Back to Journals » OncoTargets and Therapy » Volume 13
Efficacy and Safety of Iodine-125 Brachytherapy in the Treatment of Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma
Authors Wu C, Li B, Sun G, Peng C, Xiang D
Received 27 June 2020
Accepted for publication 31 August 2020
Published 29 September 2020 Volume 2020:13 Pages 9657—9666
DOI https://doi.org/10.2147/OTT.S269626
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Chunrong Wu,1 Bo Li,2 Guiyin Sun,1 Chunfang Peng,1 Debing Xiang1
1Department of Oncology, Jiangjin Central Hospital of Chongqing, Jiangjin, Chongqing 402260, People’s Republic of China; 2Department of Cardiology, Jiangjin Central Hospital of Chongqing, Jiangjin, Chongqing 402260, People’s Republic of China
Correspondence: Bo Li; Debing Xiang
Department of Cardiology; Department of Oncology, Jiangjin Central Hospital of Chongqing, No. 725 Jiangzhou Avenue, Dingshan Street, Jiangjin 402260, People’s Republic of China
Tel +86-23-47557382
Email [email protected]; [email protected]
Background: Recurrent or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) is a difficult challenge for physicians, especially when patients have been treated with external beam radiotherapy. The purpose of this study was to assess the clinical efficacy and safety of computed tomography (CT)-guided iodine-125 brachytherapy as a palliative treatment for R/M HNSCC.
Methods: From May 2011 to July 2018, we enrolled 87 patients with R/M HNSCC who had previously received external beam radiotherapy. Among these patients, 43 successfully underwent CT-guided iodine-125 brachytherapy and chemotherapy (group A); 44 patients who only received chemotherapy (group B) were matched with patients in group A. Patients’ pain score, Eastern Cooperative Oncology Group (ECOG) score, tumor compression symptoms, and side effects of iodine-125 implantation were recorded. Clinical follow-up was performed to assess progression-free survival (PFS) and overall survival (OS).
Results: Both groups of patients completed the treatment and were followed up for 9– 66 months, with a median follow-up time of 44 months. The OS was 51 months (95% CI: 42.93– 59.06 months) versus 28 months (95% CI: 23.79– 32.21 months) (p < 0.05), the PFS was 10 months (95% CI: 6.15– 13.84 months) versus 6 months (95% CI: 4.40-7.59 months) (p < 0.05) in groups A and B, respectively. The RR in group A was 25/43 (58.14%) versus 15/44 (34.10%) in group B (p < 0.05). Compared with group B, patients in group A had lower pain scores, better physical performance, and better improvement of compression symptoms. No serious treatment-related complications were observed in either group of patients.
Conclusion: Compared with chemotherapy alone, iodine-125 seed implantation combined with chemotherapy was a more effective and safer strategy for R/M HNSCC.
Keywords: iodine-125, brachytherapy, head and neck squamous cell carcinoma, recurrent, metastatic
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer worldwide. Despite diagnostic and therapeutic advances, up to 50%–60% of patients with locally advanced disease develop loco-regional relapse and/or distant metastasis within 2 years.1 Recurrent or metastatic (R/M) HNSCC often enlarges rapidly and causes a series of symptoms such as severe pain, swallowing or breathing difficulties, and hindrance of activities. Patients with advanced (R/M) HNSCC are prone to disease progression and rapid deterioration of the physical condition, such as loss of appetite, difficulty sleeping, malnutrition, and weight loss. Current conventional treatment methods, including surgery, chemotherapy, external beam radiotherapy, targeted therapy, and immunotherapy, are far from satisfactory.2,3 Treatment is difficult for patients who relapse after surgery and after radiotherapy and who cannot tolerate reoperation and do not respond to external beam radiotherapy. Therefore, it is necessary to find a treatment method with less trauma but reliable efficacy. In clinical trials, quality of life (QOL) has received increasing attention by cancer researchers and has become an important end-point in clinical cancer research, especially for patients with advanced malignant tumors.4
It is difficult to repeat external radiation therapy in patients with R/M HNSCC who had previously received radiation therapy because the dose of radiation to the surrounding organs at risk has reached its upper limit. In addition, owing to the important anatomic structures of the head and neck, such as the parotid gland, brainstem, spinal cord, optic nerve, eye lens, and temporal lobe, as well as the requirements of functional preservation and cosmetic effect, reoperation is only possible in limited cases. There is an urgent need to find methods with good palliative effect, good local control, and mild side effects. Radioactive iodine-125 implantation (I125), also known as “particle knife,” is a new complementary treatment method that can overcome the limitations of surgery and radiotherapy. The advantage of I125 seed implantation therapy is the release of a high radiation dose (100–140 Gy) to the tumor mass and a sharply reduced decline in dose at close range.5 As a result, the tumor cells are killed, leaving the surrounding normal tissue unaffected or with few side effects.
As internal radiotherapy, I125 seed implantation, which can be performed in a short time (30–45 minutes) with good patient compliance, has been successfully used to treat a series of malignant tumors, such as non-small-cell lung carcinoma,6–8 brain tumors,9,10 prostate cancer,11 and gynecological malignant tumors.12,13 I125 seed implantation therapy is rarely reported but is worth exploring for patients with R/M HNSCC.
Over the years, we have conducted computed tomography (CT)-guided I125 brachytherapy for a large number of patients with R/M HNSCC in our oncology department. The purpose of this retrospective study was to evaluate the efficacy and safety of I125 brachytherapy and chemotherapy for patients with R/M HNSCC. We were also particularly concerned about the impact of I125 seed implantation on QOL.
Materials and Methods
Patients
We enrolled a total of 87 patients with R/M HNSCC from May 2011 to July 2018. The average patient age was 62 (range, 36–78) years. All patients received radiation therapy combined with cisplatin synchronous chemotherapy at the time of initial consultation; some patients had surgery plus radiation therapy. The diagnosis of all patients was confirmed by a pathologist and imaging diagnostician. Mainly, imaging such as CT, magnetic resonance imaging (MRI), or positron emission tomography-CT (PET-CT) was used for detection and staging.
Generally, the treatment was tolerable for all patients. In total, 43 patients were enrolled in group A; these patients had a history of I125 seed implantation and met the following criteria: 1) R/M HNSCC confirmed by histological or imaging methods, 2) R/M HNSCC stage III or IV with a physical status of 0–2, or 3) life expectancy longer than 3 months, and 4) platelet count > 20.0 × 109/L and normal coagulation function. Exclusion criteria were 1) unable to tolerate the percutaneous I125 seed implantation procedure, 2) refusal of I125 seed implantation treatment, 3) unsuitable for I125 brachytherapy (tumor near or surrounding the great vessels or spinal cord, tumor rupture, local infection, and so on). Patients with R/M HNSCC who received chemotherapy were included in the control group (group B). To obtain a comparable outcome, we selected a subgroup of 44 patients who matched well with patients in group A with respect to age, sex, tumor histology characteristics, tumor location, Eastern Cooperative Oncology Group (ECOG) status, pain score, and tumor compression effects. The clinical characteristics of these patients are listed in Table 1. The tumor (T), lymph node (N), and metastasis (M) stage of tumors was assessed according to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 7th Edition.14 The research protocol was approved by the Ethics Committee of Jiangjin Central Hospital of Chongqing, and written informed consent was received from each patient, in accordance with the Declaration of Helsinki.
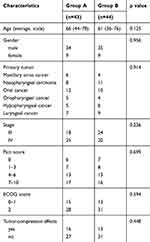 |
Table 1 Clinical Features of Patients Enrolled in This Study |
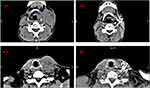
CT-Guided Implantation of I125 Seeds
The I125 implantation device includes an 18G particle implantation needle and an implantation gun. The I125 radioactive particles used in our study were produced by Beijing Zhibo Hi-Tech Biotechnology Co., Ltd., and consist of a central silver rod and a titanium alloy shell. The central silver rod has a diameter of 0.5 mm and length of 3.0 mm. A single I125 particle with radioactivity of 0.764–0.826 mCi is used clinically. Quality control including a leak detection experiment and activity measurement was performed by the manufacturer. Tumor margin and implantation of I125 seeds were calculated using a CT scanner. The precise location of the radioactive seed implant was confirmed in the preoperative treatment plan system (TPS). The planning target volume (PTV) dose was set at 100–140 Gy. We performed I125 seed brachytherapy as described in our previous research work.15
Chemotherapy
Patients in both groups A and B received chemotherapy. The chemotherapy regimen consisted of paclitaxel (135 mg/m2 on day 1) and cisplatin (30 mg/m2 on days 1, 2, and 3), with a 21-day cycle, which can be repeated for 4–6 cycles if tolerated by the patient. White blood cells (WBCs), red blood cells (RBCs), platelet count, and liver/kidney function status were measured weekly to assess chemotherapy toxicity. Tumor volume was assessed using enhanced CT every 3 months.
Evaluation of Efficacy and Safety
Efficacy and safety were evaluated 3 months after treatment. The tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria.16 The response rate (RR) was calculated as (CR + PR)/total number of patients ×100%. Progression-free survival (PFS) was based on the time interval from the treatment start to disease progression, death, or the end of the study. Overall survival (OS) was defined as the time interval from the date of diagnosis to death or the end of the study.
The toxicity criteria of the Radiation Therapy Oncology Group (RTOG) were applied to assess acute and late adverse effects of irradiation. Side effects of radiation include oral mucositis, radiation dermatitis, aggravated xerostomia, altered taste function, peripheral nerve injury, and hematology toxicity.17 The pain score was calculated using a numerical rating scale (NRS).18 The ECOG score was used to assess the patient’s physical status.19
Symptoms of tumor-induced compression are related to the anatomical location of the HNSCC and may include imaging findings suggesting that the tumor has obvious compression and is pushing on the surrounding tissues, leading to dysfunction such as difficulty opening the mouth, dysphagia, dysphonia, or nerve compression causing local pain and numbness. The improvement of tumor compression symptoms may be manifested as tumor shrinkage, swelling reduction, pain relief, and dysfunction improvement.
Follow-Up
Follow-up consisted of outpatient follow-up visits plus telephone follow-up. All patients were followed up at 3 months after chemotherapy, with or without I125 seed implantation, and then every 3 months for up to 66 months; the end of follow-up was May 2020. Patients’ imaging results, blood cell counts, physical status, pain score, side effects of radiotherapy, improvement in symptoms of compression, RR, PFS, and death were recorded in detail.
Statistical Analysis
We used IBM SPSS version 23.0 for data processing and analysis (IBM Corp., Armonk, NY, USA). Continuous data are expressed as mean ± standard deviation and were determined with Student’s t test. Categorical variables were analyzed using the χ2 test or Wilcoxon rank-sum test. The Kaplan–Meier method and Log rank test were used to analyze the survival curves of patients in the two groups. A p-value < 0.05 was considered to indicate a statistically significant difference.
Results
Patient Characteristics
The baseline characteristics of the two groups were well balanced in terms of age, sex, tumor stage, ECOG status, pain score, and tumor compression effects (Table 1). Patients in both groups A and B received chemotherapy; in addition, patients in group A successfully underwent I125 implantation therapy.
Relief of Clinical Symptoms
Compared with group B, the symptoms of patients in group A were significantly relieved after treatment. After I125 brachytherapy combined with chemotherapy, 7 patients in group A reported having no pain, 17 had mild pain, and 12 had moderate pain. However, 14 patients in group B developed moderate pain and 15 patients developed severe pain. These results indicated that pain in group A was significantly improved after treatment, whereas that in group B was aggravated (p = 0.035; Table 2).
 |
Table 2 Analysis of Pain and Physical Status Score in Patients After Treatments |
Before treatment, the ECOG scores of 15 patients in group A did not exceed 1, those of 28 patients did not exceed 2, and those of 13 patients in group B did not exceed 1; 31 patients had ECOG scores of 2, showing no significant difference (p = 0.594; Table 1). After treatment, 8 patients in group A had scores of no greater than 1, 21 patients had scores of 2; 16 patients in group B had scores of more than 3 points, and 10 patients had 4 points, with a statistically significant difference (p = 0.016; Table 2).
Among the 43 patients in group A before treatment, 16 patients had tumor compression, presenting with dysphagia, local swelling, and upper limb weakness or numbness; 13 patients in group B also experienced tumor compression (p = 0.448; Table 1). After treatment, the number of patients with tumor compression in group A was reduced to 5, whereas 14 patients in group B still had tumor compression (p = 0.023; Table 3). These findings indicated that I125 particle therapy combined with chemotherapy can significantly improve the symptoms of tumor compression and improve QOL of patients with R/M HNSCC when compared with chemotherapy alone.
 |
Table 3 Analysis of Tumor Compression in Patients After Treatments |
Response Rate (RR) and Survival Outcomes
The median follow-up time was 44 months (range: 9–66 months). After the 6-month follow-up of patients in both groups, CR and PR were observed in 7/43 (16.2%) and 18/43 (41.8%) patients in group A and in 4/44 (9.1%) and 11/44 (25%) in group B (Figure 1), respectively; 8/43 patients had PD (18.6%) in group A versus 15/44 patients (34.1%) in group B. The RR of tumors among patients in group A was 58.14% (25/43) versus 34.10% (15/44) in group B (χ2 = 5.064; p = 0.024; Table 4).
 |
Table 4 Comparasion of Treatment Effect in Both Groups |
The median PFS in group A was 10 months (95% confidence interval [CI]: 6.15–13.84), and that in group B was 6 months (95% CI: 4.40–7.59) (Log rank test, χ2 = 14.460; p < 0.001; Figure 2). The median OS in group A was 51 months (95% CI: 42.93–59.06), and that in group B was 28 months (95% CI: 23.79–32.21) (Log rank test, χ2 = 20.424, p < 0.001; Figure 3).
Complications
After brachytherapy, two patients developed oral mucositis, and three patients experienced radiation dermatitis. No patients developed seed migration, aggravated xerostomia, altered taste function, peripheral nerve injury, spinal cord injury, wound infection, or local bleeding (Table 5).
 |
Table 5 Complications After Brachytherapy with Iodine-125 Seed |
Hematological toxicity caused by chemotherapy drugs can manifest as leukopenia, anemia, and thrombocytopenia. After treatment, different degrees of decrease in WBCs, RBCs, and platelet count were observed in the two groups, but there was no significant difference (p > 0.05; Table 6). After treatment with certain drugs, affected patients’ routine blood values gradually returned to normal.
 |
Table 6 Adverse Events Observed in Both Groups Upon Follow‑up |
Discussion
HNSCC involves cancer of the oral cavity, oropharynx, hypopharynx, and larynx.20 Surgery, chemotherapy, and external radiotherapy are the standard treatment for HNSCC. Cetuximab, PD-1 inhibitor pembrolizumab used in monotherapy or in combination with platinum/5-FU, has recently been used as first-line treatment for R/M HNSCC.2,21 However, the prognosis of patients with R/M HNSCC remains poor.22 A large number of patients with R/M HNSCC have local symptoms of compression such as severe pain, difficulty breathing or swallowing, trouble carrying out activities, loss of appetite, difficulty sleeping, local bleeding, or infection, which seriously affect QOL. There is an urgent need to find treatments that improve patient symptoms, prolong survival, and reduce side effects.
The treatment of R/M HNSCC is challenging. The anatomical structure of the head and face is special and the function complicated. Reoperation may destroy function and lead to a risk of cosmetic deformities.23 Because the head and neck are adjacent to the pituitary gland, eye lens, temporal lobe, brain stem, and spinal cord, the ability to further increase the external dose is limited. In current precise radiotherapy, such as IMRT,24 IGRT,25,26 and SBRT,27 the side effects of radiotherapy remain unavoidable, such as swallowing and voice dysfunction,28 skin toxicity,29 oral mucositis,30 altered taste function,31 and xerostomia.32 Owing to the high cost of molecular targeted therapy and immunotherapy, many patients with R/M HNSCC are unable to afford this treatment. However, patients with advanced tumors still wish to alleviate their pain and achieve a good QOL. Assessment of QOL has received much attention in the treatment of patients with advanced cancer. Therefore, in cases of failure of surgery and external beam radiotherapy, there is an urgent need for effective and less toxic treatment.
I125 brachytherapy induces tumor cell apoptosis by implanting I125 particles into the tumor tissues. Radioactive I125 particles can emit γ rays at 27–35 keV with a half-life of 60.2 d.33 Beta rays exert anti-tumor effects by damaging tumor cell DNA, inducing tumor cell apoptosis, and promoting G2/M phase arrest.34 I125 particles can reach tumor sites that are difficult to reach by external irradiation and are particularly suitable for treating multifocal metastatic tumors, prolonging median survival time and PFS, and improving overall survival.
At present, I125 seed implantation therapy has been improved with novel technological advances such that patients can obtain good efficacy and experience fewer toxicities and side effects. Ji et al carried out radioactive I125 particle implantation treatment for paravertebral/postperitoneal tumors. Using 3D-printed non-coplanar templates (3D–PNCT) and CT technology, the particles can more accurately locate paravertebral/postperitoneal lesions.35 Liu et al reported that in the treatment of recurrent high-grade gliomas, 3D-PIT based on CT/MRI fusion images can enable better positioning accuracy and dose distribution of radioactive I125 particle implantation.36 Jiang et al studied the acute and late side effects of 3D-PNCT used in CT-guided radioactive I125 particles (RIS) implanted in recurrent cancer of the head and neck. Those authors found that 3D-PNCT-assisted RIS implantation can provide good positioning accuracy, and there were no obvious adverse reactions for locally recurring malignant head and neck tumors.37
Currently, the application of I125 brachytherapy in tumors continues to expand to include use in challenging tumors. Jiao et al found that patients with metastatic bone tumors treated with I125 particle brachytherapy after the failure of in vitro radiotherapy can achieve good long-term analgesic effects.38 Qu et al explored the efficacy and safety of IGRIS implantation for PRCC after EBRT; the data showed that particle therapy was particularly suitable for recurrent cervical cancer with pelvic wall involvement and could achieve a good analgesic effect.39
I125 seed implantation is particularly suitable for primary or metastatic head and neck tumors. Puthawala et al evaluated the response and long-term tumor control of recurrent head and neck cancers treated with interstitial low-dose-rate brachytherapy. At a minimum 6-month follow-up, local tumor control was achieved in 77% (217/282) of implanted tumor sites. The 2-, 5-, and 10-year disease-free actuarial survival rates for the entire group were 60%, 33%, and 22%, respectively.40
The landmark randomized study of reirradiation for head and neck cancer by Janot and colleagues should be mentioned. Those authors conducted a randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery, compared with salvage surgery alone, in head and neck carcinoma. The results showed that postoperative full-dose reirradiation combined with chemotherapy significantly improved disease-free time (DFS) but had no significant impact on OS. An increase in both acute and late toxicity was observed.41 Breen et al reviewed the records of patients who received brachytherapy for head and neck cancer in a previously irradiated field between 2007 and 2016. In total, 69 patients received brachytherapy-based reirradiation. Results showed that brachytherapy was more effective for mucosal recurrence than neck recurrence. After treatment, patients’ pain symptoms were significantly improved; however, 27% and 19% of patients experienced grade 3 adverse events of acute and chronic toxicity, respectively.42
In this study, we performed I125 implantation therapy in patients with R/M HNSCC. The results showed that I125 implantation has significant advantages in improving patient QOL and alleviating tumor-related symptoms. After receiving seed implantation plus chemotherapy, the tumor compression symptoms of group A were significantly reduced, pain was alleviated, and ECOG score improved, indicating that seed therapy significantly improved patient QOL. Patients’ compliance with treatment was better owing to faster recovery time, shorter hospital stay, lower risk of postoperative infection, and lower treatment costs.
I125 particle therapy improves RR and prolongs survival time. Compared with the control group, the total survival time was extended by 23 months, and PFS was extended by 4 months. The RR of group A was 58.14%, significantly higher than that of group B (34.10%). Similarly, Jie et al studied 76 patients who underwent I125 particle implantation after radical resection of oral squamous cell carcinoma; they found that the 5-year survival rate was 81.5%, and the 6-month local control rate was 95.3%.43 Wang et al reported the efficacy of brachytherapy combined with chemotherapy in the treatment of unresectable HNSCC. Their results showed a total RR of 78.3%, without acute complications and treatment-related radiation injury. PFS was 60.9% and OS was 52.2% during 2 years of follow-up.22 The RR of I125 brachytherapy in our study was lower than that in these studies, probably because our patients had R/M HNSCC, so the treatment difficulty was considerably increased in comparison with the initial treatment.
In our participants, there were no severe complications. Compared with the control group, hematological toxicity was not increased in the intervention group. When a patient experienced bone marrow suppression of more than 2°, their blood cell count returned to normal after treatment with CSF, EPO, IL-11, and so on. The main adverse events of I125 implantation were skin/mucosal toxicities, which improved after treatment by maintaining local cleanliness and promoting mucous membrane repair. Compared with other studies,41,42 the side effects of I125 brachytherapy in our study were relatively mild, especially in that there was no carotid artery rupture because patients with tumors close to or surrounding large blood vessels were excluded from the treatment group. Our research clarified that I125 seed implantation combined with chemotherapy was safe for R/M HNSCC, without causing greater risks to patients.
Conclusions
Our research has initially proved that I125 particle implantation combined with chemotherapy is effective and safe for patients with R/M HNSCC, with fewer side effects, higher survival rate, and better QOL. The need remains to further expand the sample size, to clarify the efficacy and safety of I125 seed implantation combined with chemotherapy for R/M HNSCC.
Acknowledgments
We thank all the people and patients who participated in this study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. Bo Li and Debing Xiang equally as co-corresponding Author.
Funding
This study was supported by District Science and Technology Commission Project of Chongqing Jiangjin (grant number Y2019067), and Health and Family Planning Commission Medical Research Project of Chongqing (grant number 2017MSXM171).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Lau A, Yang WF, Li KY, et al. Systemic therapy in recurrent or metastatic head and neck squamous cell carcinoma- a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2020;153:102984. doi:10.1016/j.critrevonc.2020.102984
2. Taberna M, Oliva M, Mesía R. Cetuximab-containing combinations in locally advanced and recurrent or metastatic head and neck squamous cell carcinoma. Front Oncol. 2019;9:383. doi:10.3389/fonc.2019.00383
3. Cramer JD, Burtness B, Ferris RL. Immunotherapy for head and neck cancer: recent advances and future directions. Oral Oncol. 2019;99:104460. doi:10.1016/j.oraloncology.2019.104460
4. Di Maio M, Perrone F. Quality of Life in elderly patients with cancer. Health Qual Life Outcomes. 2003;1:44. doi:10.1186/1477-7525-1-44
5. Skowronek J. Brachytherapy in the treatment of lung cancer-a valuable solution. J Contemp Brachytherapy. 2015;7(4):297–311. doi:10.5114/jcb.2015.54038
6. Santos R, Colonias A, Parda D, et al. Comparison between sublobar resection and 125Iodine brachytherapy after sublobar resection in high-risk patients with Stage I non-small-cell lung cancer. Surgery. 2003;134(4):691–697. doi:10.1016/S0039-6060(03)00327-1
7. Fernando HC, Landreneau RJ, Mandrekar SJ, et al. The impact of adjuvant brachytherapy with sublobar resection on pulmonary function and dyspnea in high-risk patients with operable disease: preliminary results from the American College of Surgeons Oncology Group Z4032 trial. J Thorac Cardiovasc Surg. 2011;142(3):554–562. doi:10.1016/j.jtcvs.2010.10.061
8. Yu X, Li J, Zhong X, et al. Combination of Iodine-125 brachytherapy and chemotherapy for locally recurrent stage III non-small cell lung cancer after concurrent chemoradiotherapy. BMC Cancer. 2015;15:656. doi:10.1186/s12885-015-1657-3
9. Shahzadi S, Azimi P, Long-Term PK. Results of stereotactic Brachytherapy (Temporary 125Iodine seeds) for the treatment of low-grade astrocytoma (Grade II). Iran Red Crescent Med J. 2013;15(1):49–57. doi:10.5812/ircmj.4322
10. Schwarz SB, Thon N, Nikolajek K, et al. Iodine-125 brachytherapy for brain tumours – a review. Radiat Oncol. 2012;7:30.
11. Zuber S, Weiß S, Baaske D, et al. Iodine-125 seed brachytherapy for early stage prostate cancer: a single-institution review. Radiat Oncol. 2015;10:49. doi:10.1186/s13014-015-0349-0
12. Wang Y, Zhang W, Liu P, et al. Computed tomography-guided 125I seed interstitial implantation in the treatment of recurrent ovarian cancer. Int J Gynecol Cancer. 2014;24(8):1414–1419. doi:10.1097/IGC.0000000000000244
13. Han L, Li C, Wang J, et al. Iodine-125 radioactive seed tissue implantation as a remedy treatment for recurrent cervical cancer. J Cancer Res Ther. 2016;12(Supplement):C176–C180. doi:10.4103/0973-1482.200611
14. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi:10.1245/s10434-010-0985-4
15. Wu C, Li B, Sun G, et al. Efficacy and safety of iodine-125 brachytherapy combined with chemotherapy in the treatment of advanced NSCLC in the elderly. Onco Targets Ther. 2018;11:6617–6624. doi:10.2147/OTT.S174457
16. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–216.
17. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. doi:10.1016/0360-3016(95)00060-C
18. Farčić N, Barać I, Pačarić S, et al. Acute postoperative pain in trauma patients – the fifth vital sign. Open Access Maced J Med Sci. 2017;5(3):310–315. doi:10.3889/oamjms.2017.067
19. De Kock I, Mirhosseini M, Lau F, et al. Conversion of Karnofsky Performance Status (KPS) and Eastern Cooperative Oncology Group Performance Status (ECOG) to Palliative Performance Scale (PPS), and the interchangeability of PPS and KPS in prognostic tools. J Palliat Care. 2013;29(3):163–169. doi:10.1177/082585971302900305
20. Langius JA, Zandbergen MC, Eerenstein SE, et al. Effect of nutritional interventions on nutritional status, quality of life and mortality in patients with head and neck cancer receiving (chemo)radiotherapy: a systematic review. Clin Nutr. 2013;32(5):671–678. doi:10.1016/j.clnu.2013.06.012
21. Ling DC, Bakkenist CJ, Ferris RLet al. Role of immunotherapy in head and neck cancer. Semin Radiat Oncol. 2018;28(1):12–16. doi:10.1016/j.semradonc.2017.08.009
22. Wang X, Meng J. Efficacy of brachytherapy concomitant with chemotherapy with docetaxel, cisplatin, and 5-fluorouracil in unresectable head and neck squamous cell carcinoma. JBUON. 2016;21(3):588–593.
23. Wu WJ, Guo HQ, Yu GY, et al. Iodine-125 interstitial brachytherapy for pediatric desmoid-type fibromatosis of the head and neck: a case report. J Oral Maxillofacial Surg. 2017;75(4):
24. Özcan MF, Altınova S, Atan A. Treatment approaches to small renal masses in patients of advanced age (75 years). Turk J Urol. 2018;44(4):281–286.
25. Arns A, Blessing M, Fleckenstein Jet alPhantom-based evaluation of dose exposure of ultrafast combined kV-MV-CBCT towards clinical implementation for IGRT of lung cancer. PLoS One. 2017;12(11):e0187710.
26. Deek MP, Kim S, Yue N, et al. Modern radiotherapy using image guidance for unresectable non-small cell lung cancer can improve outcomes in patients treated with chemoradiation therapy. J Thorac Dis. 2016;8(9):2602–2609. doi:10.21037/jtd.2016.08.95
27. Eriguchi T, Takeda A, Sanuki N, et al. Stereotactic body radiotherapy for operable early-stage non-small cell lung cancer. Lung Cancer. 2017;109:62–67. doi:10.1016/j.lungcan.2017.04.022
28. Carmignani I, Locatello LG, Desideri I, et al. Analysis of dysphagia in advanced-stage head-and-neck cancer patients: impact on quality of life and development of a preventive swallowing treatment. Eur Arch Oto Rhino Laryngol. 2018;275(8):2159–2167. doi:10.1007/s00405-018-5054-9
29. Bonomo P, Loi M, Desideri I, et al. Incidence of skin toxicity in squamous cell carcinoma of the head and neck treated with radiotherapy and cetuximab: a systematic review. Crit Rev Oncol Hematol. 2017;120:98–110. doi:10.1016/j.critrevonc.2017.10.011
30. Wang G, Jia L. Herb medicine for relieving radiation induced oral mucositis: a systematic review and meta-analysis protocol. Medicine (Baltimore). 2019;98(50):18337. doi:10.1097/MD.0000000000018337
31. Deshpande TS, Blanchard P, Wang L, et al. Radiation-related alterations of taste function in patients with head and neck cancer: a systematic review. Curr Treat Options Oncol. 2018;19(12):72. doi:10.1007/s11864-018-0580-7
32. Scrimger RA, Seikaly H, Vos LJ, et al. Combination of submandibular salivary gland transfer and intensity-modulated radiotherapy to reduce dryness of mouth (xerostomia) in patients with headand neck cancer. Head Neck. 2018;40(11):2353–2361. doi:10.1002/hed.25339
33. Jemal A, Bray F, Center MMet al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi:10.3322/caac.20107
34. Ma ZH, Yang Y, Zou L, et al. 125I seed irradiation induces up-regulation of the genes associated with apoptosis and cell cycle arrest and inhibits growth of gastric cancer xenografts. J Exp Clin Cancer Res. 2012;31(1):61. doi:10.1186/1756-9966-31-61
35. Ji Z, Jiang Y, Su L, et al. Dosimetry Verification of 125I seeds implantation with three-dimensional printing noncoplanar templates and CT guidance for paravertebral/retroperitoneal malignant tumors. Technol Cancer Res Treat. 2017;16(6):1533034617723221. doi:10.1177/1533034617723221
36. Liu S, Wang H, Wang C, et al. Dosimetry verification of 3D-printed individual template based on CT-MRI fusion for radioactive 125I seed implantation in recurrent high-grade gliomas. J Contemp Brachytherapy. 2019;11(3):235–242. doi:10.5114/jcb.2019.85729
37. Jiang Y, Ji Z, Guo F, et al. Side effects of CT-guided implantation of 125I seeds for recurrent malignant tumors of the head and neck assisted by 3D printing non co-planar template. Radiat Oncol. 2018;13(1):18. doi:10.1186/s13014-018-0959-4
38. Jiao D, Wu G, Ren J, et al. Radiofrequency ablation versus 125I-seed brachytherapy for painful metastases involving the bone. Oncotarget. 2016;7(52):87523–87531. doi:10.18632/oncotarget.11983
39. Qu A, Jiang P, Sun H, et al. Efficacy and dosimetry analysis of image-guided radioactive 125I seed implantation as salvage treatment for pelvic recurrent cervical cancer after external beam radiotherapy. J Gynecol Oncol. 2019;30(1):e9. doi:10.3802/jgo.2019.30.e9
40. Puthawala A, Nisar Syed AM, Gamie S, et al. Interstitial low-dose-rate brachytherapy as a salvage treatment for recurrent head-and-neck cancers: long-term results. Int J Radiat Oncol Biol Phys. 2001;51(2):354–362. doi:10.1016/S0360-3016(01)01637-6
41. Janot F, de Raucourt D, Benhamou E, et al. Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. J Clin Oncol. 2008;26(34):5518–5523. doi:10.1200/JCO.2007.15.0102
42. Breen W, Kelly J, Park HS, et al. Permanent interstitial brachytherapy for previously irradiated head and neck cancer. Cureus. 2018;10(4):e2517.
43. Jie WP, Bai JY, Li BB. Clinicopathologic analysis of oral squamous cell carcinoma after i interstitial brachytherapy. Technol Cancer Res Treat. 2018;17:1–5. doi:10.1177/1533033818806906
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.