Back to Journals » Clinical Epidemiology » Volume 15
Efficacy and Safety of Novel Oral Antivirals in Hospitalized COVID-19 Patients: A Network Meta-Analysis of Randomized Clinical Trials
Authors Liu H , Chen J, Shao W , Yan S, Ding S
Received 9 June 2023
Accepted for publication 26 September 2023
Published 1 November 2023 Volume 2023:15 Pages 1041—1053
DOI https://doi.org/10.2147/CLEP.S422386
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Vera Ehrenstein
Haoshuang Liu,1,2 Jingfeng Chen,1,2 Weihao Shao,3 Su Yan,1,2 Suying Ding1,2
1Health Management Center, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, People’s Republic of China; 2College of Public Health, Zhengzhou University, Zhengzhou, 450001, People’s Republic of China; 3School of Population Medicine and Public Health, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100730, People’s Republic of China
Correspondence: Suying Ding, Department of Health Management Center, the First Affiliated Hospital of Zhengzhou University, Longhu Zhonghuan Road, Jinshui District, Zhengzhou, Henan, 450052, People’s Republic of China, Tel +86 158 3802 3097, Email [email protected]
Objective: Numerous pharmacological interventions are now under investigation for the treatment of the 2019 coronavirus pandemic (COVID-19), and the evidence is rapidly evolving. Our aim is to evaluate the comparative efficacy and safety of these drugs.
Methods: We searched for randomized clinical trials (RCTs) on the efficacy and safety of novel oral antivirals for the treatment of hospitalized COVID-19 patients until November 30, 2022, including baricitinib, ivermectin (IVM), favipiravir (FVP), chloroquine (CQ), lopinavir and ritonavir (LPV/RTV), hydroxychloroquine (HCQ), and hydroxychloroquine plus azithromycin (HCQ+AZT). The main outcomes of this network meta-analysis (NMA) were in-hospital mortality, adverse event (AE), recovery time, and improvement in peripheral capillary oxygen saturation (SpO2). For dichotomous results, the odds ratio (OR) was used, and the 95% confidence interval (CI) was determined. We also used meta-regression to explore whether different treatments affected efficacy and safety. STATA 15.0 was used to conduct the NMA. The research protocol was registered with PROSPERO (#CRD 42023415743).
Results: Thirty-six RCTs, with 33,555 hospitalized COVID-19 patients, were included in this analysis. First, we compared the efficacy of different novel oral antivirals. Baricitinib (OR 0.56, 95% CI: 0.35 to 0.90) showed the highest probability of being the optimal probiotic species in reducing in-hospital mortality and suggested that none of the interventions reduced AE better than placebo. In terms of safety outcomes, IVM ranked first in improving the recovery time of hospitalized COVID-19 patients (mean difference (MD) − 1.36, 95% CI: − 2.32 to − 0.39). In addition, patients were most likely to increase SpO2 (OR 1.77, 95% CI: 0.09 to 3.45). The meta-regression revealed no significant differences between participants using different novel oral antivirals in all outcomes in hospitalized COVID-19 patients.
Conclusion: Currently, baricitinib has reduced in-hospital mortality in hospitalized COVID-19 patients, with moderate certainty of evidence. IVM appeared to be a safer option than placebo in improving recovery time, while FVP was associated with increased SpO2 safety outcomes. These preliminary evidence-based observations should guide clinical practice until more data are made public.
Keywords: COVID-19, network meta-analysis, pharmacological intervention, efficacy, safety
Introduction
The global impact of the 2019 coronavirus pandemic (COVID-19) has been tremendous. Worldwide, WHO has reported 637,404,847 confirmed cases of COVID-19, including 6,608,893 deaths as of November 28, 2022.1,2 COVID-19 is a newly discovered severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic that was discovered in late 2019.3 The respiratory system of the infected person acts as a conduit for the transmission of SARS-CoV-2 droplets and aerosols.4 This disease exhibits a diverse array of symptoms that range from mild flu-like symptoms to the more severe manifestation of acute respiratory distress syndrome, ultimately leading to fatality.5 SARS-CoV-2, a single-stranded RNA virus, enters via ACE2 receptors via spike protein binding.6 The replication process involves the use of RNA-dependent RNA polymerase to facilitate the synthesis of new viral particles, which then escape the host cell and initiate the replication cycle in neighboring cells.7 Numerous therapeutic drugs have been reviewed to enhance clinical outcomes and prolong life in individuals affected by COVID-19.
The fight against COVID-19 does not seem to end with acute disease screening and management. The global COVID-19 outbreak has lasted three years. Evidence from millions of cases shows that COVID-19 symptoms can occur in a significant number of people long after the first acute phase. This condition is called COVID-19 post-acute effects or long COVID.8 Long COVID symptoms are heterogeneous, indicating that the condition is multi-syndromic and multi-phased, rather than a single disease phenomenon. Patients often experience a variety of symptoms over the course of weeks and months, and some continue to struggle for two years after the first diagnosis. Symptoms are prevalent, suggest multi-system involvement, and have a negative impact on patients’ quality of life.9 Emerging evidence suggests that a proportion of symptoms resolve over time, but many continue to suffer life-altering symptoms six months after infection.10 Therefore, to accelerate the development of long COVID therapies, it is critical to first identify risk factors and then identify and understand the causes.
A vaccine against COVID-19 has been developed, as have widespread immunization campaigns. The COVID-19 vaccine may significantly reduce the mortality of individuals infected with COVID-19, according to Zambia Research.11 As of November 23, 2022, more than 3.47 billion doses of the novel coronavirus vaccine have been given to people in China.12 The COVID-19 vaccine, which is both safe and effective, is eagerly awaited. Together with vaccine efficacy, vaccine adoption plays a pivotal role in mitigating the spread of COVID-19.13 Despite the fact that vaccines are considered safe, vaccine hesitancy has grown to be a substantial obstacle to raising immunization rates.14 One of the top 10 risks to global health, according to WHO in 2019, is vaccine hesitancy.15 Vaccine hesitancy stems from a wide variety of factors. Awareness of disease risks, concerns about vaccine safety, trust (mistrust) in institutions and health care providers, and various socio-demographic characteristics (such as age, sex, income, and education level) have all been shown in previous studies to be important determinants of vaccine acceptance or rejection.16,17 However, studies have shown that even those who have been vaccinated may get novel coronavirus variants. There is a potential concern that a significant number of individuals with impaired immune systems may not receive full protection after vaccination, and that current vaccines may not be effective enough against emerging forms of the novel coronavirus.18 Therefore, it is essential to develop simple oral coronavirus drugs.
Recent clinical investigations using novel oral coronavirus drugs have shown positive results. Numerous antivirals, immunotherapies, and vaccines are being tested in response to this global health catastrophe, yet few have shown efficacy in randomized clinical trials (RCTs) to treat or prevent COVID-19. Oral antivirals offer an easier-to-administer option than monoclonal antibodies against SARS-CoV-2 and intravenous Remdesivir (RDV). Corticosteroids, tocilizumab, hydroxychloroquine (HCQ), and antiviral drugs such as RDV, a combination of lopinavir and ritonavir (LPV/RTV), ivermectin (IVM), and favipiravir (FVP) have been explored in clinical studies for COVID-19.19–22 Despite these scientific efforts, a definitive consensus on treatment is still lacking.
We report an overview of all RCTs comparing seven novel oral antivirals in terms of efficacy and safety in treating hospitalized COVID-19 patients. We employed a network meta-analysis (NMA) that allows the inclusion of data from direct (where treatments are evaluated in an RCT) and indirect comparisons (when treatments are compared between trials by combining results on how effective they are compared with a common comparator treatment). In normal clinical practice, clinicians have access to multiple treatment options and must rely on robust evidence to select the best treatment for each patient. Thus, clinically relevant summaries of NMA findings are presented, which can be used to inform treatment decisions.
Methods
This systematic review and NMA were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Reporting Guidelines (Table S1).23 The research protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO) (#CRD 42023415743).
Search Strategy
PubMed, Web of Science, Embase, and the Cochrane Library were searched from their inception until November 30, 2022. The studies were all published in English-language journals between 2020 and 2022. A combination of free and subject words was used when searching the electronic database. The full search strategy is available in Table S3.
Study Selection
All RCTs where oral antivirals were administered as therapy to hospitalized COVID-19 patients from inception until November 30, 2022, were considered for inclusion. No restrictions were placed on age, sex, race, ethnicity, or length of treatment. Case reports, conference abstracts, cross-sectional and observational studies, single-arm studies, and non-human studies were excluded.
LHS and CJF conducted an independent research search and assessed the titles and abstracts of all papers found in EndNote 20. The studies that were obtained were then assessed for eligibility through a comprehensive review of the entire text by the same reviewers. LHS and CJF reached a consensus to resolve all issues. Duplicated results were removed upon reference importation in EndNote 20 by LHS. Manual deletion was used to remove any remaining duplicates.
Outcome Measures
The primary outcome was in‐hospital mortality. Secondary outcomes were adverse event (AE), recovery time, or improvement in SpO2.
The primary outcome was to assess the effects on in-hospital mortality (ie, death during initial hospitalization; follow-up stopped at discharge), regardless of whether death occurred before or after day 28. Recovery time-the first day a patient achieved category 1, 2, or 3 on an eight-category ordinal scale within 28 days of enrollment-was a secondary outcome measure. SpO2 was measured using a calibrated pulse oximeter per clinical monitoring protocol. All AEs, regardless of severity, were recorded and included in the analysis.
Data Analysis
We conducted random effects NMA in a frequentist framework using STATA (STATA Corp, College Station, Texas, United States of America, version 15.0) and R (version 4.2.2) software.24 Direct and indirect (and mixed) comparisons were made using STATA’s self-programmed routines25 and R’s bugsnet package.26 The effect was estimated in odds ratios (OR) for dichotomous variables and mean difference (MD) for continuous variables, both with 95% confidence intervals (CI). When the median (interquartile range) was presented for continuous variables of interest, it was converted to mean (standard deviation) by calculation.27 A 2-sided p-value of less than 0.05 was considered statistically significant.
NMA was performed using STATA 15.0. A random effects model was conducted using a frequentist approach to pool direct and indirect evidence while preserving randomization, using STATA “mvmeta”, “mvmeta-make” and “network” packages. Direct evidence refers to the pooled effect based on RCTs (similar to traditional meta-analysis), while indirect is calculated from the network, eg, the difference between B and C, as extrapolated from A -v- B and A -v- C.
A network map was generated for each network. NMA provides intergroup MD effect sizes based on direct and indirect evidence between each intervention, as well as CIs and p values (calculated as 95% CIs excluding 0). Cohen’s d interpretations were used to describe the effect sizes; small 0.2, medium 0.5 and large 0.8.
Treatment strategies are ranked by surface for each outcome under the cumulative ranking curve (SUCRA) probabilities; a higher probability of SUCRA in each simulation indicates a higher probability of being the optimal treatment regimen.
Risk of Bias
To evaluate the study methodology, we used the Cochrane Collaboration’s Bias Risk Tool. The number of components for which high risk of bias (ROB) was likely to be present in trials was used to group trials into three categories of ROB: high risk, moderate risk, and low risk.28 Any differences are resolved through discussion until consensus is reached. RevMan 5.3 was used to generate risk summaries and biased risk maps.
Additional Analyses
Visual analysis of funnel plots in STATA 15.0 studies contributing to primary and secondary outcomes to assess publication bias (Figure S4). Meta-regression analysis, taking into account the different treatment of outcome assessment, was required if there was significant inconsistency (Table S4). The leverage plot can help assess the significance of heterogeneity in each outcome in the study, as high leverage values can have a substantial impact on the overall model. If a leverage plot shows low heterogeneity, meaning that most data points have relatively low leverage values, it can be considered that the study has good homogeneity, indicating low heterogeneity in the study (Comparison-adjusted funnel plots were constructed to assess publication bias and funnel asymmetry was tested using Egger’s test).
Results
Characteristics of the Included Studies
A total of 4,813 eligible studies were identified in the initial inspection using this study’s search method. The full texts of 1,392 potentially eligible studies were retrieved after screening for titles and abstracts and removing duplicates. Overall, we used 36 RCTs from 2020 to 2022 for the NMA (Table S5). Thirty-three thousand, five hundred, and fifty-five individuals were randomly assigned to one of seven novel oral antiviral drugs (baricitinib, IVM, FVP, CQ, LPV/RTV, HCQ, or HCQ+AZT) and included in an NMA. Figure 1 illustrates the systematic literature search and study selection process. Table S2 lists the trial characteristics.
 |
Figure 1 Flow diagram of the selection strategy. Notes: The search flow diagram summarizes the search, screening, retrieval, and evaluation of articles finally included in the network meta-analysis. |
Risks of Bias
Results for publication bias in studies contributing to primary and secondary outcomes are shown in Figure S1, where the risk of bias is predominantly low (Figure S2). Detailed information is provided in Table S5.
Network Meta-Analysis
The number of patients enrolled in the baricitinib trial was 1,315, with a preponderance of males. The most regularly prescribed dose of baricitinib is 4 mg/day, taken orally for 14 days. Baricitinib was primarily compared to a placebo. The length of follow-up varied between 28 and 60 days in several baricitinib studies. On the other hand, the number of patients enrolled in the IVM trial was 1,069, with a preponderance of males. With the exception of three studies where CQ, HCQ, and standard of care (SOC) were active comparators, IVM was generally compared to placebo. IVM 12 mg once daily for 3 to 7 days is the most regularly prescribed dose. The number of patients enrolled in the follow-up phase of the FVP trial was 842, with a preponderance of males. FVP was compared primarily with SOC, except in three studies where CQ, HCQ, and LPV/RTV were active comparators. For patients in the FVP group, the most regularly prescribed dose was 1,600 mg of FVP twice daily for the first day and 600 mg twice daily for the next 5 to 14 days. Follow-up ranged from 7 to 30 days across the different FVP studies. The most frequently used SOCs in all studies included antibiotics, antivirals, corticosteroids, vasopressors, and anticoagulants.
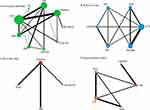
An NMA was used for indirect mixed treatment comparisons. Figure 2A–D provides an overview of the network graph. The width of the line represents the number of tests used to compare the two agents. The node size represents the number of hospitalized COVID-19 patients randomly assigned to a specific agent. Placebo and HCQ samples were found to be the highest in this NMA.
Table 1 (A–D) summarize the NMA comparison results for in‐hospital mortality, AE, recovery time, and improvement in SpO2. Concerning the primary outcome, treatment with baricitinib resulted in a statistically significant improvement in in-hospital mortality compared to placebo (OR 0.57, 95% CI: 0.36 to 0.92). In other words, individuals receiving Baricitinib are estimated to have a 43% reduction in the odds of experiencing in-hospital mortality compared to those receiving placebo. However, there are no apparent benefits of IVMs, FVPs, LPV/RTVs, or HCQs over adverse events. In terms of recovery time, IVMs (MD −1.36, 95% CI: −2.32 to −0.39) were found to be more beneficial than placebos, indicating an average reduction of 1.36 days in recovery time compared to placebo. Regarding improvement in SpO2, FVPs showed better efficacy than IVMs (OR 1.7, 95% CI: 0.09 to 3.45). The FVP group showed an average increase of 1.77 units improvement in SpO2 compared to the IVM group.
 |
Table 1 League Table of Network Meta-Analysis Results of Interventions for In-hospital Mortality(A), Adverse Event(B), Recovery Time(C), and Improvement in SpO2 (D) |
Baricitinib is the only treatment that is significantly superior to reducing in-hospital mortality (Figure 3A). IVM, FVP, CQ, LPV/RTV, HCQ, and HCQ+AZT also performed worse, but without statistical significance. These findings are supported by the SUCRA plot, which ranks placebo (SUCRA value: 0.228) and IVM as the least effective therapies (SUCRA value: 0.351). The highest ranked treatment is baricitinib (SUCRA value: 0.889). The ranking of in‐hospital mortality from high to low is baricitinib (SUCRA value: 0.889), HCQ+AZT (SUCRA value: 0.638), SOC (SUCRA value: 0.583), LPV/RTV (SUCRA value: 0.544), HCQ (SUCRA value: 0.457), FVP (SUCRA value: 0.406), CQ (SUCRA value: 0.404), IVM (SUCRA value: 0.351), and placebo (SUCRA value: 0.228). Although there was a trend towards SOC performing best and ranking highest in the SUCRA plot (Figure 3B), AEs failed to provide any discernible differences for comparisons (SUCRA value: 0.933). In the SUCRA plot, the results of IVM (SUCRA value: 0.583) and LPV/RTV (SUCRA value: 0.519) are extremely similar. In terms of recovery time, IVMs are significantly better than alternative antiviral agents due to their superior efficacy (Figure 3C). The recovery time from high to low was as follows: IVM (SUCRA value: 0.758), HCQ (SUCRA value: 0.632), baricitinib (SUCRA value: 0.576), and placebo (SUCRA value: 0.034). For SpO2, IVM performs significantly worse than FVP, as seen in the SUCRA plot (Figure 3D). The improvement in SpO2 from high to low was as follows: FVP (SUCRA value: 0.736), LPV/RTV (SUCRA value: 0.722), HCQ (SUCRA value: 0.633), placebo (SUCRA value: 0.363), and IVM (SUCRA value: 0.046).
Meta-Regression Analysis
One covariate, a different treatment, was selected to perform a meta-regression analysis of all four outcomes (in-hospital mortality, AEs, recovery time, or improvement in SpO2). There were no covariates showing a significant coefficient in the interaction model. All results are presented in Table S4.
Leverage Plot
The random-effect model was used to estimate the effect size and CIs after examining the leverage plots and comparing the deviance information criterion (DIC) values of the fixed- and random-effects models (Figure S3). The leverage plot shows low heterogeneity, as indicated by the majority of data points with small leverage values. Thus, it can be inferred that the study exhibits good homogeneity, suggesting low heterogeneity in the study.
Publication Bias
Publication bias was examined using the funnel plot adjusted for comparison. We hypothesized that research reporting positive findings was more likely to be published. A comparison adjusted funnel plot coupled with Egger’s test was used to detect a small study effect (Figure S4). Although a few estimates were lying away from the centre, Egger’s test P value indicated that statistical significance was not reached (p=0.18, p=0.49, p=0.69, p=0.26).
Discussion
Our analysis is based on 36 studies, 33,555 individuals randomly assigned to seven novel oral antivirals. Our findings may be useful in the selection of novel oral antivirals for optimal treatment of hospitalized COVID-19 patients. Some antiviral drugs are statistically and clinically different. In terms of in-hospital mortality, a significantly lower risk of COVID-19 was observed in the study group receiving anti-viral baricitinib compared to the control group receiving placebo. IVM was more effective than placebos in terms of recovery time. For SpO2, FVPs outperformed other novel oral antivirals. Overall, we found that baricitinib outperformed other agents in terms of in-hospital mortality based on SUCRA values. IVM is recommended for recovery time and FVP for SpO2. We also found that none of the interventions resulted in a reduction in AEs compared to placebo.
Priority was given in the current study to evaluating outcomes for in-hospital mortality, AEs, recovery time, or improvement in SpO2. Due to the limited sample size of clinical trials of novel oral antivirals, it is difficult to analyze the distribution of data from these small studies. Baricitinib may be superior to other drugs in reducing in-hospital mortality, according to our findings from NMA comparisons for antiviral treatments (which were measured according to SUCRA values). Notably, baricitinib inhibits JAK1/JAK2 to regulate downstream inflammatory responses and prevents IL-6-induced phosphorylation of STAT3 in a dose-dependent manner.29,30 It has antiviral activity because it prevents SARS-CoV-2 from entering and infecting alveolar lung cells.31 This mechanism may help reduce cytokine bursts in COVID-19 because the JAK-STAT signaling pathway is crucial for its development.32,33 The use of baricitinib in hospitalized COVID-19 patients significantly reduced the risk of death.34–37 IVM is a macrocyclic lactone antiparasitic drug that has been extensively studied due to its potent antiparasitic, antibacterial, antiviral, and anticancer effects.38 Recent studies have demonstrated IVM’s antiviral activity against a variety of RNA viruses, suggesting that it may be effective against SARS-CoV-2.39–43 The use of IVM in severe COVID-19 improved laboratory prognostic parameters, increased clinical recovery, and reduced mortality.44 In contrast, in a retrospective study, IVM was reported to have failed to reduce the length of symptoms in COVID-19 patients, although the reduction was not statistically significant. The results may only apply to groups like these because the study cohort was young, had few comorbidities, and had liver enzyme levels less than 1.5 times normal.45 FVP is an orally administered, broad-spectrum inhibitor of viral RNA-dependent RNA polymerase that induces viral mutations.46 Its phosphorylate derivative (T-705RTP), which corresponds to broad-spectrum suppression of RNA viruses, selectively inhibits viral RNA polymerase in vivo.47,48 In another study, patients with moderate COVID-19 who were given FVP had lower viral clearance rates.49
Other agents other than the three mentioned above did not exhibit strong antiviral activity. HCQ was not shown to reduce in-hospital mortality, AEs, recovery time, or SpO2. A recent in vitro investigation that found that CQ does not inhibit SARS-CoV-2 entry into human lung cells and subsequent diffusion across lung tissue supports these cumulative empirical results on HCQ.50
On November 22, after nearly two years of repeated reviews, the Japanese government officially approved the release of XOCOVA, a domestic oral treatment for COVID-19 developed by Shionogi Pharmaceutical Co., Ltd., for the treatment of infected people, according to final approval by the Expert Committee on New Drugs (Clinicaltrials.gov. NCT: 05305547). Oral drugs are mainly developed by US pharmaceutical giants Merck and Pfizer. They are aimed primarily at patients with moderate symptoms and severe cases and are limited to those at risk for severe conditions such as diabetes, various respiratory diseases and obesity, reducing severe illness and mortality, but only marginally for those with early symptoms. The oral drug, developed by Japan’s Shionogi Pharmaceuticals, is specifically targeted at people with early symptoms and mild infections. In addition, there are not too many restrictions on targets for use, except for pregnant women and other patients with special conditions (ClinicalTrials.gov. NCT: 05605093). Like cold medicine, it is widely available to anyone over the age of 12. However, it should not be combined with 36 other drugs, such as those for hypertension and hyperlipidemia. XOCOVA’s approval adds a new treatment option to the medical field. In this sense, it is a good thing that people who might not have been able to use oral medications before can now. But frankly, it is an emergency-approved drug. So expect new updates in the future.
Since the outbreak of COVID-19, scientists around the world have been exploring suitable therapeutics for COVID-19. To counter the threat of COVID-19, researchers are looking for long-term vaccinations as well as short-term preventative options. Despite significant improvements and positive results from vaccine candidate trials, many challenges remain, including vaccine hesitancy and logistical challenges of mass production and delivery of millions or billions of doses to the global population, which would almost certainly be the most significant constraint.51 Due to vaccine limitations and the time-consuming process of generating novel drugs, FDA-approved compounds with proven efficacy for viruses and inflammatory disorders are preferred for COVID-19 treatment. However, proof of the safety and efficacy of these drugs against COVID-19 is required, and this can only be achieved through successful clinical studies.
Clinical progress towards severe illness has important implications for both patients and health care systems, as it increases the likelihood of patients requiring mechanical respiration and facing mortality. Moreover, during periods of increased COVID-19 cases, this progression has the potential to strain local and regional hospital resources. It is important, then, to reduce COVID-19 and possibly community transmission by helping patients clear infectious viruses more quickly. Monoclonal antibodies bamlanivimab-etese-vimab, casirivimab-imdevimab, and sotrovimab are currently approved treatments for at-risk outpatients with COVID-19. Because monoclonal antibodies require administration by infusion or injection in a medical setting, oral agents such as molnupiravir that can be administered by the patient at home shortly after diagnosis may be more practical and patient-friendly for non-hospitalized patients; such agents would be important new tools in the COVID-19 treatment armamentarium.
It should be noted that this study has several limitations. First, the number of included studies is limited because only RCTs are included in this study, and we exclude observational or cohort studies. Therefore, the level of evidence in the current RCT-based meta-analysis should be considered robust. Second, the effect of vaccination status is an essential factor in determining the generalizability of the population to be treated. Vaccination rates for SARS-CoV-2 are assessed by various ethnic groups, making it necessary to carefully assess whether a particular treatment is acceptable for a population that has not received the vaccine. Although the overall vaccination status of participants in the included trials was not disclosed, given the timing of the research, it is likely that the majority of enrolled patients were unvaccinated. Third, our study examined only acute efficacy/AEs and required more data on potential long-term effects. In addition, CIs of effect size were relatively large, which may affect the reliability of our findings in this NMA. Finally, evidence on how treatment with antiviral drugs affects the long-term sequelae of COVID-19, including long COVID-19, is unclear.
The study of oral antiviral drugs for COVID-19 can provide references for the development and clinical application of coronavirus drugs, contributing to the prevention and response to potential future infectious disease pandemics. Treatment guidelines should be updated to reflect differences in infection levels, but treatment interventions should be made on a case-by-case basis, taking into account the clinical circumstances and preferences of patients and clinicians. We anticipate that these findings will contribute to shared decision-making between patients and their clinicians. To be sure, more large-scale RCTs / RPCTs and big data analysis should be collaborated on and conducted for the treatment of COVID-19 infection. Therefore, preventing and treating COVID-19 will change for the better in the future.
Conclusion
In summary, the currently recommended anti-viral agents baricitinib, IVM, and FVP should be continued for disease progression prevention in hospitalized COVID-19 patients. These findings highlight the importance of public health measures to address infectious waves caused by novel variants and the development of novel oral antivirals to counter viral evolution. While this NMA included the highest level of certainty available at the time, it was crucial for researchers to share their data for greater transparency. Meta-analysis of individual patient data from RCTs would be the next logical step in tailoring treatments based on patient characteristics.
Acknowledgments
We thank all the reviewers for their assistance and support.
Author Contributions
All authors have made significant contributions to the reported work, whether in conception, study design, execution, data acquisition, analysis, and interpretation, or in all of these areas; participated in the drafting, revision, or critical review of the article; gave final approval to the version to be published; agreed on the journal to which the article was submitted; and agreed to be responsible for all aspects of the work.
Funding
This research was supported and funded by the Collaborative Innovation Project of Zhengzhou City (XTCX2023006) and the Nursing Team Project of the First Affiliated Hospital of Zhengzhou University (HLKY2023005). Funders had no role in designing, collecting, and analyzing data, deciding to publish, or preparing the manuscript.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. World Health Organization. WHO COVID-19 dashboard. World Health Organization; 2022. Available from: https://covid.19.who.int/data.
2. Shao W, Chen X, Zheng C, et al. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern in real-world: a literature review and meta-analysis. Emerg Microbes Infect. 2022;11(1):2383–2392. doi:10.1080/22221751.2022.2122582
3. Umakanthan S, Sahu P, Ranade AV, et al. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad Med J. 2020;96(1142):753–758. doi:10.1136/postgradmedj-2020-138234
4. Meselson M. Droplets and Aerosols in the Transmission of SARS-CoV-2. N Engl J Med. 2020;382(21):2063. doi:10.1056/NEJMc2009324
5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
6. Lamers MM, Haagmans BL. SARS-CoV-2 pathogenesis. Nat Rev Microbiol. 2022;20(5):270–284. doi:10.1038/s41579-022-00713-0
7. Wang Q, Wu J, Wang H, et al. Structural Basis for RNA Replication by the SARS-CoV-2 Polymerase. Cell. 2020;182(2):417–428.e13. doi:10.1016/j.cell.2020.05.034
8. Umakanthan S, Monice M, Mehboob S, Jones CL, Lawrence S. Post-acute (long) COVID-19 quality of life: validation of the German version of (PAC19QoL) instrument. Front Public Health. 2023;11:1163360. doi:10.3389/fpubh.2023.1163360
9. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi:10.1016/j.eclinm.2021.101019
10. Michelen M, Manoharan L, Elkheir N, et al. Characterising long COVID: a living systematic review. BMJ Glob Health. 2021;6(9):e005427. doi:10.1136/bmjgh-2021-005427
11. Chanda D, Hines JZ, Itoh M, Fwoloshi S, Minchella PA, Zyambo KD. COVID-19 vaccine effectiveness against progression to in-hospital mortality in Zambia, 2021–2022. Open Forum Infect Dis. 2022;9(9):ofac469. doi:10.1093/ofid/ofac469
12. National health commission of the people’s republic of China. Available from: https://www.nhc.gov.cn/xcs/yqjzqk/202212/a87d93dbfbd14fc1b7837f1a20092cbe.sht.mL.
13. Hong J, Xu XW, Yang J, et al. Knowledge about, attitude and acceptance towards, and predictors of intention to receive the COVID-19 vaccine among cancer patients in Eastern China: a cross-sectional survey. J Integr Med. 2022;20(1):34–44. doi:10.1016/j.joim.2021.10.004
14. Majeed A, Molokhia M. Vaccinating the UK against covid-19. BMJ. 2020;371:m4654. doi:10.1136/bmj.m4654
15. World Health Organization. Ten Threats to Global Health in 2019; 2019. Available from: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
16. Umakanthan S, Lawrence S. Predictors of COVID-19 vaccine hesitancy in Germany: a cross-sectional, population-based study. Postgrad Med J. 2022;98(1164):756–764. doi:10.1136/postgradmedj-2021-141365
17. Umakanthan S, Bukelo MM, Bukelo MJ, Patil S, Subramaniam N, Sharma R. Social environmental predictors of COVID-19 vaccine hesitancy in India: a population-based survey. Vaccines. 2022;10(10):1749. doi:10.3390/vaccines10101749
18. Christie A, Mbaeyi SA, Walensky RP. CDC interim recommendations for fully vaccinated people: an important first step. JAMA. 2021;325(15):1501–1502. doi:10.1001/jama.2021.4367
19. National Institutes of Health. Management. COVID-19 Treatment Guidelines. Available from: https://www.covid19.treatment.guidelines.nih.gov/ma-nagement/.
20. Kumari M, Lu RM, Li MC, et al. A critical overview of current progress for COVID-19: development of vaccines, antiviral drugs, and therapeutic antibodies. J Biomed Sci. 2022;29(1):68. doi:10.1186/s12929-022-00852-9
21. Ferner RE, Aronson JK. Remdesivir in covid-19. BMJ. 2020;369:m1610. doi:10.1136/bmj.m1610
22. Drożdżal S, Rosik J, Lechowicz K, et al. An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resist Updat. 2021;59:100794. doi:10.1016/j.drup.2021.100794
23. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Inter J Surg. 2021;88:105906. doi:10.1136/bmj.n71
24. Xu C, Niu Y, Wu J, Gu H, Zhang C. Software and package applicating for network meta-analysis: a usage-based comparative study. J Evid Based Med. 2018;11(3):176–183. doi:10.1111/jebm.12264
25. Shim S, Yoon BH, Shin IS, Bae JM. Network meta-analysis: application and practice using Stata. Epidemiol Health. 2017;39:e2017047. doi:10.4178/epih.e2017047
26. Béliveau A, Boyne DJ, Slater J, Brenner D, Arora P. BUGSnet: an R package to facilitate the conduct and reporting of Bayesian network Meta-analyses. BMC Med Res Methodol. 2019;19(1):196. doi:10.1186/s12874-019-0829-2
27. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi:10.1186/1471-2288-14-135
28. Higgins JPT, Altman DG. Assessing risk of bias in included studies. Cochrane Handbook Syst Rev Inter. 2011;2011:187–241.
29. Bronte V, Ugel S, Tinazzi E, et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest. 2020;130(12):6409–6416. doi:10.1172/JCI141772
30. Cherian JJ, Eerike M, Bagepally BS, Das S, Panda S. Efficacy and safety of baricitinib and tocilizumab in hospitalized patients with COVID-19: a comparison using systematic review and meta-analysis. Front Pharmacol. 2022;13:1004308. doi:10.3389/fphar.2022.1004308
31. McInnes IB, Byers NL, Higgs RE, et al. Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res Ther. 2019;21(1):183. doi:10.1186/s13075-019-1964-1
32. Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30–e31. doi:10.1016/S0140-6736(20)30304-4
33. Hoang TN, Pino M, Boddapati AK, et al. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell. 2021;184(2):460–475.e21. doi:10.1016/j.cell.2020.11.007
34. U.S. Food and Drug Administration. U.S. Food and drug administration; 2022. Available from: https://www.fda.gov/media/143822/download.
35. RECOVERY Collaborative Group. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis. Lancet. 2022;400(10349):359–368. doi:10.1016/S0140-6736(22)01109-6
36. Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo- controlled Phase 3 trial. Lancet Respir Med. 2021;9(12):1407–1418. doi:10.1016/S2213-2600(21)00331-3
37. Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infect. 2020;81(2):318–356. doi:10.1016/j.jinf.2020.04.017
38. Awad H, Hassan B, Dweek S, Aboelata Y, Rawas-Qalaji M, Ahmed IS. Repurposing potential of the antiparasitic agent ivermectin for the treatment and/or prophylaxis of COVID-19. Pharmaceuticals. 2022;15(9):1068. doi:10.3390/ph15091068
39. Formiga FR, Leblanc R, de Souza Rebouças J, Farias LP, de Oliveira RN, Pena L. Ivermectin: an award-winning drug with expected antiviral activity against COVID- 19. J Control Release. 2021;329:758–761. doi:10.1016/j.jconrel.2020.10.009
40. Krolewiecki A, Lifschitz A, Moragas M, et al. Antiviral effect of high-dose ivermectin in adults with COVID-19: a proof-of-concept randomized trial. EClinicalMedicine. 2021;37:100959. doi:10.1016/j.eclinm.2021.100959
41. Rakedzon S, Neuberger A, Domb AJ, Petersiel N, Schwartz E. From hydroxychloroquine to ivermectin: what are the anti-viral properties of anti-parasitic drugs to combat SARS-CoV-2? J Travel Med. 2021;28(2):taab005. doi:10.1093/jtm/taab005
42. Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. doi:10.1016/j.antiviral.2020.104787
43. Okumuş N, Demirtürk N, Çetinkaya RA, et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infect Dis. 2021;21(1):411. doi:10.1186/s12879-021-06104-9
44. Jean SS, Hsueh PR. Old and re-purposed drugs for the treatment of COVID-19. Expert Rev Anti Infect Ther. 2020;18(9):843–847. doi:10.1080/14787210.2020.1771181
45. López-Medina E, López P, Hurtado IC, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA. 2021;325(14):142 6–1435. doi:10.1001/jama.2021.3071
46. Hung DT, Ghula S, Aziz JMA, et al. The efficacy and adverse effects of favipiravir on patients with COVID-19: a systematic review and meta-analysis of published clinical trials and observational studies. Int J Infect Dis. 2022;120:217–227. doi:10.1016/j.ijid.2022.04.035
47. Doi Y, Hibino M, Hase R, et al. A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19. Antimicrob Agents Chemother. 2020;64(12):e01897–20. doi:10.1128/AAC.01897-20
48. Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100(2):446–454. doi:10.1016/j.antiviral.2013.09.015
49. Vashchenko I, A. A, Dmitriev KA, et al. AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (COVID-19): interim results of a Phase II/III multicenter randomized clinical trial. Clin Infect Dis. 2021;73(3):531–534. doi:10.1093/cid/ciaa1176
50. Hoffmann M, Mösbauer K, Hofmann-Winkler H, et al. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature. 2020;585(7826):588–590. doi:10.1038/s41586-020-2575-3
51. Umakanthan S, Patil S, Subramaniam N, Sharma R. COVID-19 vaccine hesitancy and resistance in India explored through a population-based longitudinal survey. Vaccines. 2021;9(10):1064. doi:10.3390/vaccines9101064
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.