Back to Journals » Cancer Management and Research » Volume 10
Efficacy and safety of crizotinib in patients with anaplastic lymphoma kinase-positive advanced-stage non-small-cell lung cancer
Authors Mohieldin A , Rasmy A, Ashour M , Al-Nassar M, Ali RH , El-Enezi FG
Received 4 May 2018
Accepted for publication 24 September 2018
Published 29 November 2018 Volume 2018:10 Pages 6555—6561
DOI https://doi.org/10.2147/CMAR.S173084
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Ahmed Mohieldin,1,2 Ayman Rasmy,1,3,4 Mohamed Ashour,2,5 Muath Al-Nassar,6 Rola H Ali,7,8 Fahad G El-Enezi6
1Medical Oncology, Zagazig University Hospitals, Zagazig, Egypt; 2Medical Oncology Department, Sheikha Badriya Alsabah Centre, Kuwait Cancer Control Center, Shuwaikh, Kuwait; 3Medical Oncology Department, King Saud Medical City, Riyadh, Saudi Arabia; 4Medical Oncology Department, King Fahad Specialist Hospital, Dammam, Saudi Arabia; 5Clinical Oncology, Al-Azhar University, Cairo, Egypt; 6Thoracic Oncology – Sheikha Badriya Alsabah Centre, Kuwait Cancer Control Center, Shuwaikh, Kuwait; 7Department of Pathology, Faculty of Medicine, Kuwait University, Safat, Kuwait; 8Molecular Laboratory, Kuwait Cancer Control Center, Shuwaikh, Kuwait
Introduction: Lung cancer is the leading cause of cancer mortality worldwide, despite advances in management, especially with targeted agents and immunotherapy. Numerous oncogenes have been identified that control the growth of these malignancies. Anaplastic lymphoma kinase (ALK) is a tyrosine kinase that develops distorted functioning as a result of chromosomal rearrangement. Crizotinib, a tyrosine kinase inhibitor (TKI), was approved by the Food and Drug Administration (FDA) in 2011 for the treatment of advanced ALK-positive non-small-cell lung cancer (NSCLC).
Patients and methods: In this chart review, we compiled data from two cancer hospitals in Kuwait and Saudi Arabia which were collected from patients with advanced NSCLC treated between January 2013 and September 2017 with crizotinib after diagnosed with ALK-positive disease. Crizotinib 250 mg BID was given orally with/without food intake. We assessed overall survival (OS), objective response rate (ORR), progression-free survival (PFS), duration of the response, and dose reduction/cessation.
Results: De-identified data from 38 subjects were compiled. Their median age was 53 years, 65.8% were male, the 1-year OS was 88%, and the PFS was 16.5 months. Two cases (5.3%) had a complete response (CR), while 17 (44.7%) had a partial response (PR). Side effects of grade III/IV occurred, including elevated transaminase levels, diarrhea, and prolonged QT intervals, in 8% patients, with dose reduction in six patients (15.8%).
Conclusion: In NSCLC, crizotinib is a viable treatment option with good response and tolerable toxicity for patients with ALK-positive advanced disease.
Keywords: non-small-cell lung cancer, anaplastic lymphoma kinase gene, crizotinib, overall survival, progression-free survival
Introduction
Although lung malignancies are the second most common type of cancers, they are consistently the main cause of malignancy-related mortality in the world (in about 14% of all cases). The lung cancer incidence estimated by the American Society of Clinical Oncology (ASCO) for USA in 2018 is 234,030 new cases (121,680 in men and 112,350 in women), with mortality estimated at 154,050 (including 83,550 men and 70,500 women).1 During the past 15 years, the approach to treat non-small-cell lung cancer (NSCLC) cases has changed from “chemo-for-all” to the use of a “tailored medicine” strategy. Oncologists now have identified that at least one driver change, including gene mutation, rearrangement, or amplification, occurs in 50% of the cases of metastatic lung adenocarcinoma.2,3
Chromosomal rearrangement has a role in the activation of tyrosine kinase inhibitor (TKI; anaplastic lymphoma kinase [ALK]), which leads to the expression of “EML4-ALK” as an “oncogenic fusion kinase.”4,5 Additional ALK gene changes similarly happen in anaplastic large-cell lymphoma,6 pediatric neuroblastoma,7 and inflammatory myofibroblastic tumors.8
For patients having advanced NSCLC with ALK-positive subtypes, new treatment options have been developed using ALK gene rearrangement studies confirmed by “Food and Drug Administration (FDA)-approved tests”. Currently, the FDA has granted different levels of approval for the various small molecule TKIs developed for the management of NSCLC with different ALK rearrangements, in that these agents confer different levels of clinical improvement in comparison to standard chemotherapy.
Crizotinib as a TKI has been approved for the management of cancers utilizing tyrosine kinases such as ALK,5 MET,9 or ROS110 since August 2011, when the FDA recognized it as a first-generation ALK inhibitor for treating such cases.10 After 2 years, regular approval for crizotinib was granted by the FDA.
In the cases where ALK rearrangements occurred, leading to disease recurrence or progression after crizotinib treatment, the second- and third-generation oral ALK inhibitors (such as ceritinib, alectinib, or brigatinib) have been approved.11–13 These newer inhibitors have more potent effects against the different EML4-ALK resistance point mutations.
Based on the ALEX trial, in November 2017, alectinib was approved by the FDA for the management of NSCLC in ALK-positive cases.14 In February 2018, the FDA announced the auditing of another application (for lorlatinib, a third-generation ALK inhibitor) regarding the ability to resensitize cancer cells to crizotinib.15
Patients and methods
This chart review “retrospective” study focused on the outcomes of advanced NSCLC “stage IIIB and IV” diagnosed at the oncology departments of the Kuwait Cancer Control Center (KCCC) in Kuwait and the King Fahad Specialist Hospital (KFSH) in Saudi Arabia between January 2013 and September 2017. All cancer diagnoses were confirmed by surgical pathology from tissues obtained from either primary lung lesions or metastatic sites. Each diagnosis of adenocarcinoma thus was confirmed by immunohistochemistry (IHC) by an expert pathologist. Evaluations of EGFR mutations, ALK rearrangements, and ROS1 rearrangements were carried out to identify potentially targetable drive alterations. The ALK FISH gene rearrangement evaluation was performed in all patients centrally using CE-marked Vysis LSI ALK Break Apart Rearrangement Probe Kits (Abbott Laboratories, Abbott Park, IL, USA) that detect ALK locus rearrangements on chromosome 2p23.
Patient characteristics, such as age; sex; comorbidities; initial symptoms; metastatic site; prior therapy including surgery, chemotherapy, crizotinib, and local treatment by radiotherapy (RT); response to therapy; pattern of failure; time to progression (TTP); and survival outcome, were documented. All cases had initial staging by “computed tomography (CT) and/or magnetic resonance imaging (MRI) scans” prior to starting crizotinib. Platinum combination chemotherapy was allowed as the first-line treatment. Palliative RT was given at any time to control localized pain or for hemostatic purposes using three-dimensional (3D) conformal RT.
All patients were given crizotinib either as the first-line treatment and/or subsequent treatment upon failure of platinum-based chemotherapy or as a switch maintenance after achieving the maximum response on platinum-based chemotherapy. Crizotinib was recommended as standard dose “250 mg orally every 12 hours with or without meal” until treatment failure or intolerable therapy-related side effects. Dose modification was allowed according to patient tolerance.
Outpatient treatment was carried out with regular evaluation during visits by clinical and laboratory assessment including complete blood counts (CBCs), liver and renal function tests, bone panels, and electrocardiograms. Evaluations of the response were carried out by repeating the CT and/or MRI scans by 3–4-month interval. The response status was recorded according to RECIST 1.0 criteria (Response Evaluation Criteria in Solid Tumors). In the case of solitary recurrence, metastasectomy, stereotactic, and/or gamma knife therapy were performed.
Statistical analyses
Statistical evaluations were carried out by SPSS software version 23.0 (IBM Corporation, Armonk, NY, USA).
All the following are considered as per standard guidelines: response duration, time to objective response (TOR), overall survival (OS), and progression-free survival (PFS). For patients without disease progression at the time of analysis, the date of the last follow-up was considered right censored. Log-rank tests were used for comparisons of the Kaplan–Meier survival curves. All tests of hypotheses were conducted at the alpha of 0.05 level with a 95% CI.16
Results
We identified 386 adult patients diagnosed by histopathology and IHC with advanced-stage lung adenocarcinoma treated between January 2013 and September 2017 at both centers. We identified ALK rearrangement in 38 of these using FISH. The median age of the study subjects was 53 years (41–82), with five patients above 65 years and a male predominance (70.8%). Nonsmokers composed more than half of the patients (57.9%). The majority of patients (36 patients; 94.7%) had a good performance status (PS) according to “Eastern Cooperative Oncology Group (ECOG)”. One patient had an ECOG PS of 3 due to an extensive lumber vertebral metastasis that needed fixation and postoperative irradiation. He moved with the assistance of a walker or by wheelchair. Another patient had ECOG PS of 4, on mechanical ventilation secondary to respiratory failure, and we initiated crizotinib therapy through a nasogastric tube.17
Five patients (13.2%) had stage IIIB disease, while the rest (86.8%) had stage IV disease, of whom 11 patients (28.9%) were diagnosed with a single-site metastasis. A pretreatment staging workup identified that bones (24.1%), liver (23.7%), and brain (21.1%) were the most common sites for metastases (Table 1). Three patients (7.9%) were known to have NSCLC after lobectomy for early-stage disease. At follow-up, these patients had developed distant metastases. Biopsies from the metastatic sites confirmed ALK-positive disease.
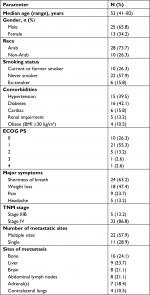  | Table 1 Patient characteristics at baseline (n=38) Abbreviations: BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; PS, performance status. |
Treatment received, treatment interruptions, and dose modification
More than half of the patients (55.3%) were treated with a platinum-based combination at some point before crizotinib initiation, either as a postoperative adjuvant therapy or as a palliative therapy for a metastatic disease before the availability of the ALK testing results. A wide variety of platinum-based combinations were used: pemetrexed, paclitaxel, gemcitabine, or vinorelbine plus a platinum component with/without bevacizumab. Before the initiation of crizotinib, seven patients (18.4%) received palliative RT either as a whole brain irradiation for multiple brain metastases, for palliation of tender bony metastasis, or as a hemostatic dose. Three patients (7.9%) were treated for solitary brain metastasis by gamma knife brain surgery.
On the initiation of crizotinib, the majority of patients (32 patients; 84.2%) tolerated the 250 mg oral (PO)/BID dose with no significant interruptions. Treatment interruptions and dose modification were recommended by the treating physician for six patients (15.8%) because of intolerable adverse events (Table 2).
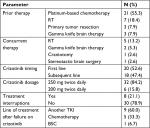  | Table 2 Treatment received, interruptions, and dose modifications Abbreviations: BSC, best supportive care; RT, radiotherapy; TKI, tyrosine kinase inhibitor. |
Failure of crizotinib was reported in 16 patients (42.1%), of whom 15 patients had clinical and radiological disease progression on crizotinib. During crizotinib therapy, intracranial relapse with a limited number of brain lesions occurred in four patients. These patients had good extracranial disease control and continued on crizotinib therapy after controlling the intracranial relapse; two patients (12.5%) received gamma knife brain therapy, one patient (6.3%) received stereotactic brain surgery, and another patient underwent a craniotomy (Table 3A).
  | Table 3 Crizotinib failure and adverse events (38 patients) |
Adverse events were recorded in 38 patients and are summarized in Table 3B. The most commonly reported events were fatigue (68.4%), peripheral edema (63.2%), and transaminitis (50%). Grade III transaminitis occurred in two patients. After a few days of treatment, enzyme titers returned to basal levels and crizotinib was resumed and continued at a lower dosage (200 mg orally twice daily) without further interruptions. Grade IV transaminitis was reported in another patient. After normalization of the hepatic enzymes, this patient was changed to alectinib treatment, but unfortunately, the same adverse event was reported. Finally, this patient commenced chemotherapy and showed a good tolerance to it.
Diarrhea (grade III/IV) occurred in three of the cases (7.9%), leading to dehydration and a slight elevation of blood urea nitrogen in one patient. Uncomplicated neutropenia was reported in six patients (15.8%).
Bradycardia with prolonged QT interval occurred in 10 patients (26.3%). Three were grade III/IV. One of these patients was a 72-year-old female, who was morbidly obese, with a cardiac pacemaker implanted for the previous 7 years. On day 9 of crizotinib usage, she fainted and was found to have severe sinus bradycardia (heart rate was 30 beats/min) and significant prolongation of the QT interval. She was admitted to a cardiac care unit where crizotinib was immediately stopped. After 3 days of monitoring, her heart rate returned to her baseline level. Because of her advanced age, multiple comorbidities, and her advanced cancer with expected poor tolerance to chemotherapy, our thoracic multidisciplinary team and her cardiologist adjusted the pacemaker to a higher rate and resumed crizotinib at 250 mg PO BID. Another two patients, with unknown history of cardiac disease, developed sinus bradycardia and prolongation of the QT interval. They showed good tolerance on the lower dosage.
Of the 38 patients included in efficacy analyses, 36 were suitable for response evaluation (two patients died early from the disease). The objective response rate (ORR) was 50%. Two cases (5.3%) were with a complete response (CR), and 17 cases (44.7%) were with a partial response (PR). Two cases (5.3%) achieved stable disease (SD), while progressive disease (PD) occurred in four cases (16.7%). The median time to an objective response in 19 patients was 3.4 months (range 2.2–5.2 months, 95% CI: 3.2–3.7). The time of the response was 19.9 months.
The 1-year survival rate was 88.6%, while 5-year survival rate was 34.8%. The mean PFS was 17.678± 4.227 months (rang: 9.393- 25.963). Patients with first -line crizotinib had a lower mean PFS of 14.834 ± 6.789 months (range: 1.527–28.141) when compared to the patients who received crizotinib as a subsequent line of treatment with PFS of 20.928±4.958 months (range: 11.210–30.646) with no statistically significant outcome (P=0.487).
The mean OS was 50.097±6.547 months (rang: 37.265–62.928 months). Patients with first-line crizotinib treatment had longer mean OS of 48.794 ±8.941months (rang: 31.270–66.319 months) when compared to patients who received crizotinib as subsequent lines with mean OS of 46.790±7.827 months (range: 48.794–62.131) with no statistically significant outcome (P=0.456) (Table 4 and Figures 1 and 2).
  | Table 4 Response and survival outcome Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease; TOR, time to objective response. |
Discussion
Worldwide, lung cancer has been described as the highest malignancy as per public health view. In Kuwait, lung cancer is the sixth most frequent cancer (6.2% of all malignancies, the fifth most common in males and the ninth in females). Lung cancer is the most common reason for malignancy-related mortality (15.9%) in Kuwaiti males, while among Kuwaiti females, it is the fifth most common reason for malignancy-related mortality (4.9%). In Saudi Arabia, lung cancer is the sixth most frequent cancer (4.7% for all malignancies). In Saudi males, lung cancer is the fourth highest malignancy (7.4%) and the second most common cause of malignancy-related mortality (11.2%). Lung cancer in Saudi females is very rare (2.2%), and it is the eighth most common cause of malignancy-related mortality (4.4%).18
The OS is the most consistent end point in estimating any investigational new treatment for cancer, with significant progress in OS needed for getting regulatory approval. Post approval, local research is important as well to assess the drug’s effectiveness and tolerability.
We performed a retrospective study on the management of cases confirmed as advanced-stage ALK-positive NSCLC. This study used existing data available in the medical charts and electronic medical records of two of the biggest tertiary referral centers for cancer management on the western coast of the Arabian Gulf, the KCCC and the KFSH, from January 2013 to September 2017.
Crizotinib is a first-generation ALK-TKI that has previously been shown to have remarkable activity for the treatment for ALK-positive NSCLC, considering the response rate and PFS results. In August 2011, crizotinib was approved by the FDA for the management of ALK-positive advanced-stage NSCLC based on the outcomes of the PROFILE 1014 trial, where crizotinib showed superiority to the first pemetrexed/platinum combination in cases with advanced ALK-positive NSCLC not previously treated. Treatment with crizotinib resulted in longer PFS, higher ORR, and more tolerable adverse events than chemotherapy.19 Although there were fewer subjects in our study than in the PROFILE 1014 trial (38 vs 172 subjects), there were some differences. We detected ALK rearrangement in 9.8% of cases of lung adenocarcinoma, while in most of the published studies, ALK rearrangement was reported in 5–7% of cases.4,5 Most of the subjects in our study were young, Asian, and nonsmoker females, and this may explain the high prevalence of ALK-positive NSCLC in our study. The median age of our cases was 53 years, and most of these cases were nonsmokers (57.9%).
Similar results were shown in the PROFILE 1014 trial (age 52 years, and 65% nonsmokers). Because of the toxicity profiles of all the approved ALK-TKIs used in clinical practice, we were able to administer crizotinib via nasogastric tube in a patient with poor PS (ECOG PS 3–4).17
At the time of initial diagnosis, central nervous system (CNS) metastasis was reported in 21.1% of our patients vs 38% in the PROFILE 1014 trial. The strategy we applied for controlling CNS disease is supported by the results of this trial, including the effects of whole-brain irradiation, gamma knife brain surgery, stereotactic brain surgery, craniotomy, and following up of asymptomatic small brain lesions.20
In the PROFILE 1014 trial, the ORR for crizotinib was 74%. The median PFS was extended more with crizotinib than with chemotherapy “10.9 vs 7.0 months”. The median OS was not reached by crizotinib; the likelihood of 1-year survival with crizotinib was stable at 84%. In our study, the ORR for crizotinib was 50%, and the median PFS was 16.5 months (95% CI: 1.6–31.4). The median OS from the initiation of crizotinib was 48.0 months (95% CI: 18.2–77.8). In the near future, ALK mutation analysis may play a role in explaining why some ALK-positive NSCLC patients do not respond well to crizotinib.21
When the use of standard chemotherapeutic agents results in clinical failure, it is feasible to switch to crizotinib as per prior first- and second-line registration trials of crizotinib. In our study, the survival differences between the cases that received standard chemotherapy before and chemotherapy-naive cases were challenging based on the confounding factors involved in patient selection and previous chemotherapy exposure. Our analysis revealed no substantial difference in PFS (P-value was 0.49) or OS (P-value was 0.46) between cases exposed to standard platinum-based combination chemotherapy before crizotinib and those who received crizotinib as the first-line treatment. These differences could be elucidated in a randomized controlled study.22
Lee et al23 concluded in their study that ALK positivity was unconventionally predictive of pemetrexed effectiveness in NSCLC cases. ALK-positive crizotinib-naive patients have a significantly longer PFS when receiving pemetrexed.
Conclusion
Crizotinib seems to be a promising agent for the management of advanced ALK-positive NSCLC. Crizotinib had a good response rate, a very acceptable side effect profile, promising median PFS, and median OS.
Ethical approval
This study did not entail any experimental procedures or treatments of human participants by any of the authors. The research ethics committees of the Sheikha Badriya Alsabah Center, KCCC, Kuwait, and KFSH, Saudi Arabia, approved this study. Given the retrospective nature of this study and its use of deidentified data, a waiver for obtaining individual patient consent was requested and approved by the ethics committees. All patient data were stored safely, securely coded, and de-identified to ensure confidentiality with limited access by the corresponding author.
Acknowledgment
The abstract of this paper was presented at the ASCO Annual Meeting, USA (June 2017), as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in the Journal of Clinical Oncology 35, no. 15_suppl, DOI: 10.1200/JCO.2017.35.15_suppl.e20516 https://meetinglibrary.asco.org/record/146588/abstract.
Disclosure
The authors report no conflicts of interest in this work.
References
American Cancer Society’s Cancer Statistics Center [webpage on the Internet]. Key Statistics for Lung Cancer; 2018. Available https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/key-statistics.html. Accessed October 16, 2018. | ||
Kohno T, Nakaoku T, Tsuta K, et al. Beyond ALK-RET, ROS1 and other oncogene fusions in lung cancer. Transl Lung Cancer Res. 2015; 4(2):156. | ||
Li T, Kung HJ, Mack PC, Gandara DR. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol. 2013;31(8):1039–1049. | ||
Camidge DR, Doebele RC. Treating ALK-positive lung cancer--early successes and future challenges. Nat Rev Clin Oncol. 2012;9(5):268–277. | ||
Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. | ||
Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263(5151):1281–1284. | ||
Chen Y, Takita J, Choi YL, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455(7215):971–974. | ||
Coffin CM, Patel A, Perkins S, Elenitoba-Johnson KS, Perlman E, Griffin CA. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001;14(6):569–576. | ||
Christensen JG, Zou HY, Arango ME, et al. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther. 2007;6(12 Pt 1):3314–3322. | ||
Kazandjian D, Blumenthal GM, Luo L, et al. Benefit-risk summary of crizotinib for the treatment of patients with ROS1 alteration-positive, metastatic non-small cell lung cancer. Oncologist. 2016;21(8):974–980. | ||
Khozin S, Blumenthal GM, Zhang L, et al. FDA approval: ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer. Clin Cancer Res. 2015;21(11):2436–2439. | ||
Larkins E, Blumenthal GM, Chen H, et al. FDA Approval: Alectinib for the Treatment of Metastatic, ALK-Positive Non-Small Cell Lung Cancer Following Crizotinib. Clin Cancer Res. 2016;22(21):5171–5176. | ||
Markham A. Brigatinib: First Global Approval. Drugs. 2017;77(10):1131–1135. | ||
Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377(9):829–838. | ||
Shaw AT, Friboulet L, Leshchiner I, et al. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N Engl J Med. 2016;374(1):54–61. | ||
Pazdur R. Endpoints for assessing drug activity in clinical trials. Oncologist. 2008;13(Suppl 2):19–21. | ||
Tamai K, Nagata K, Otsuka K, et al. Crizotinib administered via nasogastric and percutaneous endoscopic gastrostomy tubes for the successful treatment of ALK-rearranged lung cancer in a patient with poor performance status. Respir Investig. 2013;51(1):46–48. | ||
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015 ;136(5):E359-86. | ||
Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–2177. | ||
Bruynzeel AM, Lagerwaard FJ. Whole brain radiotherapy for brain metastases from non-small cell lung cancer: the end of an era? J Thorac Dis. 2016;8(11):E1525–E1527. | ||
Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18(5):1472–1482. | ||
Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12(11):1004–1012. | ||
Lee JO, Kim TM, Lee SH, et al. Anaplastic lymphoma kinase translocation: a predictive biomarker of pemetrexed in patients with non-small cell lung cancer. J Thorac Oncol. 2011;6(9):1474–1480. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


