Yu C, Zhang N, Zhu W, et al. Does Moxa Smoke Have Significant Effect on the Acupuncturist’s Respiratory System? A Population-Based Study. Evid Based Complement Alternat Med. 2019;2019:4873235. doi:10.1155/2019/4873235
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
