Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Efficacy and Safety of Esketamine Combined with Antidepressants for Treatment-Resistant Depression: A Meta-Analysis
Authors Liu P , Zhang SS, Liang Y, Gao ZJ, Gao W, Dong BH
Received 12 September 2022
Accepted for publication 14 November 2022
Published 7 December 2022 Volume 2022:18 Pages 2855—2865
DOI https://doi.org/10.2147/NDT.S388764
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Peng Liu,1,2 Shan-Shan Zhang,1,2 Yun Liang,1,2 Zi-Jun Gao,1 Wei Gao,3 Bu-Huai Dong1
1Department of Anesthesiology, Xi’an Honghui Hospital, Affiliated Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China; 2Xi’an Medical University, Xi’an, People’s Republic of China; 3Department of Anesthesiology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China
Correspondence: Bu-Huai Dong, Department of Anesthesiology, Xi’an Honghui Hospital, Affiliated Hospital of Xi’an Jiaotong University, No. 555, Youyi East Road, Nanqimen, Xi’an, 710054, People’s Republic of China, Tel +86 29-85260965, Email [email protected]
Objective: To evaluate the efficacy and safety of esketamine + antidepressant in treatment-resistant depression.
Methods: We searched PubMed, Web of Science, Embase, CNKI, and Wanfang databases to obtain published information on esketamine + antidepressant from inception to July 2022. We searched for randomized controlled studies on the treatment of depression with a double-blind induction phase. Outcome indicators included changes in Montgomery-Asberg Depression Rating Scale (MADRS) scores before and after treatment, effective response rate, remission rate, and changes in self-rating depression scale (SDS). We analyzed data using Review Manager 5.4 and assessed the quality of evidence using Grading of Recommendations Assessment, Development, and Evaluation (GRADE) analysis.
Results: A total of seven articles were included, including 701 patients in the esketamine + antidepressant group and 551 in the placebo group. Meta-analysis results showed that esketamine + antidepressant could improve the MADRS score in patients with treatment-resistant depression (MD = − 2.68, 95% CI − 3.98 to − 1.37, P < 0.0001), SDS (MD = − 2.9, 95% CI − 4.01 to − 1.79, P < 0.00001), response rate at the end of the double-blind induction period (RR = 1.28, 95% CI 1.12 to 1.46, P = 0.0002), remission rate at the end of the double-blind induction period (RR = 1.39, 95% CI 1.18 to 1.63, P < 0.0001), Five-Dimensional Health Scale (EQ-5D-5L) (MD = 0.05, 95% CI 0.02 to 0.08, P = 0.00009), Visual Analogue Scale of Health Status (EQ-VAS) (MD = 5.54, 95% CI 2.37 to 8.71, P = 0.0006).
Conclusion: Esketamine + antidepressant has an obvious curative effect in treatment-resistant depression and can rapidly improve depression in patients, quality of life and satisfaction, but minor adverse reactions can occur.
Keywords: esketamine, treatment-resistant depression, refractory depression, antidepressant, meta-analysis
Introduction
Major depressive disorder is a common mental illness. The prevalence of major depressive disorder varies widely in countries worldwide, but about 6% of cases seriously affect social and psychological functions and reduce the quality of life. The mechanism of action is unclear,1 but about 10% to 30% of cases are refractory.2 Treatment-resistant depression is defined as the failure to achieve clinical remission even after receiving a sufficient amount and course of two completely different drugs with good patient compliance.3,4 Authoritative experts worldwide believe that the direction of depression treatment should be to improve health status, reduce recurrence, and improve quality of life.5 However, the ineffectiveness of existing clinical therapy prompted the US Food and Drug Administration to approve a new nasal spray antidepressant in 2019, esketamine, for treating adult patients with refractory depression. Due to its special development history (K powder) and mild side effects, it requires strict and standardized use.
Esketamine, the S enantiomer of racemic ketamine, belongs to the NMDA receptor blocker. Compared with racemic ketamine, esketamine has less psychomimetic effect, more anesthetic, and analgesic activity, and 3 to 4 times more affinity for NMDA receptors than R-ketamine.6 The literature has reported that the radioligand-binding properties of NMDA receptors produce region-specific adaptive changes in patients with long-term antidepressant use,7 suggesting that it may be one of the antidepressant mechanisms. A review reported that esketamine could also activate the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAr),8 which can regulate multiple signaling pathways of synaptic plasticity; induce brain-derived neurotrophic factor expression; increase the release of monoamine transmitters in the central nervous system; promote angiogenesis and synapse regeneration, and enhance neuronal activity.9
Despite the growing interest in the treatment of refractory depression, current treatment options still do not improve the patient’s symptoms well, nor do they improve patient and physician satisfaction, and the application of esketamine nasal spray is a major innovation in this area in anticipation of satisfactory results. Therefore, the present study included more studies, increased the sample size and indicators, and set stricter inclusion and exclusion criteria, to provide more evidence to support the application of esketamine + antidepressant in treatment-resistant depression.
Methods
Study Design
Since this is a meta-analysis of published studies, ethical approval and informed consent were not necessary, and we calculated all pooled results based on published data. This systematic review and meta-analysis adhered to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.10 The data relevant to our study are available from the corresponding author upon request.
Eligibility Criteria
Original research studies published in English and meeting the following criteria were included:1) the patient met the diagnostic criteria of the US Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV); 2) the patient met the criteria for treatment-resistant depression; 3) gender and age are not limited; (4) the types of studies included in this analysis were published randomized controlled studies. Exclusion criteria: 1) non-randomized controlled trial, animal experiments, reviews, inconsistent outcome indicators, and others; 2) non-double-blind induction period in the experimental phase, such as maintenance phase, open-label phase; 3) experimental design is not rigorous; 4) the subjects of the study were patients with non-refractory depression.
Search Strategy
We searched PubMed, Web of Science, Embase, CNKI, and Wanfang databases to obtain information on esketamine + antidepressant published from the establishment of the databases to July 2022. Boolean logic was used for the database search, and Boolean search operators “AND” and “OR” were used to link search terms. Search terms were as follows: “treatment-resistant depression” AND (“esketamine” AND “antidepressant”) AND “randomized controlled trial”. Moreover, the reference section of the included literature and previously published articles for other studies that met the criteria were searched.
Study Selection
Two researchers (PL and SS-Z) screened the first-time searched literature according to the guidelines, extracted data, and checked the data. In case of discrepancies, a third person was consulted (BH-D or WG).
Data Extraction
The two reviewers (YL and ZJ-G) collated the final included studies and used the standardized data extraction format to extract the data. After data extraction, the reviewers matched the data before rereading the papers wherever discrepancies arose. Any discrepancies were resolved through discussion with a third reviewer (BH-D or WG). The extracted data included the following: first author, year, basic demographic characteristics, intervention protocol, outcome indicators, and pre-and post-change values for cognitive assessments. If the required data were missing, not reported in the paper, or reported in an unusual form, we contacted the corresponding author of the related paper for clarification.
Assessment of Risk of Bias
The other authors independently evaluated the risk of bias of the included studies by using the RCT risk of bias assessment tool recommended in Cochrane Handbook 5.1, and finally cross-checked the results. Evaluation entries include random sequence generation, allocation concealment, performer and participant blinding, outcome assessment blinding, outcome data completeness, selective reporting, and other biases. Each project was described as “low risk”, “high risk” or “unclear risk”, depending on the judgment of the two authors.+97.
Statistical Analysis
We used risk ratio (RR) and 95% confidence interval (CI) to express dichotomous variables and used mean difference (MD) and 95% CI to express continuous variables; I2 values were used to determine the magnitude of heterogeneity, and smaller heterogeneity (I2 < 50%, P > 0.1) was used for meta-analysis with the fixed-effects model, and vice versa with a random-effects model. We used Stata 16.0 software to detect publication bias, and P > 0.05 indicated no bias. For statistical analysis, we used Review Manager 5.4 software.Stata 16.0 was used to test publication bias by Egger’s method. Empirically, the funnel plot asymmetry test should be used only if at least 10 studies are included in the analysis.
Assessment of the Quality of Evidence
The quality of evidence for each outcome indicator was judged according to the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) grading system11 and evaluated using the GRADE profiler 3.6 software.
Results
Literature Search
A total of 122 relevant papers were searched through the databases, including 11 in Chinese and 160 in English. We used the endnote software to exclude 56 duplicate papers and 97 irrelevant studies. Eighteen papers were excluded after reading through the full text according to the literature screening the PRISMA flowchart. Seven studies were included in the final meta-analysis. The literature screening process is shown in Figure 1.
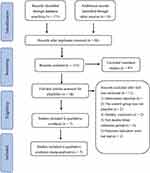 |
Figure 1 PRISMA flowchart for the article screening and selection.10 Notes: PRISMA figure adapted from Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. Creative Commons. |
Characteristics of Eligible Studies
Of the 171 identified articles, 18 were comprehensively evaluated for content and quality, leaving 7 articles. All seven studies were randomized controlled trials, most of which were in Europe and the United States. All of them were conducted in the last 3 years, the treatment phase of the patients was a double-blind induction treatment period, and the patients were all treated with conventional antidepressants combined with esketamine or placebo. The main characteristics of the included literature are shown in Table 1.
 |
Table 1 Basic Information of the Included Literature |
Risk of Bias
Seven studies12–18 adopted the random number table method and the random timetable generated by the computer system for randomization. Only one study12 did not specify allocation concealment; all seven studies12–18 were double-blind, except for some missing data in the study by Popova et al 2019;18 the rest of the results were found to be complete, but it was unclear whether other sources of bias existed (Figure 2).
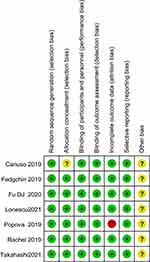 |
Figure 2 Risk of bias summary of each eligible study. |
Changes in Montgomery-Asberg Depression Rating Scale (MADRS) Score Before and After Treatment
A total of 10 active arms in the seven articles12–18 reported the change value of MADRS at the end of the double-blind induction period from baseline (I2 = 0%, P = 0.56). The fixed effect model was selected, and the results showed that the combined effect value was located on the left side of the null line. Compared with the control group, the esketamine + antidepressant group showed significantly improved MADRS score of patients with treatment-resistant depression (MD = −2.68, 95% CI −3.98 to −1.37, P < 0.0001) (Figure 3).
 |
Figure 3 Forest plot of esketamine + antidepressant to improve MARDS scores. |
Effective Response Rate at the Endpoint of the Double-Blind Induction Period
The effective response rate was defined as a ≥ 50% reduction in MADRS score at the endpoint of the double-blind induction period. Five articles12,15–18 in eight active arms documented the above data, involving 911 study subjects. No significant heterogeneity was observed among study subjects (P = 0.64, I2 = 0%), analyzed using a fixed-effects model. Compared with the control group, esketamine + antidepressant significantly improved the response to antidepressants in patients with treatment-resistant depression (RR = 1.28, 95% CI 1.12 to 1.46, P = 0.0002) (Figure 4).
 |
Figure 4 Forest plot for effective response rate comparison. |
Remission Rate at the Endpoint of the Double-Blind Induction Period
Remission was defined as a MADRS score ≤ 12 at the endpoint of the double-blind induction period, and data were recorded in six active arms of five articles13–15,17,18 with 1112 study subjects. No significant heterogeneity was observed among study subjects (P = 0.56, I2 = 0%). The results of the fixed-effects model analysis showed significant differences. Compared with placebo + antidepressants, esketamine + antidepressants significantly improved remission in patients with treatment-resistant depression (RR = 1.39, 95% CI 1.18 to 1.63, P < 0.0001) (Figure 5).
 |
Figure 5 Forest plot of remission rate comparison. |
Depression Self-Rating Scale (SDS) Changes
Four articles15–18 with seven activity arms documented SDS changes from baseline in 758 study subjects, with little heterogeneity between study subjects (P = 0.74, I2 = 0%); compared with the control group, esketamine + antidepressant significantly improved the SDS score in patients (MD = −2.9, 95% CI −4.01 to −1.79, P < 0.00001) (Figure 6).
 |
Figure 6 Forest plot comparing changes in SDS. |
Other Outcome Indicators
Other outcome indicators were summarized, and the European Five-Dimensional Health Scale (EQ-5D-5L), health status visual scale (EQ-VAS), depression screening scale (PHQ-9), and 24-hour (next day) remission rate at the end of the double-blind induction period were statistically analyzed. No significant heterogeneity was observed among the studies, and the differences in the meta-analysis results were significant (P < 0.05). However, no large heterogeneity was observed among the included studies in terms of sustained effective response rate; the results of the meta-analysis were not significant (P > 0.05) (Table 2).
 |
Table 2 Summary of Remaining Outcome Indicators |
Adverse Events
A total of seven studies12–19 documented the occurrence of adverse reactions during treatment. With the current focus on common adverse events during treatment, the data showed that the experimental group was significantly more prone to symptoms such as nausea, dizziness, schizophrenia, vomiting, vertigo, dysgeusia, lethargy, dysesthesia, increased blood pressure, and sedation (P < 0.05). However, the difference in adverse reactions such as headache and anxiety was not significant (P > 0.05) (Table 3).
 |
Table 3 Summary of Adverse Events |
Sensitivity Analysis
When comparing the sustained effective response rate of esketamine + antidepressant for patients, a certain degree of heterogeneity was observed among the studies. After excluding the study by Takahashi et al 2021,16 the heterogeneity between the experimental group and the control group changed (P = 0.44, I2 = 0%), and the difference was significant (RR = 2.79, 95% CI 1.42 to 5.50, P = 0.003).
Publication Bias
Stata 16.0 was used to test publication bias by Egger’s method. Due to the lack of included literature, only the changes in MADRS scores recorded in seven studies were tested. The results showed no obvious publication bias among the studies (P = 0.1416).
GRADE Level of Evidence
The GRADE systematic review results showed that the evidence for remission at the end of the double-blind induction period was of high quality. The evidence for the effective response rate, changes in MADRS score before and after, and changes in SDS at the end of the double-blind induction period were moderate (Table 4).
 |
Table 4 Quality of Evidence |
Discussion
This study found that in the treatment of refractory depression, the clinical efficacy of esketamine nasal spray combined with conventional antidepressants in the double-blind treatment period was significantly better than that of the control group, and it could treat clinical symptoms quickly and effectively. Esketamine can act as an antidepressant as early as 2 hours.19 Esketamine nasal spray, in combination with antidepressants, may help relieve depressive symptoms based on its role as an N-methyl-D-aspartate (NMDA) receptor antagonist.20,21 This study conducted a comprehensive analysis of depression remission from multiple-scale scores. The meta-analysis results showed that the clinical efficacy and statistics of the experimental group were significantly different. However, in terms of adverse reactions, the incidence of side effects in the experimental group was higher. One long-term safety data showed that most adverse events were mild or moderate and were transient, self-limited, and resolved within the same day,22,23 consistent with the results of the included study.
In recent years, studies conducted in European countries have found that treatment-resistant depression can increase patients’ anxiety and comorbidities.24,25 Although many treatment options are currently available for treatment-resistant depression,20,21 studies suggest that these therapies may lack consistent long-term efficacy,26 and approximately one-third of patients with major depressive disorder do not respond significantly to first-line treatment.27 Even when two antidepressants with different pharmacological mechanisms are combined, treatment in 10% to 30% of patients is ineffective.28 As early as 2003, Daly et al29 evaluated the efficacy of intranasal esketamine and placebo in treating treatment-resistant depression and then re-evaluated clinical trials at the international level to verify the safety and efficacy of the experiment. Another study reported that using esketamine nasal spray for treating patients with suicidal tendencies and refractory depression could also achieve rapid and effective relief.13 Currently, esketamine is not recommended as a first-line drug in China’s treatment guidelines for treatment-resistant depression, but it has been approved as a first-line drug for treatment-resistant depression in Europe and the United States.30 The use of esketamine to treat treatment-resistant depression is an advance in the understanding and treatment of the disorder.31 Another psychedelic drug was reported to produce a significant antidepressant effect one day after dosing,32,33 which may be another approach for treating refractory depression.
Our study focused on the efficacy of esketamine in refractory depression and differs significantly from published studies. For example, there are studies34,35 that evaluate the effect of ketamine and esketamine on depression or evaluate the efficacy of ketamine on major depression.36 However, combined with the current research reports, the exact mechanism of esketamine in the treatment of antidepressants is still not very clear.37 Individual patients and their conditions lead to different responses to drugs, so optimizing the treatment strategy, ie, individualized treatment is the best choice to effectively improve patients’ quality of life.38
Limitations
The main limitation of the study is the small number of included references. A large number of clinical studies are needed for further verification; secondly, the allocation concealment of some included studies is not very clear, so a potential bias may be possible; thirdly, the dose and frequency of esketamine are inconsistent, so the optimal dose has not yet been obtained in this analysis; finally, this analysis only focused on the double-blind treatment period, and did not analyze the subsequent open treatment period.
Conclusions
This analysis showed that the application of esketamine nasal spray for refractory depression could relieve and improve depressive symptoms quickly and effectively. Since the current relevant data are still relatively small, further experimental evaluation is needed. At the same time, esketamine, as an anesthetic drug, has the characteristics of analgesia and sedation, which provides new ideas and development prospects for the treatment of patients with depression. It is possible to treat patient’s symptoms of depression while operating on them, but this requires extensive research validation and high quality data.
Data Sharing Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Acknowledgments
We sincerely thank the editor and the reviewers who participated in the review, and thank you for your constructive comments.
Funding
This research received no external funding.
Disclosure
The authors declare that they have no competing interests.
References
1. Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299–2312. doi:10.1016/s0140-6736(18)31948-2
2. Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369–388. doi:10.2147/ppa.S29716
3. Demyttenaere K. What is treatment resistance in psychiatry? A”difficult to treat” concept. World Psychiatry. 2019;18(3):354–355. doi:10.1002/wps.20677
4. Trevino K, McClintock SM, McDonald Fischer N, et al. Defining treatment-resistant depression: a comprehensive review of the literature. Ann Clin Psychiatry. 2014;26(3):222–232.
5. Lam RW, McIntosh D, Wang J, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 1. disease burden and principles of care. Can J Psychiatry. 2016;61(9):510–523. doi:10.1177/0706743716659416
6. Muller J, Pentyala S, Dilger J, et al. Ketamine enantiomers in the rapid and sustained antidepressant effects. Ther Adv Psychopharmacol. 2016;6(3):185–192. doi:10.1177/2045125316631267
7. Boyer PA, Skolnick P, Fossom LH. Chronic administration of imipramine and citalopram alters the expression of NMDA receptor subunit mRNAs in mouse brain. A quantitative in situ hybridization study. J Mol Neurosci. 1998;10(3):219–233. doi:10.1007/BF02761776
8. Borbely E, Simon M, Fuchs E, et al. Novel drug developmental strategies for treatment-resistant depression. Br J Pharmacol. 2022;179(6):1146–1186. doi:10.1111/bph.15753
9. Yang C, Kobayashi S, Nakao K, et al. AMPA receptor activation-independent antidepressant actions of ketamine metabolite (S)-norketamine. Biol Psychiatry. 2018;84(8):591–600. doi:10.1016/j.biopsych.2018.05.007
10. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi:10.1136/bmj.n160
11. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi:10.1136/bmj.39489.470347.AD
12. Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;175(7):620–630. doi:10.1176/appi.ajp.2018.17060720
13. Ionescu DF, Fu DJ, Qiu X, et al. Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a phase 3, double-blind, randomized study (ASPIRE II). Int J Neuropsychopharmacol. 2021;24(1):22–31. doi:10.1093/ijnp/pyaa068
14. Fu DJ, Ionescu DF, Li X, et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I). J Clin Psychiatry. 2020;81(3). doi:10.4088/JCP.19m13191
15. Ochs-Ross R, Daly EJ, Zhang Y, et al. Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression-TRANSFORM-3. Am J Geriatr Psychiatry. 2020;28(2):121–141. doi:10.1016/j.jagp.2019.10.008
16. Takahashi N, Yamada A, Shiraishi A, et al. Efficacy and safety of fixed doses of intranasal esketamine as an add-on therapy to oral antidepressants in Japanese patients with treatment-resistant depression: a phase 2b randomized clinical study. BMC Psychiatry. 2021;21(1):526. doi:10.1186/s12888-021-03538-y
17. Fedgchin M, Trivedi M, Daly EJ, et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol. 2019;22(10):616–630. doi:10.1093/ijnp/pyz039
18. Popova V, Daly EJ, Trivedi M, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019;176(6):428–438. doi:10.1176/appi.ajp.2019.19020172
19. Kishimoto T, Chawla JM, Hagi K, et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med. 2016;46(7):1459–1472. doi:10.1017/S0033291716000064
20. Ide S, Ikeda K. Mechanisms of the antidepressant effects of ketamine enantiomers and their metabolites. Biol Psychiatry. 2018;84(8):551–552. doi:10.1016/j.biopsych.2018.07.018
21. Murrough JW, Perez AM, Pillemer S, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74(4):250–256. doi:10.1016/j.biopsych.2012.06.022
22. Wajs E, Aluisio L, Holder R, et al. Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: assessment of long-term safety in a phase 3, open-label study (SUSTAIN-2). J Clin Psychiatry. 2020;81(3). doi:10.4088/JCP.19m12891
23. Ceban F, Rosenblat JD, Kratiuk K, et al. Prevention and management of common adverse effects of ketamine and esketamine in patients with mood disorders. CNS Drugs. 2021;35(9):925–934. doi:10.1007/s40263-021-00846-5
24. Fabbri C, Hagenaars SP, John C, et al. Genetic and clinical characteristics of treatment-resistant depression using primary care records in two UK cohorts. Mol Psychiatry. 2021;26(7):3363–3373. doi:10.1038/s41380-021-01062-9
25. Jaffe DH, Rive B, Denee TR. The humanistic and economic burden of treatment-resistant depression in Europe: a cross-sectional study. BMC Psychiatry. 2019;19(1):247. doi:10.1186/s12888-019-2222-4
26. Akil H, Gordon J, Hen R, et al. Treatment resistant depression: a multi-scale, systems biology approach. Neurosci Biobehav Rev. 2018;84:272–288. doi:10.1016/j.neubiorev.2017.08.019
27. Denee T, Kerr C, Ming T, et al. Current treatments used in clinical practice for major depressive disorder and treatment resistant depression in England: a retrospective database study. J Psychiatr Res. 2021;139:172–178. doi:10.1016/j.jpsychires.2021.05.026
28. Mrazek DA, Hornberger JC, Altar CA, et al. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatr Serv. 2014;65(8):977–987. doi:10.1176/appi.ps.201300059
29. Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139–148. doi:10.1001/jamapsychiatry.2017.3739
30. McIntyre RS, Rosenblat JD, Nemeroff CB, et al. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatry. 2021;178(5):383–399. doi:10.1176/appi.ajp.2020.20081251
31. Sanders B, Brula AQ. Intranasal esketamine: from origins to future implications in treatment-resistant depression. J Psychiatr Res. 2021;137:29–35. doi:10.1016/j.jpsychires.2021.02.020
32. Palhano-Fontes F, Mota-Rolim S, Lobo-Soares B, et al. Recent evidence on the antidepressant effects of ayahuasca. In: Ayahuasca Healing and Science. Springer; 2021:21–41.
33. Palhano-Fontes F, Barreto D, Onias H, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. 2019;49(4):655–663. doi:10.1017/S0033291718001356
34. Ng J, Rosenblat JD, Lui LMW, et al. Efficacy of ketamine and esketamine on functional outcomes in treatment-resistant depression: a systematic review. J Affect Disord. 2021;293:285–294. doi:10.1016/j.jad.2021.06.032
35. Bahji A, Vazquez GH, Zarate CA
36. Papakostas GI, Salloum NC, Hock RS, et al. Efficacy of esketamine augmentation in major depressive disorder: a meta-analysis. J Clin Psychiatry. 2020;81(4). doi:10.4088/JCP.19r12889
37. Bozymski KM, Crouse EL, Titus-Lay EN, et al. Esketamine: a novel option for treatment-resistant depression. Ann Pharmacother. 2020;54(6):567–576. doi:10.1177/1060028019892644
38. Li Z, Ruan M, Chen J, et al. Major depressive disorder: advances in neuroscience research and translational applications. Neurosci Bull. 2021;37(6):863–880. doi:10.1007/s12264-021-00638-3
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
