Back to Journals » Journal of Hepatocellular Carcinoma » Volume 11
Efficacy and Safety of HepaSphere Drug-Eluting Bead Transarterial Chemoembolization Combined with Hepatic Arterial Infusion Chemotherapy as the Second-Line Treatment in Advanced Hepatocellular Carcinoma
Authors Liu B , Gao S, Guo J, Kou F, Liu S, Zhang X, Feng A, Wang X, Cao G , Chen H, Liu P, Xu H, Gao Q, Yang R, Xu L, Zhu X
Received 8 December 2023
Accepted for publication 23 February 2024
Published 4 March 2024 Volume 2024:11 Pages 477—488
DOI https://doi.org/10.2147/JHC.S452120
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Gerber
Baojiang Liu,* Song Gao,* Jianhai Guo, Fuxin Kou, Shaoxing Liu, Xin Zhang, Aiwei Feng, Xiaodong Wang, Guang Cao, Hui Chen, Peng Liu, Haifeng Xu, Qinzong Gao, Renjie Yang, Liang Xu, Xu Zhu
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Interventional Therapy, Peking University Cancer Hospital & Institute, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xu Zhu, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Interventional Therapy, Peking University Cancer Hospital & Institute, Beijing, People’s Republic of China, Tel + 86-10-8819-6059, Email [email protected]
Purpose: Recently, hepatic arterial infusion chemotherapy (HAIC) has also gained popularity for hepatocellular carcinoma (HCC). Several studies have compared HAIC and Transarterial chemoembolization (TACE). However, comparisons between TACE plus HAIC and HAIC are rarely reported. Here, we evaluated the performance of HepaSphere DEB-TACE combined with HAIC (Hepa-HAIC) compared to HAIC in patients with advanced HCC.
Patients and Methods: In this retrospective study, we enrolled 167 patients diagnosed with advanced HCC and treated at Peking University Cancer Hospital from May 2018 to May 2022. The cohort comprised 74 patients who received HepaSphere DEB-TACE combined with HAIC-FOLFOX (Hepa-HAIC) and 93 patients who received HAIC-FOLFOX. Over 60% of patients received prior treatments. To avoid selection bias, propensity score matching was applied to the efficacy and safety analyses. The primary endpoints are progression-free survival (PFS) and overall survival (OS); the secondary endpoints include objective response rate (ORR), disease control rate (DCR), and safety.
Results: Propensity-matching yielded 48 pairs, and group baselines were almost equal after matching. Median PFS and median OS were both higher in the matched Hepa-HAIC cohort (median PFS: 8.9 vs 5.8 months, p = 0.035; median OS: 22.4 vs 9.5 months, p = 0.027), which was consistent with pre-matching analysis. The ORR in the Hepa-HAIC and HAIC cohorts was 75.0% and 37.5%, respectively; the DCR was 93.8% after Hepa-HAIC and 81.3% after HAIC. There was no treatment-related death. Grade 3– 4 ALT elevation was more frequent in the Hepa-HAIC group (33.3% vs 8.3%, p = 0.003), while vomiting was more frequent in the HAIC group (29.2% vs 12.5%, p = 0.084).
Conclusion: The Hepa-HAIC group is superior to the HAIC group in metrics of PFS, OS, ORR, and DCR, which indicates the combination of HepaSphere DEB-TACE and HAIC may lead to improved outcomes with a comparable safety profile in advanced HCC.
Keywords: transarterial chemoembolization, advanced hepatocellular carcinoma, hepatic arterial infusion chemotherapy, propensity score matching
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and a leading cause of cancer-related deaths worldwide.1 More than 70% of patients with HCC are diagnosed with advanced disease, which is a challenging clinical scenario.2 Transarterial chemoembolization (TACE) and hepatic arterial infusion chemotherapy (HAIC) are two widely used locoregional therapies for the treatment of advanced HCC.3
Atezolizumab and bevacizumab are the preferred first-line treatments for advanced HCC, with a median overall survival (OS) of 19.2 months and a median progression-free survival (PFS) of 6.9 months.4 Lenvatinib is an optional first-line treatment for unresectable HCC, with a median survival time of 13.6 months.5 Treatment with regorafenib and cabozantinib is a second-line option for patients with HCC, with a median OS of 10.2–10.6 months and a significantly longer OS than with placebo.6,7 As all currently approved and recommended clinical trials of second-line agents have been conducted in populations after progression on first-line sorafenib, treatment selection following resistance to first-line ICI and targeted therapy remains a challenge. The choice of second-line therapy after first-line (ICI combined with targeted treatment) is empirical and largely depends on drug safety, patient comorbidities, physician and patient preferences, and local reimbursement policies.In advanced HCC, there is an urgent need for additional treatment options for HCC that has already received targeted treatment and immunotherapy.
DEB-TACE (Drug-Eluting Bead Transarterial Chemoembolization) and HAIC (Hepatic Arterial Infusion Chemotherapy) are common treatments for unresectable HCC; the efficacy is less certain for HAIC alone or DEB-TACE combined with HAIC. DEB-TACE is a minimally invasive procedure that delivers chemotherapy drugs directly to the tumor through the hepatic artery and is often used to treat patients who are not candidates for surgery or liver transplantation.8,9
HAIC delivers high doses of chemotherapy drugs directly to the tumor through the hepatic artery and has demonstrated efficacy in unresectable HCC.10–12 TACE combined with HAIC, also known as TACE-HAIC, is a multimodal approach for the treatment of HCC. This combination therapy has shown improved treatment outcomes in patients with unresectable HCC.13–16
Here, we pursue improved treatments for advanced HCC by evaluating the efficacy and safety of DEB-TACE combined with HAIC and HAIC alone.
Materials and Methods
Study Design and Patient Selection
This single-center retrospective study was approved by the Research Ethics Committee of our hospital (2023YJZ76), and the requirement for informed consent was waived because this was a retrospective study involving no interventions and because all data were deidentified. Patient data and confidentiality were respected by the Declaration of Helsinki.
We reviewed data from 167 patients with advanced HCC who underwent HAIC alone (HAIC group) or HepaSphere DEB-TACE combined with HAIC (Hepa-HAIC group) from May 2018 to May 2022. All patients were diagnosed based on their pathology findings or using the criteria of the American Association for the Study of Liver Disease (AASLD).17
Study participants were enrolled based on specific inclusion and exclusion criteria. Inclusion criteria were: advanced HCC, aged 18–85 years, Child–Pugh grade A–B, an Eastern Cooperative Oncology Group-Performance Status (ECOG-PS) ≤ 2, and at least one measurable intrahepatic lesion according to the modified response evaluation criteria in solid tumors (mRECIST).18 Exclusion criteria were: patients receiving other antitumor therapies (ie, chemotherapy, immunotherapy, or targeted therapy), portal hypertension with massive ascites, patients with missing data on their first imaging assessments, and those who were lost to follow-up.
Drug-Eluting Bead Transarterial Chemoembolization (DEB-TACE)
Pre-procedural imaging techniques, such as computed tomography (CT) or magnetic resonance imaging (MRI), were used to assess tumor size, location, and vascular supply. The Seldinger technique was used to access the femoral artery after an injection of a local anesthetic.
HepaSphere microspheres are expandable biocompatible microspheres made of sodium acrylate/vinyl alcohol copolymer with an estimated loading capacity of 40–60 mg/vial of beads. The chemotherapeutic solution was mixed with physiologic saline or nonionic isotonic contrast medium (270 mg/mL Visipaque [iodixanol]; Amersham Health) in direct contact with the dehydrated microspheres by injecting the mixture directly into the vacuum-sealed vial of HepaSphere. After at least 20 min, >90% of the chemotherapeutic solution had been absorbed by the microspheres. The drug-loaded microspheres were then aspirated from the vial, and additional contrast medium or saline was added to obtain a final injectable volume of 20 mL.
The arterial branches feeding the tumor were selectively cannulated by microcatheters to proceed with TACE and to ensure better preservation of the surrounding nontumoral liver tissue. Spheres were injected far from the origin of the gastroduodenal, right gastric, and cystic arteries.
TACE was administered as a slow injection of the HepaSphere microsphere mixture loaded with chemotherapeutic agent and the nonionic isotonic contrast medium, which improves the radiopacity of the mixture for a controlled injection under fluoroscopic guidance. We avoided any reflux that may have occurred and revealed contrast graphic impregnation within and around the target lesions, up to the complete embolization of the arteries feeding the lesions, thus preserving the blood flow of the main artery to perform HAIC. In cases with multiple large or diffuse lesions, the embolization was done in 2–3 stages to prevent hepatic infarction or failure. Patients received intravenous analgesic and antiemetic medications before the procedure.
Hepatic Arterial Infusion Chemotherapy
For the patients who underwent HepaSphere DEB-TACE combined with HAIC (Hepa-HAIC) or HAIC alone, the tip of a 2.4 French microcatheter was set at the proper hepatic artery, with appropriate placement confirmed using DSA (Innova 4100IQ; General Electric Company, Boston, MA, USA). The right gastric, gastroduodenal, and posterior superior pancreaticoduodenal arteries were occluded with coils (Boston Scientific, Marlborough, MA, USA) to prevent gastroduodenal ulcers caused by anticancer agents. The catheter was then safely secured to the skin to prevent shifting.
An artery infusion pump was connected to the microcatheter to administer HAIC in the ward. Oxaliplatin (OXA) (60 mg/m2 for the Hepa-HAIC group; 85 mg/m2 for the HAIC group) was administered intra-arterially for 4 h, leucovorin (200 mg/m2) was administered intravenously for 2 h, and 5-fluorouracil (5-FU) (1.2 g for the Hepa-HAIC group; 2.4 g/m2 for the HAIC group) was administered intra-arterially for 20 h. Hepa-HAIC was repeated at intervals of 4–6 weeks and HAIC was performed every 3–4 weeks if the treatment produced a response; treatment continued until the intrahepatic lesions progressed or toxicity became unacceptable Hepa-HAIC and HAIC were administered until complete remission, disease progression, the occurrence of intolerable toxicity, or withdrawal from treatment at the patient’s request.
Follow-Up
Adverse effects were recorded as the number of patients with any adverse effect(s), or of particular adverse effects. Safety was assessed among all the patients treated using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to evaluate the severity of adverse events of Hepa-HAIC or HAIC. Within 1–2 weeks after treatment, the patients underwent physical examinations and blood tests to assess their hematologic profiles and liver function, and to perform coagulation screens. In addition, the serum concentration of alpha-fetoprotein was checked every 4–6 weeks. Abdominal contrast-enhanced three-phase dynamic spiral CT or MRI and chest CT were performed to evaluate efficacy after each treatment cycle using RECIST 1.0.
Tumor Response and Survival Assessments
The primary endpoints are PFS and OS. PFS was defined as the time from date of treatment initiation until progression and OS was defined as the interval between the time of treatment initiation and death or the last follow-up assessment. Progressive disease (PD) was defined as tumor progression and clinical symptom progression after Hepa-HAIC or HAIC. The secondary endpoint includes objective response rate (ORR), disease control rate (DCR), and safety. ORR was defined as CR plus PR, whereas DCR was defined as the best tumor response of CR plus PR plus SD. A tumor’s response was assessed using the Mrecist.18 Assessment was performed by two experienced radiologists, both with >10 years of experience.
Statistical Analyses
Continuous variables were analyzed using the Wilcoxon rank-sum test and the independent t-test, while categorical variables were analyzed using the chi-square test and Fisher’s exact test. To reduce selection bias and the impact of possible confounds related to the clinical features of the groups, propensity score matching (PSM) was employed.19 To estimate the propensity scores, logistic regression was used to forecast the likelihood that patients will fall into the Hepa-HAIC group. Age, sex, hepatitis, Child-Pugh score, previous treatment, ECOG-PS score, intrahepatic tumor size, extrahepatic metastasis, AFP, tumor number, and vein invasion were balanced by p <0.05.
The Kaplan-Meier technique, the Log rank test, and Cox proportional hazards regression models were used to estimate OS and PFS. Statistical analyses were conducted using IBM®SPSS® software (v.22.0; IBM Corporation, Armonk, NY, USA) and R (v.2.15.x; The R Foundation for Statistical Computing, Vienna, Austria). P-values <0.05 were considered statistically significant.
Results
Patient Characteristics
In total, 167 patients satisfied the inclusion criteria for this trial between May 2018 and May 2022, with 74 receiving Hepa-HAIC treatment and 93 receiving HAIC treatment (Figure 1). The mean number of treatments for patients of the Hepa-HAIC and HAIC groups was 4.1±2.8 and 3.5±2.4, respectively. Follow-ups lasted through December 2022. This study had 94 patients (56.3%) with tumor sizes > 5 cm and 88 patients (62.7%) with vein invasion. There were 158 patients (94.6%) who had received previous treatment, that including immunotherapy and TKIs. Hepatitis B virus infection (HBV) was the primary cause of HCC (85.0%).
All patients with BCLC stage C in our study received TACE, tyrosine kinase inhibitors, or immune checkpoint inhibitors. A significant difference in the percentage of vein invasion was noted between the two groups before matching (Table 1). After PSM, we were able to create matched cohorts of 48 patients each. The baseline characteristics are shown in Table 2, and there was no significant difference between the two groups.
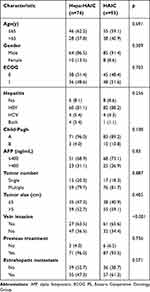 |
Table 1 Baseline Characteristics of Patients with Advanced HCC (Before PSM) |
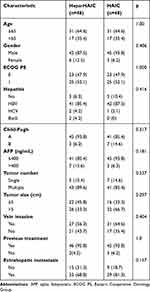 |
Table 2 Baseline Characteristics of Patients with Advanced HCC (After PSM) |
Efficacy
For all patients, the median follow-up time was 24.2 months (range 1.9–46.8 months). During follow-up, 59 of 96 patients died; the median OS in the Hepa-HAIC group (22.4 months) was longer than that in the HAIC group (9.5 months) after PSM (p = 0.027) (Figure 2A). The median PFS was significantly higher for patients who received Hepa-HAIC than HAIC (8.9 months vs 5.8 months, p = 0.035) alone (Figure 2B). The ORR in Hepa-HAIC and HAIC cohorts was 75.0% and 37.5% (p < 0.001), respectively; the DCR was 93.8% after Hepa-HAIC and 81.3% after HAIC (p < 0.001). Specific values are shown in Table 3. Subgroup comparisons of OS (Figure 3A) and PFS (Figure 3B) for the prespecified subgroups sex, age, ECOG, hepatitis, Child-Pugh, AFP, previous treatment, vein invasion, extrahepatic metastasis, tumor size, and tumor number are shown in. Multivariate analyses of the matched cohort (n=96) showed that Hepa-HAIC p=0.030) was independent prognostic factor associated with OS (Table 4). Multivariate analyses showed that Hepa-HAIC (p = 0.017) and the age (p = 0.009) were independent prognostic factors associated with PFS (Table 5).
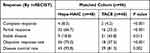 |
Table 3 Tumor Response |
 |
Table 4 Univariate and Multivariate Analysis of Risk Factors for OS (After PSM) |
 |
Table 5 Univariate and Multivariate Analysis of Risk Factors for PFS (After PSM) |
 |
Figure 2 Overall survival curves (A) and progression-free survival curves (B) for patients who received Hepa-HAIC and HAIC. |
 |
Figure 3 Treatment effect on overall survival (A) and progression-free survival (B) by subgroup. |
Safety
Adverse events were generally manageable in both groups. The most common grade 1 or 2 adverse events in the Hepa-HAIC group included fever (71.7%), vomiting (56.3%), and elevated aminotransferase. The most common grade 3 or 4 adverse events were thrombocytopenia (14.6% in Hepa-HAIC group; 20.8% in HAIC group) and elevated TBIL (22.9% in Hepa-HAIC group; 41.7% in HAIC group). Grade 3–4 ALT elevation was more frequent in the Hepa-HAIC group (33.3% vs 8.3%, p = 0.003) while vomiting was more frequent in the HAIC group (29.2% vs 12.5%, p = 0.084). There was no treatment-related death. In addition, despite repeated punctures, no serious vascular problems were seen (Table 6).
 |
Table 6 Treatment-Related Adverse Events in the Groups (Matched) |
Discussion
Advanced HCC is associated with a very poor prognosis, and localized treatments such as TACE, HAIC, and radiotherapy are critical. Atezolizumab combined with bevacizumab, lenvatinib, and sorafenib have been the first-line treatments for advanced HCC,4,5,20 with regorafenib and cabozantinib as second-line treatment.6,7 Targeted combination immunotherapies have been increasingly administered to patients worldwide, especially in Asian countries, and are significantly improving patients’ survival.4,21,22 However, more follow-up studies are needed on HCC patients who have received targeted and immunotherapy.
TACE and HAIC have shown promising results in patients with unresectable HCC, particularly in Asian countries where it has been extensively utilized.11,12,23 Regarding the efficacy of TACE and HAIC in treating HCC, there has been continued attention,14–16,24 but few studies have evaluated the efficacy and safety of Hepa-HAIC compared to HAIC alone in patients with advanced HCC, especially those who have received systemic therapy. Therefore, we conducted this study.
In our study, the median OS (22.4 months vs 9.0 months, p = 0.027) and the median PFS (8.9 vs 5.8 months, P = 0.035) in the Hepa-HAIC group was longer than that in the HAIC group after PSM. With regorafenib and cabozantinib as the second-line treatment for patients with HCC, the median OS was 10.2–10.6 months and the median PFS was 3.1–5.2 months.6,7 A retrospective study found a median PFS of 3.1 months and a median OS of 8.3 months in HCC patients treated with GEMOX after sorafenib progression,25 while HAIC with FOLFOX results in a longer OS (9.0 vs 5.9 months) than intravenous FOLFOX4.26
Second-line combination therapy may offer better survival for patients who have received immunotherapy and targeted therapy.25 Hepa-HAIC therapy may achieve a longer PFS and OS by providing an effective, low-toxicity treatment option. It is challenging to reduce tumor blood supply in a blood-rich HCC without TACE or targeted therapy. The dose of OXA and 5-FU was lower in the Hepa-HAIC group than the HAIC group (OXA: 60 vs 85 mg/m2; 5-FU: 1.0 vs 1.5 g/m2). In the HAIC group, patients were treated every 3–4 weeks, while Hepa-HAIC patients received treatment every 6 weeks. Lower chemotherapy doses and extended treatment intervals can facilitate longer recuperation periods. Hepatic sinusoidal obstruction syndrome (HSOS) induced by OXA has become a key concern for patients with CRC receiving OXA chemotherapy in recent years.27 Notably, HSOS caused by OXA is significantly correlated with both the cumulative dose of drugs and the treatment cycle.28,29
Moreover, after a short period of high-dose HAIC (FOLOFX), drug-resistant HCC may emerge as a barrier, and the treatment is poorly tolerated by patients. Research is ongoing to study the mechanisms of oxaliplatin resistance and find solutions to overcome it.30–32
There were no significant differences in grade 3–4 adverse events between the two groups, except for ALT elevation in the Hepa-HAIC group. The embolization resulted in a short-term increase in transaminases, similar to previous studies.13,14 This also suggests that Hepa-HAIC need to preserve liver function. Liver function indicators may directly reflect the liver state and were associated with HCC prognosis.
Our study has several limitations. First, this was a retrospective study with a limited sample size, and the retrospective analysis may have influenced the results, although the data were adjusted using PSM. Second, the role of treatment after tumor progression is crucial for survival. Patients who have undergone HAIC therapy may experience diminished liver function and have limited access to alternative treatments.
In previous years, increasing attention has been focused on TACE combined with HAIC.16 Hepa-HAIC may have a better OS for advanced HCC than HAIC alone. This study presents an alternative treatment option for patients with advanced HCC following progression on targeted and immunotherapy treatments.
Abbreviations
AASLD, American Association for the Study of Liver Disease; CT, Computed tomography; DCR, Disease control rate; HAIC, Hepatic arterial infusion chemotherapy; HBV, Hepatitis B virus; HSOS, Hepatic sinusoidal obstruction syndrome; MRI, Magnetic resonance imaging; ORR, Objective response rate; OS, Overall survival; PD, Progressive disease; PFS, Progression-free survival; PSM, Propensity score matching.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, XZ, upon reasonable request.
Ethics Approval and Informed Consent
Approval of the research protocol by an Institutional Reviewer Board. The study was conducted in accordance with the principles of the Declaration of Helsinki. This study was approved by the research ethics committee of Peking University Cancer Hospital (2023YJZ76).
Acknowledgments
We would like to thank Charlesworth Author Services for English language editing.
The abstract of this paper was presented at The ASCO Annual Meeting as a poster presentation with interim findings. The poster’s abstract was published as Poster Abstracts in the Journal of Clinical Oncology: https://ascopubs.org/doi/10.1200/JCO.2023.41.16_suppl.4122.
Author Contributions
Baojiang Liu and Song Gao contributed equally to this work. All authors made significant contributions to the work reported herein. Particularly, they contributed toward study conception; study design; execution; data collection, analysis, and interpretation; and drafting, revising, or critically reviewing the manuscript. All authors provided final approval of the manuscript version to be published and agreed on the journal to which the manuscript has been submitted. They also agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (81971717), Key Projects of CSCO-Bayer Cancer Research Fund (Y-bayer202002-0091), and Beijing Hospital authority of Hospitals’ Ascent Plan (DFL20220903).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015;35(9):2155–2166. doi:10.1111/liv.12818
3. Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236. doi:10.1002/hep.20933
4. Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi:10.1016/j.jhep.2021.11.030
5. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
6. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi:10.1016/S0140-6736(16)32453-9
7. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi:10.1056/NEJMoa1717002
8. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
9. Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol. 2015;62(5):1187–1195. doi:10.1016/j.jhep.2015.02.010
10. Lyu N, Kong Y, Mu L, et al. Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2018;69(1):60–69. doi:10.1016/j.jhep.2018.02.008
11. He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953–960. doi:10.1001/jamaoncol.2019.0250
12. Li QJ, He MK, Chen HW, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized Phase III trial. J Clin Oncol. 2022;40(2):150–160. doi:10.1200/JCO.21.00608
13. Liu BJ, Gao S, Zhu X, et al. Sorafenib combined with embolization plus hepatic arterial infusion chemotherapy for inoperable hepatocellular carcinoma. World J Gastrointest Oncol. 2020;12(6):663–676. doi:10.4251/wjgo.v12.i6.663
14. Liu BJ, Gao S, Zhu X, et al. Combination therapy of chemoembolization and hepatic arterial infusion chemotherapy in hepatocellular carcinoma with portal vein tumor thrombosis compared with chemoembolization alone: a propensity score-matched analysis. BioMed Res Int. 2021;2021:6670367. doi:10.1155/2021/6670367
15. Liu B, Gao S, Guo J, et al. A novel nomogram for predicting the overall survival in patients with unresectable HCC after TACE plus hepatic arterial infusion chemotherapy. Transl Oncol. 2023;34:101705. doi:10.1016/j.tranon.2023.101705
16. Ge N, Wang H, He C, Wang X, Huang J, Yang Y. Optimal interventional treatment for liver cancer: HAIC, TACE or iTACE? J Interv Med. 2023;6(2):59–63. doi:10.1016/j.jimed.2023.03.001
17. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
18. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
19. Pattanayak CW, Rubin DB, Zell ER. Propensity score methods for creating covariate balance in observational studies. Rev Esp Cardiol. 2011;64(10):897–903. doi:10.1016/j.recesp.2011.06.008
20. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Eng J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
21. Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, Phase 2-3 study. Lancet Oncol. 2021;22(7):977–990. doi:10.1016/S1470-2045(21)00252-7
22. Qin S, Chan SL, Gu S, et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. 2023;402(10408):1133–1146. doi:10.1016/S0140-6736(23)00961-3
23. Liu BJ, Gao S, Zhu X, et al. Real-world study of hepatic artery infusion chemotherapy combined with anti-PD-1 immunotherapy and tyrosine kinase inhibitors for advanced hepatocellular carcinoma. Immunotherapy. 2021;13(17):1395–1405. doi:10.2217/imt-2021-0192
24. Gao S, Zhang PJ, Guo JH, et al. Chemoembolization alone vs combined chemoembolization and hepatic arterial infusion chemotherapy in inoperable hepatocellular carcinoma patients. World J Gastroenterol. 2015;21(36):10443–10452. doi:10.3748/wjg.v21.i36.10443
25. Patrikidou A, Sinapi I, Regnault H, et al. Gemcitabine and oxaliplatin chemotherapy for advanced hepatocellular carcinoma after failure of anti-angiogenic therapies. Investig New Drugs. 2014;32(5):1028–1035. doi:10.1007/s10637-014-0100-y
26. Qin S, Cheng Y, Liang J, et al. Efficacy and safety of the FOLFOX4 regimen versus doxorubicin in Chinese patients with advanced hepatocellular carcinoma: a subgroup analysis of the EACH study. Oncologist. 2014;19(11):1169–1178. doi:10.1634/theoncologist.2014-0190
27. Zhu C, Ren X, Liu D, Zhang C. Oxaliplatin-induced hepatic sinusoidal obstruction syndrome. Toxicology. 2021;460:152882. doi:10.1016/j.tox.2021.152882
28. Cayet S, Pasco J, Dujardin F, et al. Diagnostic performance of contrast-enhanced CT-scan in sinusoidal obstruction syndrome induced by chemotherapy of colorectal liver metastases: radio-pathological correlation. Eur J Radiol. 2017;94:180–190. doi:10.1016/j.ejrad.2017.06.025
29. Han NY, Park BJ, Kim MJ, Sung DJ, Cho SB. Hepatic parenchymal heterogeneity on contrast-enhanced CT scans following oxaliplatin-based chemotherapy: natural history and association with clinical evidence of sinusoidal obstruction syndrome. Radiology. 2015;276(3):766–774. doi:10.1148/radiol.2015141749
30. Liu S, Bu X, Kan A, et al. SP1-induced lncRNA DUBR promotes stemness and oxaliplatin resistance of hepatocellular carcinoma via E2F1-CIP2A feedback. Cancer Lett. 2022;528:16–30. doi:10.1016/j.canlet.2021.12.026
31. Ma L, Xu A, Kang L, et al. LSD1-demethylated LINC01134 confers oxaliplatin resistance through SP1-induced p62 transcription in HCC. Hepatology. 2021;74(6):3213–3234. doi:10.1002/hep.32079
32. Shi Y, Niu Y, Yuan Y, et al. PRMT3-mediated arginine methylation of IGF2BP1 promotes oxaliplatin resistance in liver cancer. Nat Commun. 2023;14(1):1932. doi:10.1038/s41467-023-37542-5
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

