Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Efficacy and Safety of Combined Ethanol-Lipiodol Mixture and Drug-Eluting Bead TACE for Large HCC
Authors Chuang YH , Cheng YF, Tsang LLC , Ou HY , Hsu HW, Lim WX, Huang PH , Weng CC, Yu CY
Received 20 November 2022
Accepted for publication 7 January 2023
Published 15 January 2023 Volume 2023:10 Pages 81—90
DOI https://doi.org/10.2147/JHC.S398434
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Imam Waked
Yi-Hsuan Chuang,* Yu-Fan Cheng,* Leo Leung-Chit Tsang, Hsin-You Ou, Hsien-Wen Hsu, Wei-Xiong Lim, Po-Hsun Huang, Ching-Chun Weng, Chun-Yen Yu
Departments of Diagnostic Radiology, Kaohsiung Chang Gung Memorial Hospital, and Chang Gung University College of Medicine, Kaohsiung, 833401, Taiwan
*These authors contributed equally to this work
Correspondence: Chun-Yen Yu, Department of Diagnostic Radiology, Kaohsiung Chang Gung Memorial Hospital, 123 Ta-Pei Road, Niao-Sung District, Kaohsiung, 833401, Taiwan, Tel +886-7-731-7123 #3027, Email [email protected]
Purpose: To evaluate treatment response, survival and safety of a novel TACE using combination of ethanol-Lipiodol mixture and drug-eluting beads in patients with large unresectable HCC, single tumor > 8 cm or multiple tumors with the largest tumor diameter > 5 cm and total tumor diameter > 10 cm.
Patients and Methods: Between June 2016 and February 2020, a total of 27 patients were enrolled in this retrospective cohort study. Treatment response was assessed at first month after the treatment; progression-free survival (PFS) and overall survival (OS) were evaluated. The prognostic factors associated with patient survival were statistically analyzed by the Cox regression model. Adverse events were recorded.
Results: The maximum diameter of the tumors ranged from 5 cm to 17 cm (mean 10.48 cm). The objective response and disease control rates were 56% and 78%, respectively, at 1-month follow-up. The median survival time was 15.9 months (95% CI, 9.03– 34.76 months). The OS rates were 76.9% at six months, 65.2% at one year and 44.8% at two years. AFP > 400 ng/mL (p = 0.0306), maximum tumor size > 10cm (p = 0.0240) were potential risk factors for OS. Regarding safety, major complications occurred in one patient (1/27, 3.7%), presenting with transient hepatic encephalopathy.
Conclusion: Combined DEB-TACE appeared to have favorable objective tumor response. It can be an effective treatment option for large unresectable HCC.
Keywords: hepatocellular carcinoma, transarterial chemoembolization, treatment response, prognosis
Introduction
Hepatocellular carcinoma (HCC) is the sixth common cancer but the third leading cause of cancer-related death worldwide, with almost 75% of all cases found in the Asia-Pacific region because of the high prevalence of chronic hepatitis B and C virus infection in this region.1,2 The 5-year overall survival (OS) rate has been reported to be 10%–18%, and is considerably shorter in cases of large HCCs that are >5 cm in size. The Barcelona Clinic of Liver Cancer (BCLC) algorithm is the most widely used staging system in HCC.3 Owing to the observed clinical heterogeneity of the intermediate stage BCLC-B, the updated 2022 version stratifies the BCLC-B into three groups of patients according to tumor burden and underlying liver function. It also highlights the concept of treatment stage migration. Patients can be eligible for transplantation in case of successful downstaging by TACE or switch to systemic therapy after TACE failure/refractoriness or deterioration of liver function.
Many patients present with intermediate or advanced stages and thus are not candidates for curative treatment. For large HCC, more extended hepatectomy is required, and it is associated with a higher risk of postoperative morbidity and mortality. Inadequate hepatic reserve remains a great concern, especially in patients with chronic liver diseases. Transarterial chemoembolization (TACE) has been the de facto mainstay of treatment for unresectable HCC. Drug-eluting bead TACE (DEB-TACE) uses nonabsorbable microspheres loaded with chemotherapy drug to embolize the target tumor, and this new device has shown encouraging results, with lower rates of systemic complications and greater efficacy in advanced stage or large tumors compared with conventional TACE (cTACE).4–6 Yet, it is often difficult to perform complete embolization for large hypervascular HCC in a single session because of the presence of abundant extrahepatic collateral arteries and dilated intratumoral vessels which pose a problem of insufficient beads. This may cause disease progression later owing to neoangiogenesis arising from the hypoxic environment of tumor blood vessels. Ethanol-Lipiodol mixture, a hybrid of bland embolization and chemical ablation, causes long-lasting occlusion of the arterioles and portal venules supplying the tumor.7,8 Lipiodol (ethiodized oil), which is selectively retained by HCC, may also act as a microembolic agent.
Herein, we demonstrated a combination therapy involving the administration of ethanol-Lipiodol mixture followed by DEB-TACE for treating large HCC. The clinical efficacy and adverse events were evaluated.
Materials and Methods
Study Population
This retrospective study was approved by the institutional review board of Kaohsiung Chang Gung Memorial Hospital (IRB No. 202201671B0). All patients provided written informed consent before treatment.
Between June 2016 and February 2020, we enrolled 27 patients. These patients had unresectable large HCC who received combined DEB-TACE as the initial treatment. The selection criteria were the presence of a single tumor >8 cm in size, or multiple tumors with the largest tumor diameter >5 cm and total tumor diameter >10 cm. Other eligibility criteria were as follows: Eastern Cooperative Oncology Group performance status of 0–1, no previous treatment for HCC, and well preserved hematologic and liver functions. Patients with lymph node and extrahepatic metastasis were excluded. Demographic, procedural, and follow-up data were collected. The diagnosis of HCC was based on the results of multiphase, contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI) with or without positive alpha-fetoprotein (AFP) (>20 ng/mL).
Pretreatment Investigations
Laboratory testing included blood count; biochemistry; coagulation; AFP assessment; hepatitis virus B and C serology; and serum levels of total bilirubin, alanine transaminase (ALT), aspartate transaminase (AST), and albumin. Moreover, HCC lesion number and size, and the presence of portal tumor thrombosis were recorded.9,10
Combined TACE Involving Ethanol-Lipiodol Mixture and DEB-TACE
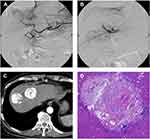
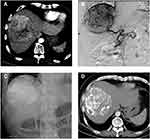
A 4-F RH catheter was inserted into the common hepatic or superior mesenteric artery to assess vascular anatomy, tumor location and extent, and the patency of portal flow. We super-selectively catheterized into the distal feeding artery using a 2.2-F microcatheter (Progreat, Terumo). Before embolization, 5 mL lidocaine (100 mg) diluted with 5 mL normal saline was given for pain control. Then, ethanol-Lipiodol mixture (1:2 ratio) was infused through the microcatheter. The dose was determined by diameter and vascular supply of targeted HCC, generally 9–18 mL, and no more than 27 mL once. Subsequently, the microcatheter was withdrawn to a more proximal location followed by slow injection of HepaSphere microspheres (30–60/50–100 µm; Merit Medical) applied as carriers to load doxorubicin or epirubicin, with a total of one vial for each patient. Gelfoam cubes were optionally administered for further embolization until sufficient arterial flow stasis was achieved (Figures 1 and 2).
After TACE, patients were transferred to the hospital ward for overnight observation and management of possible post embolization syndrome (PES) or other complications.
Follow-Up After Combined DEB-TACE
A follow-up CT/MRI was performed 4–6 weeks after the procedure and then every 3–6 months. The need for subsequent therapies was decided when residual tumor, tumor growth, or a new tumor was noted. Repeated TACE was continued until disease progression or intolerance. Patients would be recommended to undergo alternative treatments such as radiotherapy, Sorafenib, immunotherapy or supportive care. The modified Response Evaluation Criteria in Solid Tumors were adapted to evaluate the therapeutic response, which was classified into complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD), according to the European Association for the Study of the Liver criteria.11 Tumor measurements were performed by experienced abdominal radiologists. The final date of follow-up was August 31, 2022, based on which progression-free survival (PFS) and overall survival (OS) were calculated for prognostic analysis.
Statistical Analysis
Demographic and clinical features have been presented as either continuous numbers with means ± SDs or counts with proportions (percentages). Given the small sample size, Fisher’s exact test was applied to analyze tumor response between the subgroups. Survival rates were estimated using the Kaplan–Meier method. The Cox proportional hazards regression model was used to determine the factors associated with survival. Comparisons of pre- and post-treatment tumor size and liver function were evaluated by paired t-test. All statistics were analyzed using MedCalc Version 20.115 (MedCalc Software Ltd, Belgium), and two-sided P-values <0.05 were considered statistically significant.
Results
Baseline Clinical Characteristics
There were 27 patients (20 men and 7 women), with a mean age of 66.53 ± 9.55 years and age range of 45–79 years. A total of 18 patients (66%) were hepatitis B and/or C carriers. The number of patients in Child-Pugh class A was 23 and in class B was 4. About half of the patients (13/27, 48%) had HCC with the largest diameter >10 cm, and 11 patients (41%) had a single HCC. Portal vein tumor thrombosis (PVTT) was observed in 7 patients (26%), and it was graded Vp1 (n = 3) or Vp2 (n = 4). The number of TACE sessions ranged from 1 to 4. Post-progression therapies included radiotherapy, Sorafenib and immunotherapy. The data are presented in Table 1.
 |
Table 1 Patient Demographic and Clinical Characteristics |
Radiologic Tumor Response After Combined DEB-TACE
At 1 month after TACE, 4 patients (15%) showed CR, 11 (41%) showed PR, 6 (22%) showed SD, and 6 (22%) showed PD, as summarized in Table 2. Objective response (CR or PR) was achieved in 15 patients (54%). Figure 3 demonstrates the response of target lesions. There was significant reduction in viable tumor size after combined TACE (10.48 ± 3.34 vs 6.43 ± 5.67, p = 0.0001). There were no significant differences between the responder (CR+PR) and non-responder (SD+PD) groups in terms of BCLC stage B or C, maximum tumor size, tumor number, and presence of PVTT (Table 3).
 |
Table 2 Tumor Response of the 27 Patients 1 Month Following Combined DEB-TACE |
 |
Table 3 Subgroup Analysis of 1-Month Overall Response |
 |
Figure 3 Change in viable target lesion size after combined DEB-TACE. |
Overall Survival and Progression-Free Survival
During the follow-up period (0.7–72 months), 21 patients died and 6 remained alive, with a median OS of 15.9 months (95% CI, 9.03–34.76 months). Of these 6 patients who were alive, 3 underwent liver transplantation, 1 underwent right lobectomy, and 1 underwent left lobectomy. The OS rates at 6 months, 1 year, and 2 years after chemoembolization were 76.9%, 65.2%, and 44.8%, respectively. Moreover, the PFS rates at 6 months, 1 year, and 2 years were 64.0%, 46.9%, and 40%, respectively (Figure 4).
 |
Figure 4 Kaplan–Meier curves of (A) OS and (B) PFS in patients with unresectable HCC following combined DEB-TACE. |
Factors Associated with Patient Survival
AFP level >400 ng/mL (hazard ratio [HR] 4.52; 95% CI, 1.15–17.79) and maximum tumor size >10 cm (HR 5.07; 95% CI, 1.23–20.79) were potential risk factors for OS (Table 4). By contrast, neutrophil-to-lymphocyte ratio (NLR) >3 and PVTT were not related to survival in these TACE patients.
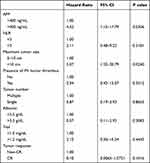 |
Table 4 Multivariate Cox Regression Analysis of Prognostic Factors for Overall Survival |
Complications
Post embolization syndrome, including fever, abdominal pain, and nausea/vomiting, developed in 22 of the 27 study patients (81.4%) and resolved without any treatment (Table 5). Chemoembolization-related major complications occurred in 1 patient (3.7%), with manifestation by transient hepatic encephalopathy. There was no in-hospital mortality. The albumin level and liver enzymes showed no significant changes at 1-month follow-up visit (Table 6).
 |
Table 5 Adverse Events and Complications |
 |
Table 6 Liver Function at Baseline and at Follow-Up Visit One Month After Embolization |
Discussion
Patients with a large HCC measuring ≥5 cm fall into the intermediate stage or stage B, according to the Barcelona Clinic Liver Cancer (BCLC) staging system, and are not amenable to curative treatment. TACE has been applied as a global standard of care for intermediate-stage HCC.12–15 In the current study, we introduced a new procedure using a combination of ethanol-Lipiodol mixture and DEB-TACE for large unresectable HCC. We enrolled BCLC-B and BCLC-C HCC patients who had a single tumor >8 cm or multiple tumors with the largest tumor diameter >5 cm and a total tumor diameter >10 cm. The combined DEB-TACE showed disease control rates of 78%, and the 1-year survival rate was 65.2%. Considering its safety and feasibility, it could be a preferred choice for large HCC control.
The therapeutic efficacy of transarterial ethanol ablation (TEA) with ethanol-Lipiodol mixture is determined by the embolization of tumor feeding arteries. In an animal experiment conducted by Kan et al, ethanol caused permanent embolization of both the hepatic artery and portal vein, and inhibited tumor growth by denaturing protein, causing vascular endothelial injury, and causing platelet cohesion in tumors, without promoting collateral circulation.4 Cheung et al, in a 100-patient review, reported that a solitary HCC with a maximum diameter over 5 cm and multiple HCCs had 1-year survival rates of 75% and 63%, respectively, after TEA.5 In a randomized controlled trial by Yu et al comparing the effectiveness of TEA and TACE, TEA was associated with better tumor response in terms of CR at 3 months (70% vs 51%, P = 0.012), 6 months (73% vs 54%, P = 0.012), and 12 months (75% vs 59%, P = 0.031), and longer PFS. More recently, a study focused on large HCCs ≥10 cm revealed that TEA had better tumor response and survival outcomes as compared to TACE or transarterial radioembolization (TARE).16,17 In our new combination TACE, we used ethanol-Lipiodol mixture as the first embolizer for delivering ethanol diffuse into the HCC to destroy tumor cells and to devascularize tumor tissue. Hepasphere DEB-TACE was added to create an ischemic environment while keeping the cytotoxic agents in the tumors with sustained and slow release. We aim for complete occlusion of the blood supplying arteries of the tumor for better therapeutic effect.
The median survival duration of our 27 study patients was 15.90 months. The 1-year and 2-year survival rates were 65.2% and 44.8%, respectively, which are quite similar to the rates reported by Wu et al in a TACE-sorafenib-thermal ablation group (68.2% and 40.9%, respectively).18 For HCCs measuring over 10 cm, Min et al compared survival after surgical resection and TACE, and the 1-year OS rates were 73.8% and 37.8%, respectively.19 Kim et al retrospectively evaluated 283 patients with large HCCs treated with TACE plus radiotherapy. The median OS duration with TACE plus radiotherapy was 15 months significantly better than that of TACE alone (8 months, p = 0.04).20 In our study, patients with complete control of large HCC (4/27, 15%) showed a longer survival, but the finding did not reach a statistical significance. Our results suggested that OS outcomes are highly dependent on subsequent aggressive surgical resection or liver transplantation. Similar results were seen in a retrospective analysis performed by Chen et al. Of the 110 patients with large/multifocal HCCs, patients who underwent TACE followed by resection were found to have improved OS compared to those who underwent TACE alone (OS duration: 47.00 ± 2.87 vs 20.00 ± 1.85 months), and the finding was statistically significant.21 Li et al also found that hepatic artery infusion chemotherapy more often downstaged patients to resection and ultimately prolonged overall survival compared with those treated with chemoembolization.22 This may be because HCC responsiveness to TACE indicates a favorable biology.23,24
Our multivariate analysis showed that AFP levels >400 ng/mL and maximum tumor size >10 cm were significant prognostic factors for patient survival, with HRs of 4.5279 (P = 0.0306) and 5.0751 (P = 0.0240), respectively. AFP has been widely adopted in the surveillance, diagnosis, and prognostic prediction of HCC, and a high AFP level is associated with a more aggressive behavior of HCC.25,26 Liu et al investigated patients with BCLC stage B treated with TACE and showed that median survival was better in AFP responders than in nonresponders (20 vs 12 months, P = 0.002).27 Unsurprisingly, large tumor size (>10 cm) yielded worse treatment response and limited survival benefit of TACE. In a 302 patient cohort, Kim et al concluded that the use of TACE for the treatment of single huge HCC (>10 cm) remains questionable.28 Another study on 821 BCLC B patients treated with TACE, Kim et al proposed a new subclassification pushing the tumoral limits to the up to 11 criteria.29 According to the survival results of the new B3 class (median survival 11.3 months), TACE is not recommended in this new class. So far, TACE for patients with large HCC (>10 cm) remains a challenge.
The NLR, an inflammation-based score, has been investigated as a prognostic indicator in post-therapeutic recurrence and survival of patients with HCC, but it was not found to be predictive of survival in our study.30 PVTT is associated with a high recurrence rate and worse prognosis. The median 1- and 3-year OS rates after surgery were reported to be 52.8% and 23.4%, respectively, for those with main portal or 1st branch involvement.31 Previously, TACE was commonly contraindicated in cases of HCC with portal vein invasion. There is emerging evidence that TACE is safe and improves the outcome of selected patients with unresectable HCC, provided the collateral blood circulation around the obstructed PVTT is sufficient, but further data are needed to demonstrate the influence on OS. Silva et al included 13 studies with 1933 TACE patients having PVTT, and reported a 1-year survival rate of 29%.31 In our study, PVTT was not a significant prognostic factor for survival.
The safety issue of TACE in treating patients with large HCCs is of utmost importance. In our study, up to 81.4% of patients experienced PES, which can be related to extensive tumor necrosis. A major complication was seen in 1 Child-Pugh class B patient (3.7%), and this rate is slightly higher than the reported 3% in previous studies involving patients with smaller HCCs. A recent study involving patients with large HCCs of Child-Pugh class A treated with TEA, TACE, and TARE showed comparable results of PES compared with our data, while no major adverse event was observed.17 We believe that appropriate patient selection, based on careful assessment of hepatic reserve, is a key factor to lower the incidence of treatment-related adverse events.
The limitations of this study are a small sample size, nonrandomized controls, and a single-center design.
Conclusion
With the advancements of technologies and innovations in HCC treatment, several trials are exploring combinations of loco-regional and systemic therapies, which need to be tailored with better algorithms to fit the patient’s stage. Our novel combined DEB-TACE provides excellent local control as well as survival benefit in patients with large unresectable HCC.
Ethics Approval and Informed Consent
Institutional Review Board (IRB) of Kaohsiung Chang Gung Memorial Hospital approved the study (IRB No. 202201671B0). All patients provided written informed consent before treatment, in compliance with the Declaration of Helsinki. The transplant and donation process conformed to the Declaration of Istanbul. All organs were donated voluntarily with written informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–1362. doi:10.1016/S0140-6736(22)01200-4
3. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
4. Kan Z, Wallace S. Transcatheter liver lobar ablation: an experimental trial in an animal model. Eur Radiol. 1997;7(7):1071–1075. doi:10.1007/s003300050256
5. Cheung YC, Ko SF, Ng SH, Chan SC, Cheng YF. Survival outcome of lobar or segmental transcatheter arterial embolization with ethanol-lipiodol mixture in treating hepatocellular carcinoma. World J Gastroenterol. 2005;11(18):2792–2795. doi:10.3748/wjg.v11.i18.2792
6. Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46(3):474–481. doi:10.1016/j.jhep.2006.10.020
7. Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(1):41–52. doi:10.1007/s00270-009-9711-7
8. Ray CE, Brown AC, Green TJ, et al. Survival outcomes in patients with advanced hepatocellular carcinoma treated with drug-eluting bead chemoembolization. AJR Am J Roentgenol. 2015;204(2):440–447. doi:10.2214/AJR.14.12844
9. Liver Cancer Study Group of Japan. The general rules for the clinical and pathological study of primary liver cancer. Jpn J Surg. 1989;19(1):98–129. doi:10.1007/BF02471576
10. Kudo M, Kitano M, Sakurai T, Nishida N. General rules for the clinical and pathological study of primary liver cancer, nationwide follow-up survey and clinical practice guidelines: the outstanding achievements of the Liver Cancer Study Group of Japan. Dig Dis. 2015;33(6):765–770. doi:10.1159/000439101
11. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
12. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma [published correction appears in J Hepatol. 2019 Apr;70(4):817]. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
13. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
14. Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, Phase 3 trial [published correction appears in Lancet Gastroenterol Hepatol. 2017 Sep;2(9):e6]. Lancet Gastroenterol Hepatol. 2017;2(8):565–575. doi:10.1016/S2468-1253(17)30156-5
15. Kudo M, Han G, Finn RS, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized Phase III trial. Hepatology. 2014;60(5):1697–1707. doi:10.1002/hep.27290
16. Yu SC, Hui JW, Hui EP, et al. Unresectable hepatocellular carcinoma: randomized controlled trial of transarterial ethanol ablation versus transcatheter arterial chemoembolization. Radiology. 2014;270(2):607–620. doi:10.1148/radiol.13130498
17. Yu SCH, Hui JW, Li L, et al. Comparison of Chemoembolization, Radioembolization, and Transarterial Ethanol Ablation for Huge Hepatocellular Carcinoma (≥ 10 cm) in Tumour Response and Long-Term Survival Outcome. Cardiovasc Intervent Radiol. 2022;45(2):172–181. doi:10.1007/s00270-021-02777-6
18. Wu Y, Qi H, Cao F, et al. TACE-sorafenib with thermal ablation has survival benefits in patients with huge unresectable hepatocellular carcinoma. Front Pharmacol. 2020;11:1130. doi:10.3389/fphar.2020.01130
19. Min YW, Lee JH, Gwak GY, et al. Long-term survival after surgical resection for huge hepatocellular carcinoma: comparison with transarterial chemoembolization after propensity score matching. J Gastroenterol Hepatol. 2014;29(5):1043–1048. doi:10.1111/jgh.12504
20. Kim KH, Kim MS, Chang JS, Han KH, Kim DY, Seong J. Therapeutic benefit of radiotherapy in huge (≥10 cm) unresectable hepatocellular carcinoma. Liver Int. 2014;34(5):784–794. doi:10.1111/liv.12436
21. Chen J, Lai L, Lin Q, et al. Hepatic resection after transarterial chemoembolization increases overall survival in large/multifocal hepatocellular carcinoma: a retrospective cohort study. Oncotarget. 2017;8(1):408–417. doi:10.18632/oncotarget.13427
22. Li QJ, He MK, Chen HW, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized Phase III trial. J Clin Oncol. 2022;40(2):150–160. doi:10.1200/JCO.21.00608
23. Sandow TA, Arndt SE, Albar AA, et al. Assessment of response to transcatheter arterial chemoembolization with doxorubicin-eluting microspheres: tumor biology and hepatocellular carcinoma recurrence in a 5-year transplant cohort. Radiology. 2018;286(3):1072–1083. doi:10.1148/radiol.2017170731
24. Tabrizian P, Holzner ML, Mehta N, et al. Ten-year outcomes of liver transplant and downstaging for hepatocellular carcinoma. JAMA Surg. 2022;157(9):779–788. doi:10.1001/jamasurg.2022.2800
25. Galle PR, Foerster F, Kudo M, et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39(12):2214–2229. doi:10.1111/liv.14223
26. Ma WJ, Wang HY, Teng LS. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J Surg Oncol. 2013;11:212. doi:10.1186/1477-7819-11-212
27. Liu G, Ouyang Q, Xia F, et al. Alpha-fetoprotein response following transarterial chemoembolization indicates improved survival for intermediate-stage hepatocellular carcinoma. HPB. 2019;21(1):107–113. doi:10.1016/j.hpb.2018.06.1800
28. Kim GH, Kim JH, Shim JH, et al. Chemoembolization for single large hepatocellular carcinoma with preserved liver function: analysis of factors predicting clinical outcomes in a 302 patient cohort. Life. 2021;11(8):840. doi:10.3390/life11080840
29. Kim JH, Shim JH, Lee HC, et al. New intermediate-stage subclassification for patients with hepatocellular carcinoma treated with transarterial chemoembolization. Liver Int. 2017;37(12):1861–1868. doi:10.1111/liv.13487
30. Mouchli M, Reddy S, Gerrard M, Boardman L, Rubio M. Usefulness of neutrophil-to-lymphocyte ratio (NLR) as a prognostic predictor after treatment of hepatocellular carcinoma”. Review article. Ann Hepatol. 2021;22:100249. doi:10.1016/j.aohep.2020.08.067
31. Cerrito L, Annicchiarico BE, Iezzi R, Gasbarrini A, Pompili M, Ponziani FR. Treatment of hepatocellular carcinoma in patients with portal vein tumor thrombosis: beyond the known frontiers. World J Gastroenterol. 2019;25(31):4360–4382. doi:10.3748/wjg.v25.i31.4360
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.