Back to Journals » Drug Design, Development and Therapy » Volume 17
Efficacy and Safety of Different Doses of Remimazolam Tosilate Applied in Upper Gastrointestinal Endoscopy: A Prospective Randomized Controlled Double-Blind Trial
Authors Cui X, Cheng Z, Li H, Zhang X, Luan H, Zhao Z, Zhu P
Received 13 June 2023
Accepted for publication 31 August 2023
Published 20 September 2023 Volume 2023:17 Pages 2889—2896
DOI https://doi.org/10.2147/DDDT.S422531
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Video abstract of "Efficacy and safety of different doses of Remimazolam" [ID 422531].
Views: 187
Xiaozhen Cui,1 Zhi Cheng,2 Han Li,2 Xiaobao Zhang,2 Hengfei Luan,2 Zhibin Zhao,2 Pin Zhu2
1Department of Anesthesiology, Graduate Training Base of Lianyungang First People’s Hospital of Jinzhou Medical University, Lianyungang, Jiangsu, People’s Republic of China; 2Department of Anesthesiology, The First People’s Hospital of Lianyungang, Lianyungang, Jiangsu, People’s Republic of China
Correspondence: Pin Zhu, The First People’s Hospital of Lianyungang, No. 6 East Zhenhua Road, Lianyungang, Jiangsu, People’s Republic of China, Email [email protected]
Background: Remimazolam is a novel benzodiazepine narcotic. When used for gastrointestinal endoscopy or bronchoscopy, it provides adequate sedation and rapid recovery. However, studies on the optimal initial loading dose of remimazolam remain inadequate. Therefore, we conducted a randomized controlled clinical trial to investigate the efficacy and safety of different doses of remimazolam applied in upper gastrointestinal endoscopy.
Methods: A total of 218 patients scheduled for upper gastrointestinal endoscopy were included in our trial and divided into experimental and control groups: the experimental groups were the remimazolam groups (R1 of 0.2 mg/kg, R2 of 0.3 mg/kg, and R3 of 0.4 mg/kg), and the control group was the propofol group. Following a single injection of trial drugs during the induction period, operational requirements were evaluated based on MOAA/S scores. When the sedation was successfully achieved, safety was evaluated based on the incidence of various intraoperative and postoperative adverse events.
Results: The success rates of intraoperative sedation were 82% in group R1, 98% in group R2, 96% in group R3, and 100% in group P. The incidence of hypotension was lower in the remimazolam groups than in the propofol group (16%), 4% in group R1, 6% in group R2, and 6% in group R3. The incidence of postoperative vertigo was significantly higher, and sedation recovery time was prolonged in high-concentration remimazolam group.
Conclusion: Satisfactory efficacy can be obtained with higher concentrations of remimazolam tosilate in patients undergoing upper gastrointestinal endoscopy with ASA grade I or II. However, as the dose is progressively increased, the incidence of adverse reactions by remimazolam tosilate are also significantly increased, such as vertigo and prolonged sedation recovery time.
Trial Registration: The trial was registered prior to enrollment at the Chinese Clinical Trial Registry (ChiCTR 2000032067).
Keywords: remimazolam, sedation, hypotension, hypoxemia, upper gastrointestinal endoscopy
Introduction
Endoscopy is a reliable and indispensable tool in clinical work. Its quality and safety are our focus. Sedation is frequently used to minimize the potential for injury during endoscopic surgery, reduce procedure-related anxiety, improve patient tolerance and satisfaction, and increase the quality and safety of endoscopic procedure.1
Propofol, as a short-acting anesthetic, has good sedative effect and rapid recovery,2 and is widely used for sedation in endoscopy.3 However, propofol also has significant cardiovascular and respiratory effects, and its adverse effects include hemodynamic instability, respiratory depression, pain at the injection site, and propofol infusion syndrome.4
Remimazolam tosilate is a benzodiazepine that differs from other drugs in that it contains a carboxylate linkage, which enables it to be rapidly hydrolyzed into inactive metabolites by tissue esterases.5 According to previous studies, remimazolam tosilate provided adequate duration of sedation and rapid recovery when used in gastrointestinal endoscopy, bronchoscopy, and was well tolerated by patients.6–10 However, studies on its optimal initial loading dose are still inadequate and need to be continued. Therefore, primary objective of this study was to investigate the safety and efficacy of different doses of remimazolam tosilate applied in upper gastrointestinal endoscopy.
Materials and Methods
Study Design
Our protocol was approved by the Medical Ethics Committee of the First People’s Hospital of Lianyungang. The study protocol complies with the Declaration of Helsinki. The trial was registered prior to enrollment at the Chinese Clinical Trial Registry (ChiCTR 2000032067) on 19/04/2020. The 220 subjects were recruited over a period of 8 months (21/04/2020 to 31/12/2020). It has been confirmed that the informed consent had been obtained from all subjects. Due to the different education levels of patients, some patients cannot write their own names and therefore cannot sign the informed consent form in person. In this case, we should confirm that verbal informed consent was obtained from the patient himself/herself while the informed consent document was signed by his/her legal guardian.
Participants
Eligible patients must meet all of the following inclusion criteria to be enrolled in this study: aged between 18 and 60 years, male or female; patients undergoing routine gastroscopic diagnosis and treatment; ASA physical status rating of Class I or II; 18 kg/m2 < BMI < 30 kg/m2; the expected gastroscopic operation time is not more than 15 min; clearly understand and voluntarily participate in the study, and their own informed consent is signed.
Patients with any of the following will not be enrolled in this study: 1) those requiring sophisticated endoscopic diagnostic and therapeutic techniques (such as cholangiopancreatography, endoscopic ultrasonography, endoscopic mucosal resection, endoscopic submucosal dissection, transoral endoscopic myotomy); 2) those who are scheduled for endotracheal intubation; 3) those who are judged to have respiratory tract management difficulties (modified Mahalanobis score IV); 4) hypertensive patients whose blood pressure is not satisfactorily controlled by antihypertensive drug therapy (sitting systolic blood pressure ≥ 160 mmHg, and/or diastolic blood pressure ≥ 100 mmHg); 5) sitting systolic blood pressure ≤ 90 mmHg; 6) pregnant or lactating women; 7) those who are allergic or contraindicated to benzodiazepines, propofol, dyclonine, and their drug components; 8) patients who are considered by the investigator to be inappropriate for this clinical study.
Randomization and Blinding
During 0–24 hours before the start of gastroscopy, the patients were randomized 1:1:1:1 to the remimazolam groups (group R1, group R2, and group R3) and propofol group (group P). Considering that there is a big difference in the appearance between the investigational drug remimazolam tosilate (powder for injection) and the control drug propofol (white or off-white uniform emulsion), in order to ensure that this study is not affected by subjective factors and achieve the double-blind effect as far as possible, the evaluation investigator and administration investigator were respectively set up in this study. The administration investigator only participated in the randomization, dispensing and administration process, and the patient screening, informed consent process and evaluation of various observation indicators were completed by the evaluation investigator. In addition to blinding the subjects, the evaluation investigator was also blinded throughout the trial.
Outcome Measure
The primary outcome is the success rate of sedation with remimazolam tosilate compared with propofol. Sedation success was defined as: 1) completion of the entire gastroscopic procedure; 2) absence of remedial sedative medication; 3) addition of ≤ 3 additional doses in any 15-minute period after the end of the initial dose of trial drug. Success rate of sedation was defined as the proportion of subjects with successful sedation in each group.
Procedures
Laboratory parameters such as electrocardiogram (ECG), heart rate (HR), blood pressure (BP), oxygen saturation (SpO2), respiratory rate, and BIS values in all eligible patients were monitored upon arrival in the gastroscopy room. All patients received lidocaine hydrochloride mucilage 0.1 g orally before induction of sedation. And supplemental oxygen at a rate of 2 to 4 L/min was administered by the nasal cannula to all patients throughout the entire gastroscopic diagnosis and treatment process. They were then randomized to either the remimazolam groups or the propofol group using a centralized randomization method. Remimazolam groups: The initial doses of remimazolam tosilate were 0.2 mg/kg (group R1), 0.3 mg/kg (group R2), and 0.4 mg/kg (group R3), respectively. Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale (see Appendix) was used to assess sedation levels. Within 1 minute (inclusive) after the end of the initial dose, if the patient achieved adequate sedation (MOAA/S score ≤ 2), gastroscopic access was started; if the patient had MOAA/S score > 2 or MOAA/S score ≤ 2 but failed gastroscopic access attempts, additional doses were allowed 1 minute after the end of the initial dose, and were administered at 0.05 mg/kg/dose via intravenous bolus at least 1 minute apart until the MOAA/S score was ≤ 2. Propofol group: Propofol (group P) was administered initially at 2.0 mg/kg. If the patients did not achieve adequate sedation (MOAA/S score ≤ 2) or MOAA/S score ≤ 2 but failed gastroscopic access attempts, within 1 minute, additional propofol of 0.5 mg/kg was allowed via intravenous bolus at least 1 minute apart until the MOAA/S score was ≤ 2.
After gastroscopic endoscopy, in order to maintain a certain degree of sedation (MOAA/S score ≤ 2), additional remimazolam tosilate (group R1, group R2, and group R3) or propofol (group P) was allowed in different groups when necessary.
Sedation failure was defined as: 1) From the end of the initial dose of the trial drug, the number of additional doses was greater than 3 in any 15-minute period; remedial sedative can be decided by the investigator; 2) The entire gastroscopic diagnosis and treatment process was not completed or remedial sedative were used during the trial. The trial data were collected according to the schedule. Rescue sedative remedial measures were determined by the investigator, including the choice of sedative remedial drugs (such as propofol), remedial dosage, remedial frequency, etc.
Management of adverse events during operation: Give mask oxygen inhalation if respiratory rate < 8 times/min and/or oxygen saturation < 90%; give esmolol 10 mg (without contraindication) if heart rate > 100 times/min, or increases by more than 20% from baseline if baseline value > 83 times/min; give intravenous atropine 0.3 mg (without contraindication) if heart rate < 55 times/min, or decreases by more than 20% from baseline if baseline value < 69 times/min; give norepinephrine 4 ug as needed if systolic blood pressure < 90 mmHg, or decreases by more than 20% from baseline if baseline value < 113 mmHg; give symptomatic drug treatment according to diagnosis if cardiac rhythm abnormalities occur.
Sample Size and Statistical Analysis
According to our preliminary results, the success rate of sedation in each remimazolam group was 0.2 mg/kg (91%), 0.3 mg/kg (93%), and 0.4 mg/kg (100%), respectively, and that in propofol group was 100%. PASS (version 21.0.3) was used to estimate that 183 patients in total would provide a power of 80% to detect an effect size (W) of 0.207 using Chi-Square Test with a significance level (alpha) of 0.05. Considering the dropout rate of each group is 20%, we enrolled 220 patients for four groups. Data from 218 patients were analyzed after two protocol violations.
All statistical analyses in this trial were programmed and calculated using SPSS statistical analysis software (version 20.0). Continuous variables were presented as mean ± SD and median (inter-quartile range) if normality was not met; t-test or Wilcoxon rank sum test was used for comparison of continuous data between groups. Categorical variables were presented as number of cases (constituent ratio); Chi-square test or Fisher exact test was used to compare categorical variables between groups. For repeated measures data, repeated measures analysis of variance or generalized estimating equations was used, and if missing values were present, generalized linear mixed models were used based on the normality test results for the data. In this study, except for non-inferiority one-sided test for primary efficacy endpoint, two-sided test was used for all other tests. P value ≤ 0.05 would be considered statistically significant for the tested difference. Confidence interval was 95%.
Results
A total of 220 patients were randomized and 218 were enrolled in our study, including 55 to group P, 54 to group R1, 55 to group R2, and 54 to group R3. Two patients were excluded because of protocol violation (Figure 1). There were similarities in the demographics of patients (age, gender, BMI) across all treatment groups, and baseline demographics (ASA score, pulse, MAP, systolic pressure, diastolic pressure, operation time) were no significant differences across the four groups (Table 1).
 |
Table 1 Baseline Characteristics |
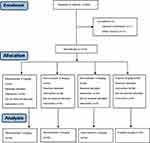 |
Figure 1 Flowchart of patient selection. |
Figure 2 shows the primary outcome (sedation success rate) for this study. The success rate of sedation in the propofol group was 100%, and that of remimazolam groups was 82% in the group R1, 98% in group R2, and 96% in group R3, respectively. Among them, the success rate of sedation in group R1 was lower than that in group P, and there was a statistically significant difference between the two groups (p < 0.05). In addition, there was no significant difference between group R2 and group P, or group R3 and group P (p > 0.05).
 |
Figure 2 The success rate of sedation with remimazolam group compared with propofol group. *p < 0.05 group R1 compared with group P. |
Intraoperative and postoperative adverse events are presented in Table 2 and Figure 3. According to the Table 2, sedation induction time was longer in the remimazolam groups than in the group P, and was more pronounced in group R1, which was statistically different compared with the other groups.
 |
Table 2 Intraoperative and Postoperative Adverse Events |
The incidence of hypotension during procedure was lower in the remimazolam groups compared with the P group (16%): 4% in group R1 (p < 0.05), 6% in group R2 (p < 0.05), and 6% in group R3 (p < 0.05). Additionally, the incidence of body movement in group R1 (80%) was higher than that in other groups, with a statistically significant difference (p < 0.01).
Among postoperative adverse events, the incidence of postoperative hypotension in the remimazolam groups was lower than that in the propofol group (30%), particularly in the group R1 (4%) and group R2 (7%), with a statistically significant difference (p < 0.01). Besides, the incidence of postoperative vertigo was significantly higher and sedation recovery time was prolonged in the remimazolam groups. In particular, group R3 had statistically significant differences in the incidence of postoperative vertigo (41%) and sedation recovery time (13.2±3.4 min) compared with the other three groups (p < 0.05).
Discussion
According to Xiaoyan Sheng et al study, a single injection of remimazolam tosilate was considered to have good safety and tolerability at doses of 0.025 to 0.4 mg/kg.11 In our trial, the sedative drugs were administered as a single injection, and we compared the sedative effects of remimazolam tosilate at three concentrations with propofol on the basis of meeting the needs of gastroscopic procedures. The results showed that higher concentrations of remimazolam tosilate resulted in satisfactory sedation success rates (98% and 96%) and were not inferior to propofol (100%), which is consistent with the results of some previous studies.8,9
The adverse effects of hypotension following induction of anesthesia sedation have been acknowledged.12 And there is a clear relationship between arterial blood pressure and organ dysfunction, which may cause harmful effects even if mean arterial pressure < 60–70 is only transiently sustained.13 In this regard, although both remimazolam tosilate and propofol may cause transient cardiovascular depression, a lower incidence of intraoperative hypotension was reported in the remimazolam groups (4%, 6%, 6%) compared with the propofol group (16%) in this trial. The same result was also noted in an incidence of hypotension developed on patients at postoperative stage: compared with an incidence of 30% with propofol, patients taking remimazolam tosilate were less likely to suffer from hypotension. This is consistent with our expectation that remimazolam tosilate has a significant advantage in maintaining hemodynamic stability in the patients with ASA grade I or II.
Regarding other adverse events, only group R1 showed a higher incidence of intraoperative body movement than the other treatment groups. The differences shown above are theoretically related to different sedation levels.14 Previous studies have shown that propofol usually causes deep sedation while remimazolam tosilate causes moderate sedation.9
However, in our trial, hemodynamic stability maintenance with remimazolam tosilate sacrificed with prolonged sedation recovery time and resulted in a significant increase in the incidence of vertigo as concentration increased. For slower recovery times, remimazolam tosilate sedation can be effectively antagonized with the benzodiazepine antagonist flumazenil. In Doi et al study,15 9% of patients in the remimazolam group used flumazenil and all awoke within 2 minutes. In addition, although at higher concentrations, the time for remimazolam tosilate to achieve adequate sedation (42.4s ± 14.1s and 42.1s ± 13.9s) was remarkably faster than other commonly used sedative drugs, such as midazolam,16,17 it took slightly longer time to achieve adequate sedation than propofol (33.6 s ± 12.9 s), which was related to its drug onset time.
Overall, both agents had acceptable safety and tolerability from a safety perspective, and remimazolam tosilate was more effective in reducing the incidence of intraoperative and postoperative hypotension. Higher doses lead to more complete sedation, but also increase the probability of causing perioperative adverse events to some extent. In our study, 0.3mg/kg seems to be a better dose for patients undergoing upper gastrointestinal endoscopy with ASA grade I or II. At this dose, sedation success and adverse event rates can be better balanced. Therefore, it is believed that remimazolam tosilate, as an ultra-short-acting sedative, can meet the need for anesthesia sedation in upper gastrointestinal endoscopy procedures, with non-inferior effects compared to propofol. The potential of remimazolam tosilate was specifically demonstrated in short-term surgery.
Our study also suffers several limitations. First, our study did not include ASA III patients, indicating that our results may not be used in vulnerable patients. Second, it is difficult to keep anesthesiologist fully blinded to the trial drugs because the property and color are different between remimazolam tosilate and propofol, which may cause theoretical bias. Third, since this is a single-center study, the generalizability of the study sample may be compromised, and therefore the applicability of the study results may be limited.
Conclusion
In summary, satisfactory efficacy can be obtained with higher concentrations of remimazolam tosilate in patients undergoing upper gastrointestinal endoscopy with ASA grade I or II. Its main advantage is that it has less effect on hemodynamics, but as the dose is progressively increased, the adverse reactions caused by high concentration (0.4 mg/kg) of remimazolam tosilate are also significantly increased, such as nausea, vertigo, and prolonged sedation recovery time. The general applicability of remimazolam tosilate needs to be confirmed by further clinical trials with other types of surgery.
Data Sharing Statement
All data generated or analyzed during this study are included in the article. Further inquiries about the datasets can be directed to the corresponding author on reasonable request. The patient datasets anonymity will be preserved prior to distribution.
Funding
This work was funded by China Health Promotion Foundation(HX2020013739002), Lianyungang 521 project and Clinical Research Fund of The Affiliated Lianyungang Hospital of Xuzhou Medical University (LC13), Scientific Research Development Fund of Kangda College of Nanjing Medical University(KD2021KYJJZD113).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wiggins TF, Khan AS, Winstead NS. Sedation, analgesia, and monitoring. Clin Colon Rectal Surg. 2010;23(1):14–20. doi:10.1055/s-0030-1247852
2. Sacchetti A, Senula G, Strickland J, Dubin R. Procedural sedation in the community emergency department: initial results of the ProSCED registry. Acad Emerg Med. 2007;14(1):41–46. doi:10.1197/j.aem.2006.05.023
3. Triantafillidis JK, Merikas E, Nikolakis D, Papalois AE. Sedation in gastrointestinal endoscopy: current issues. World J Gastroenterol. 2013;19(4):463–481. doi:10.3748/wjg.v19.i4.463
4. Chang ACY, Chang ACH, Nicin L, et al. An in vitro model for identifying cardiac side effects of anesthetics. Anesth Analg. 2020;130(1):e1–e4. doi:10.1213/ANE.0000000000003757
5. Kilpatrick GJ, McIntyre MS, Cox RF, et al. CNS 7056: a novel ultra-short-acting Benzodiazepine. Anesthesiology. 2007;107:60–66. doi:10.1097/01.anes.0000267503.85085.c0
6. Pambianco DJ, Borkett KM, Riff DS, et al. A phase IIb study comparing the safety and efficacy of remimazolam and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2016;83(5):984–992. doi:10.1016/j.gie.2015.08.062
7. Jhuang BJ, Yeh BH, Huang YT, Lai PC. Efficacy and safety of remimazolam for procedural sedation: a meta-analysis of randomized controlled trials with trial sequential analysis. Front Med. 2021;8:641866. doi:10.3389/fmed.2021.641866
8. Rex DK, Bhandari R, Desta T, et al. A Phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88(3):427–437.e6. doi:10.1016/j.gie.2018.04.2351
9. Chen SH, Yuan TM, Zhang J, et al. Remimazolam tosilate in upper gastrointestinal endoscopy: a multicenter, randomized, non-inferiority, phase III trial. J Gastroenterol Hepatol. 2021;36(2):474–481. doi:10.1111/jgh.15188
10. Dai G, Pei L, Duan F, et al. Safety and efficacy of remimazolam compared with propofol in induction of general anesthesia. Minerva Anestesiol. 2021;87(10):1073–1079. doi:10.23736/S0375-9393.21.15517-8
11. Sheng XY, Liang Y, Yang XY, et al. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol. 2020;76(3):383–391. doi:10.1007/s00228-019-02800-3
12. Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KG, Kalkman CJ. Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology. 2007;107(2):213–220. doi:10.1097/01.anes.0000270724.40897.8e
13. Sessler DI, Bloomstone JA, Aronson S, et al. Perioperative quality initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122(5):563–574. doi:10.1016/j.bja.2019.01.013
14. Amornyotin S. Sedation-related complications in gastrointestinal endoscopy. World J Gastrointest Endosc. 2013;5:527–533. doi:10.4253/wjge.v5.i11.527
15. Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34(4):543–553. doi:10.1007/s00540-020-02788-6
16. Khurmi N, Patel P, Kraus M, Trentman T. Pharmacologic considerations for pediatric sedation and anesthesia outside the operating room: a review for anesthesia and non-anesthesia providers. Paediatr Drugs. 2017;19(5):435–446. doi:10.1007/s40272-017-0241-5
17. Kress JP, O’Connor MF, Pohlman AS, et al. Sedation of critically ill patients during mechanical ventilation. A comparison of propofol and midazolam. Am J Respir Crit Care Med. 1996;153(3):1012–1018. doi:10.1164/ajrccm.153.3.8630539
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

