Back to Journals » Journal of Pain Research » Volume 17
Efficacy and Safety of Loxoprofen Sodium Hydrogel Patch in Patients with Chronic Inflammatory Pain: A 4-Week Real-World Study
Authors Chen Y , Bian X, Wang J , Yan F, Gao J, Sun T
Received 28 August 2023
Accepted for publication 26 January 2024
Published 5 February 2024 Volume 2024:17 Pages 535—541
DOI https://doi.org/10.2147/JPR.S437462
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Krishnan Chakravarthy
Yang Chen, Xiaoen Bian, Junnan Wang, Fang Yan, Jing Gao, Tao Sun
Department of Pain Management, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, 250021, People’s Republic of China
Correspondence: Tao Sun, Shandong Provincial Hospital Affiliated to Shandong First Medical University, 324 Jingwu Road, Jinan, Shandong, 250021, People’s Republic of China, Email [email protected]
Purpose: Chronic inflammatory pain is usually treated with oral non-steroidal anti-inflammatory drugs (NSAIDs). However, oral NSAIDs can cause some adverse events, and local preparation is an important alternative drug. Currently, small sample clinical studies show that loxoprofen sodium hydrogel patch (LX-P) has good analgesic and anti-inflammatory effects; however, there is a lack of real-world clinical research data.
Patients and Methods: This study included 60 patients with chronic inflammatory pain. They were treated with LX-P without affecting their real-world treatment for two weeks.
Results: After 2 weeks of continuous medication, 93.33% of the patients stated that the treatment was effective. Only 3.33% of the patients had a relapse after 4 weeks. Moreover, the swelling range and degree of swelling decreased markedly and the dysfunction of the pain site was markedly alleviated. The total satisfaction of patients after treatment reached 90.00%.
Conclusion: In this real-world observational study, LX-P showed good efficacy and safety in patients with chronic inflammatory pain.
Keywords: loxoprofen sodium hydrogel patch, real-world study, chronic inflammatory pain, non-steroidal anti-inflammatory drugs
Introduction
Chronic inflammatory pain, such as pain resulting from osteoarthritis, frozen shoulder, cervical spondylosis, and myofasciitis, has a high incidence and recurrence rate.1,2 Chronic inflammatory pain tends to have a low age of onset and significantly increases the medical and economic burden of patients, which can markedly reduce the quality of life without timely intervention.3 According to the United Kingdom Health and Safety Executive, 5.7 million working days were lost between 2001 and 2002 due to low back pain, whereas 4.1 million working days were lost due to upper limb, neck, and shoulder pain, resulting in an annual economic loss of approximately £5.7 billion.4 In China, the years of living with disability increased dramatically from 17.6 million to 28.1 million between 1990 and 2019.5 Therefore, the treatment of chronic inflammatory pain is essential.
Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used to treat pain and inflammation associated with chronic diseases.6,7 However, oral NSAIDs may increase the risk of cardiovascular and renal diseases and are known to interact with various medications, including antihypertensive agents, antithrombotic agents, antidepressants, and corticosteroids.8,9 The use of oral non-selective NSAIDs [ie, cyclooxygenase (COX)-1 and COX-2 inhibitors] also increases the risk of upper gastrointestinal complications such as erosive gastritis and bleeding.10 Topical NSAIDs have analgesic and anti-inflammatory effects similar to those of oral NSAIDs but with a potentially improved safety profile due to their reduced systemic absorption.11 Therefore, the United States and European guidelines for osteoarthritis usually recommend topical NSAIDs rather than oral NSAIDs.12,13
Loxoprofen (LX) is a non-selective NSAID prodrug that became the second most commonly used NSAID (after diclofenac) in China in 2007.14 The LX in the loxoprofen sodium hydrogel patch (LX-P) penetrates directly into the affected area and relieves pain.15 Currently, small-sample clinical studies have shown that LX-P has good analgesic and anti-inflammatory therapeutic effects;7,15–17 however, there is a lack of data from real-world clinical studies. Real-world study (RWS) is a patient-centered outcome research that aims to evaluate the external validity and safety of intervention measures in real clinical settings.18,19 Compared to RWS, randomized controlled trials have stricter inclusion criteria and intervention protocols, making it difficult to reflect the true clinical use of LX-P. This 4-week study aimed to evaluate the efficacy, safety, and patient compliance of topical LX-P in the treatment of chronic inflammatory pain in a real-world setting to provide a theoretical basis for future clinical treatment of patients with chronic inflammatory pain.
Materials and Methods
Patients
Patients aged 18 years or older diagnosed with chronic inflammatory pain, including osteoarthritis, frozen shoulder, myofascial pain syndrome, tendinitis, tennis elbow, rheumatoid arthritis, gout, sacroiliac arthritis, and Achilles tendinitis, were included. The patients had no previous history of hemorrhage; asthma; severe cardiac, hepatic, or renal insufficiency; or allergy to NSAIDs.
The exclusion criteria included: (1) patients with bleeding disorders, bronchial asthma, or known allergy to NSAIDs; (2) pregnant and lactating women or women planning to become pregnant; (3) patients who had participated in clinical studies with other drugs within 1 month or had other concomitant diseases or complications (local skin lesions, infections, or rashes) that may have interfered with the observation of efficacy; and (4) other conditions that the investigators considered inappropriate for participation in the trial.
Study Design
This RWS was conducted at Shandong Provincial Hospital Affiliated to Shandong First Medical University. The study’s protocol was approved by the Institutional Review Board of Shandong Provincial Hospital Affiliated to Shandong First Medical University (SWYX: NO. 2021–405), in accordance with the Declaration of Helsinki and the guidelines for Good Clinical Practice.20 Informed consent was obtained from the participants. The participants were screened based on the inclusion and exclusion criteria.
LX-P (Jiudian Pharmaceutical, H20173272) was used in combination with the original conventional baseline treatment and examination without any modifications. LX-P contains 100 mg LX per patch, and the participants had to use one patch daily for 2 weeks. A case report form was completed by the investigator or operating physician at baseline to record the patient’s demographic characteristics, medical history, diagnosis, and real-world treatment plan and to collect data on the patient’s pain level, degree of swelling, and functional impairment (Supplemental File). The overall improvement in pain and efficacy rates, patch comfort and satisfaction, and adverse effects were assessed at weeks 1 and 2. The patients were assessed for recurrence at week 4.
Clinical Evaluation
The level of pain and its improvement were assessed using a numerical rating scale (NRS). The NRS is a classification of pain on a scale of 0–10, where a score of 0 represents no pain, and 10 represents severe pain. The reduction in pain level was calculated using the following formula:  .
.
Pain improvement was classified as (1) cured, with normal activity and ≥ 95% reduction in the NRS score; (2) highly effective, with significant symptomatic relief, basic recovery of functional activities, and ability to engage in normal work with 75–89% reduction in the NRS score; (3) effective, with symptomatic relief, improvement in functional activities, and ability to engage in light work with 30–74% reduction in NRS score; and (4) ineffective, with no significant improvement in symptoms and signs and < 30% reduction in the NRS score. The final effective rate was calculated using the following formula: (cured + highly effective + effective) / total number of cases.17
The secondary indicators included the degree of swelling at the site of pain and the degree of functional impairment. The degree of swelling was scored on a scale of 0 to 3, where 0 indicated no swelling; 1 indicated relatively normal skin swelling with stretch marks; 2 indicated skin swelling with loss of stretch marks, slightly elevated skin temperature, but no blister formation; and 3 indicated significant swelling, shiny skin, tension blisters, and significantly elevated skin temperature. The degree of functional impairment was also scored on a scale of 0 to 3, where 0 indicated normal function; 1 indicated mild impairment, such as mild functional limitation and no obvious impacts on life and work, such as pain in the joint with a degree of functional limitation less than one-third of normal; 2 indicated moderate impairment, such as obvious functional impairment and some difficulty or inconvenience in living and work activities, such as pain in the joint with a degree of functional limitation greater than one-third or less than two-thirds of normal; and 3 indicated severe impairment, such as severe functional impairment and living and work difficulties, such as pain in the joint with a degree of functional limitation greater than two-thirds of normal.
The patient’s comfort was assessed and scored using the degree of skin redness, rash, sticky hair, residual patch marks, odor, and pulling and constricting sensation during activity. Satisfaction included five levels: very satisfied, satisfied, neutral, dissatisfied, and very dissatisfied. Finally, if an adverse reaction occurred during the follow-up period, it was recorded in detail in the case report form (Supplemental File).
Statistical Analysis
Statistical analysis was performed using Statistical Package for the Social Sciences version 25.0 software. The Newcombe–Wilson method was used to calculate 95% confidence intervals (CIs) for the overall efficacy and recurrence rates. The independent samples t-test was used to analyze the differences in secondary indicators before and after treatment. Differences with P < 0.05 were considered statistically significant.
Results
Study Population and Baseline Characteristics
Sixty patients were screened and enrolled in this study between October 2021 and March 2022. The demographic characteristics of the 60 patients are presented in Table 1. There were 21 men, 38 women, and 1 patient whose sex was not accurately recorded. The patients’ ages ranged from 20 to 91 years, with a mean age of 57.10 years and a median age of 57 years.
 |
Table 1 Baseline Characteristics of the Patients |
Clinical Evaluation
The therapeutic efficacy was evaluated 1 and 2 weeks after drug administration (Table 2). After 1 week of treatment, 31 patients reported effective pain relief. However, the treatment was considered ineffective in 27 patients, with a total effective rate of 53.45% (95% CI: 40.80–65.67%). After 2 weeks, two patients reported significant therapeutic efficacy, and 54 reported effective treatment. However, the treatment was considered ineffective in four patients, with a total effective rate of 93.33% (95% CI: 84.07–97.38%). After 1 month of treatment, 2 out of 60 patients experienced recurrence, with a recurrence rate of 3.33% (95% CI: 0.9–11.4%).
 |
Table 2 (1) the Total Effective Rate After 1 Week. (2) the Total Effective Rate After 2 Weeks. (3) the Recurrence Rate After 4 Weeks |
For the secondary indicators, the average extent of swelling at the site of pain decreased from 4.38 to 2.88. The average degree of swelling decreased from 3.47 to 2.87, and the average degree of functional impairment decreased from 0.75 to 0.37, with highly significant differences in all groups. After 2 weeks, the average extent and degree of swelling and functional impairment continued to decrease to 1.60, 2.17, and 0.13, respectively (Table 3).
 |
Table 3 (1) the Secondary Indicators After 1 Week. (2) the Secondary Indicators After 2 Weeks |
After 2 weeks of treatment, patient comfort and satisfaction were assessed. The average comfort score was 0.92, representing a mild or lower degree of skin redness, residual patch marks, and odor (Table 4). Meanwhile, 53 of the 60 patients were satisfied, and 1 was very satisfied, with an overall satisfaction rate of 90.00% (95% CI: 79.85–95.34%) (Table 5). No obvious adverse reactions occurred during the follow-up period.
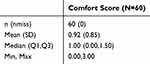 |
Table 4 The Comfort Score |
 |
Table 5 The Total Satisfaction Rate |
Discussion
Several domestic and international guidelines and consensuses currently recommend topical NSAIDs as the first choice for chronic musculoskeletal system pain.12,13 Data from some studies have shown that topical LX-P for chronic inflammatory pain significantly reduced NRS pain scores and improved quality of life. After the application of LX-P to the lesion, LX accumulates in local tissues, which can provide strong anti-inflammatory and analgesic effects and rapid relief of pain at the lesion that lasts 24 h.21 A randomized, controlled, double-blind, double-dummy, multicenter, non-inferiority trial included 169 patients with knee osteoarthritis.17 Patients were randomly assigned to either LX-P or loxoprofen tablet (LX-T) groups for a 4-week treatment. At the end of the treatment, the curative effect was similar between the two groups. There were fewer adverse events in the LX-P group, but the difference was not statistically significant. In another study, 70 patients with active ankylosing spondylitis were randomly assigned to the LX-P and LX-T groups, and there was no significant difference in efficacy and safety between the two groups after 4 weeks of treatment.15 A Phase III, randomized, double-blind, non-inferiority study included 242 patients diagnosed with lower and upper limbs post-traumatic injury who were experiencing moderate or severe pain.16 After being randomly divided into two groups and treated with LX-P and LX-T for 7 days, the pain relief of the two groups was similar, while the incidence of adverse events in the LX-P group was half that of the LX-T group. In this study, the therapeutic efficacy of topical LX-P was 93.33% after 2 weeks, effectively reducing the pain experienced by patients. In addition, there was an extremely significant reduction in local swelling and functional impairment, indicating that topical LX-P treatment was effective in improving patient quality of life. The follow-up results 2 weeks after the end of the treatment course indicated a relapse rate of only 3.33%, indicating that LX-P can lead to long-lasting therapeutic efficacy.In another double-blind, double-dummy, parallel-group, randomized controlled trial, 182 Chinese patients were enrolled and randomized equally to either LX-T or LX-P treatment for 2 weeks.7 The primary endpoint of final efficacy rate was 81.3% in the LX-P group and 72.2% in the LX-T group.No serious adverse events occurred in either group.
LX-P exhibited good tolerability and safety in this study, with no clear adverse effects and good patient comfort and satisfaction. Oral NSAIDs reduce inflammation levels and pain intensity by inhibiting COX isoenzymes and lowering prostaglandin levels. To achieve pain relief, oral NSAIDs must achieve systemic efficacy levels, which can also lead to gastrointestinal and cardiovascular complications.21,22 Topical formulations provide low systemic drug levels, producing peak blood concentrations that are less than 10% of the levels observed after the administration of oral NSAIDs, and produce adverse effects that are usually mild and transient, such as pruritus, which resolve with discontinuation of the drug.23 Recently published evidence-based guidelines for topical NSAIDs for musculoskeletal pain have also indicated no statistically significant difference in the risk of adverse events with topical NSAIDs compared with placebo.3
We have found the excellent practicability of LX-P from a number of randomized controlled trials, and this study is the earliest RWS. However, this study had some limitations. First, the sample size of this study is small, and the 1-month follow-up time is shorter. Second, there was not enough control in this study. In the future, further research is necessary to improve the limitations of the present study.
Conclusion
In this real-world observational study, LX-P showed good efficacy and safety in patients with chronic inflammatory pain, reducing pain while decreasing local swelling and functional impairment and improving patient quality of life. After 2 weeks of treatment, 93.33% of patients reported the treatment to be effective. Furthermore, during the follow-up at 1 month, only 3.33% of patients experienced recurrence. This study is the earliest RWS on the clinical application of LX-P, demonstrating its effective alleviation of chronic inflammatory pain in real clinical settings.
Acknowledgments
The authors would like to thank Hunan Jiudian Pharmaceutical Co., Ltd. for funding the study and Editage for English language editing in the manuscript. The authors are grateful for all staff involved in the study development and conduction. This study was supported by Hunan Jiudian Pharmaceutical Co., Ltd.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cieza A, Causey K, Kamenov K, et al. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2021;396(10267):2006–2017. doi:10.1016/S0140-6736(20)32340-0
2. Cooper C, Arden NK. Excess mortality in osteoarthritis. BMJ. 2011;342:d1407. doi:10.1136/bmj.d1407
3. Shi C, Ye Z, Shao Z, et al. Multidisciplinary guidelines for the rational use of topical non-steroidal anti-inflammatory drugs for musculoskeletal pain (2022). J Clin Med. 2023;12(4):1544. doi:10.3390/jcm12041544
4. Buckle P. Ergonomics and musculoskeletal disorders: overview. Occup Med. 2005;55(3):164–167. doi:10.1093/occmed/kqi081
5. Chen N, Fong D, Wong J. Trends in musculoskeletal rehabilitation needs in china from 1990 to 2030: a bayesian age-period-cohort modeling study. Front Public Health. 2022;10:869239. doi:10.3389/fpubh.2022.869239
6. Paoloni JA, Milne C, Orchard J, Hamilton B. Non-steroidal anti-inflammatory drugs in sports medicine: guidelines for practical but sensible use. Br J Sports Med. 2009;43(11):863–865. doi:10.1136/bjsm.2009.059980
7. Zhao D, Chen Z, Hu S, et al. Efficacy and safety of loxoprofen hydrogel transdermal patch versus loxoprofen tablet in Chinese patients with myalgia: a double-blind, double-dummy, parallel-group, randomized, controlled, non-inferiority trial. Clin Drug Investig. 2019;39(4):369–377. doi:10.1007/s40261-019-00756-x
8. Moore N, Pollack C, Butkerait P. Adverse drug reactions and drug-drug interactions with over-the-counter NSAIDs. Ther Clin Risk Manag. 2015;11:1061–1075. doi:10.2147/TCRM.S79135
9. Scarpignato C, Lanas A, Blandizzi C, et al. Safe prescribing of non-steroidal anti-inflammatory drugs in patients with osteoarthritis--an expert consensus addressing benefits as well as gastrointestinal and cardiovascular risks. BMC Med. 2015;13(1):55. doi:10.1186/s12916-015-0285-8
10. Scheiman JM. NSAID-induced gastrointestinal injury: a focused update for clinicians. J Clin Gastroenterol. 2016;50(1):5–10. doi:10.1097/MCG.0000000000000432
11. Rannou F, Pelletier JP, Martel-Pelletier J. Efficacy and safety of topical NSAIDs in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4 Suppl):S18–S21. doi:10.1016/j.semarthrit.2015.11.007
12. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, Hip, and knee. Arthritis Care Res. 2012;64(4):465–474. doi:10.1002/acr.21596
13. Bruyere O, Cooper C, Pelletier JP, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis-From evidence-based medicine to the real-life setting. Semin Arthritis Rheum. 2016;45(4 Suppl):S3–S11. doi:10.1016/j.semarthrit.2015.11.010
14. Arakawa T, Fujiwara Y, Sollano JD, et al. A questionnaire-based survey on the prescription of non-steroidal anti-inflammatory drugs by physicians in East Asian countries in 2007. Digestion. 2009;79(3):177–185. doi:10.1159/000211713
15. Fan M, Cao S, Tu L, et al. Efficacy and safety of loxoprofen hydrogel patch versus loxoprofen tablet in patients with ankylosing spondylitis: a 4-week randomized, open-label study. Biomed Rep. 2019;10(6):331–336. doi:10.3892/br.2019.1209
16. Fujiki EN, Netto NA, Kraychete DC, et al. Efficacy and safety of loxoprofen sodium topical patch for the treatment of pain in patients with minor acute traumatic limb injuries in Brazil: a randomized, double-blind, noninferiority trial. Pain. 2019;160(7):1606–1613. doi:10.1097/j.pain.0000000000001549
17. Mu R, Bao CD, Chen ZW, et al. Efficacy and safety of loxoprofen hydrogel patch versus loxoprofen tablet in patients with knee osteoarthritis: a randomized controlled non-inferiority trial. Clin Rheumatol. 2016;35(1):165–173. doi:10.1007/s10067-014-2701-4
18. Liu F, Panagiotakos D. Real-world data: a brief review of the methods, applications, challenges and opportunities. BMC Med Res Methodol. 2022;22(1):287. doi:10.1186/s12874-022-01768-6
19. Andrade C. Real world studies: what they are and what they are not. Indian J Psychol Med. 2023;45(5):537–538. doi:10.1177/02537176231188563
20. Grimes DA, Hubacher D, Nanda K, et al. The good clinical practice guideline: a bronze standard for clinical research. Lancet. 2005;366(9480):172–174. doi:10.1016/S0140-6736(05)66875-4
21. Greig SL, Garnock-Jones KP. Loxoprofen: a Review in Pain and Inflammation. Clin Drug Investig. 2016;36(9):771–781. doi:10.1007/s40261-016-0440-9
22. Heyneman CA, Lawless-Liday C, Wall GC. Oral versus topical NSAIDs in rheumatic diseases: a comparison. Drugs. 2000;60(3):555–574. doi:10.2165/00003495-200060030-00004
23. Lionberger DR, Brennan MJ. Topical nonsteroidal anti-inflammatory drugs for the treatment of pain due to soft tissue injury: diclofenac epolamine topical patch. J Pain Res. 2010;3:223–233. doi:10.2147/JPR.S13238
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
