Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Efficacy and Safety of Bisoprolol in Patients with Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis
Authors Feng Z , Zhang L, Wang Y, Guo H, Liu J
Received 18 September 2023
Accepted for publication 15 December 2023
Published 23 December 2023 Volume 2023:18 Pages 3067—3083
DOI https://doi.org/10.2147/COPD.S438930
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Zhouzhou Feng,1 Lu Zhang,1 Yaqin Wang,1 Hong Guo,1 Jian Liu1,2
1The First Clinical Medical College of Lanzhou University, Lanzhou City, People’s Republic of China; 2Gansu Maternal and Child Health Hospital/Gansu Central Hospital, Lanzhou City, People’s Republic of China
Correspondence: Jian Liu, The First Clinical Medical College of Lanzhou University, Lanzhou City, 730000, People’s Republic of China, Tel +86 136 0935 4197, Email [email protected]
Purpose: To evaluate the clinical efficacy and safety of bisoprolol in patients with chronic obstructive pulmonary disease (COPD).
Research Methods: This systematic review and meta-analysis was conducted following the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) statements. The primary outcome measures analyzed included: Pulmonary function(FEV1, FEV1%, FVC), 6-minute walking distance (6MWD), adverse events and inflammatory cytokines(IL-6, IL-8, CRP).
Results: Thirty-five studies were included with a total of 3269 study participants, including 1650 in the bisoprolol group and 1619 in the control group. The effect of bisoprolol on lung function in patients with COPD, FEV1, MD (0.46 [95% CI, 0.27 to 0.65], P=0.000), FEV1%, MD (− 0.64 [95% CI, 0.42 to 0.86], P=0.000), FVC, MD (0.20 [95% CI, 0.05 to 0.34], P=0.008), the results all showed a statistically significant result. The effect of bisoprolol on 6MWD in COPD patients, MD (1.37 [95% CI, 1.08 to 1.66], P=0.000), which showed a statistically significant result. The occurrence of adverse events in COPD patients treated with bisoprolol, RR (0.83 [95% CI, 0.54 to 1.26], P=0.382), resulted in no statistical significance. The effect of bisoprolol on inflammatory cytokines in COPD patients, IL-6, MD (− 1.16 [95% CI, − 1.67 to − 0.65], P=0.000), IL-8, MD (− 0.94 [95% CI, − 1.32 to − 0.56], P=0.000), CRP, MD (− 1.74 [95% CI, − 2.40 to − 1.09], P=0.000), the results were statistically significant. We performed a subgroup analysis of each outcome indicator according to whether the patients had heart failure or not, and the results showed that the therapeutic effect of bisoprolol on COPD did not change with the presence or absence of heart failure.
Conclusion: Bisoprolol is safe and effective in the treatment of COPD, improving lung function and exercise performance in patients with COPD, and also reducing inflammatory markers in patients with COPD, and this effect is independent of the presence or absence of heart failure.
Keywords: chronic obstructive pulmonary disease, bisoprolol, beta-blockers, meta-analysis
Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic disease characterized by persistent airflow limitation.1 Because of the exponential increase in morbidity and mortality, COPD affects approximately 384 million people worldwide and is considered the third leading cause of death worldwide.2 The pathogenesis of COPD may be related to smoking, endothelial dysfunction, abnormalities in airway and alveolar structure and function, inflammation, and genetic factors.3 It is well known that patients with chronic obstructive pulmonary disease (COPD) are prone to hypoxic pulmonary vasoconstriction, which may lead to pulmonary hypertension and pulmonary vascular remodeling, and ultimately right ventricular dilatation, resulting in right ventricular failure, which is the main cause of clinical deterioration and death.4 In addition to right ventricular changes, up to 30% of patients with COPD suffer from systolic or diastolic left heart failure.5 Notably, COPD exacerbations are now recognized as heterogeneous events with different underlying pathogenic mechanisms, including cardiac decompensation and thromboembolic events.6,7 Up to one-third of deaths in patients with COPD can be attributed to cardiovascular disease, and it has been reported that the risk of cardiovascular death increases by 28% for every 10% reduction in FEV1.8 Therefore, active prevention and treatment of exacerbations and concomitant cardiovascular disease in patients with COPD appears to be of paramount importance.
Bisoprolol is a type of selective beta-blockers (BBs) that is commonly used in the treatment of patients with cardiovascular diseases such as moderate or severe chronic stable heart failure, coronary artery disease and hypertension.9–11 Studies have shown that the use of cardioselective beta blockers in patients with COPD has no significant adverse effects on FEV1, respiratory symptoms, or responsiveness to beta agonist inhalation therapy.12 Several studies have shown that beta blockers reduce mortality by 28% and acute exacerbations by 37% in patients with COPD.13–15 BBs may have a positive impact on COPD patients with cardiovascular disease (CVD) and even on those without CVD.16,17, Additional studies suggest that selective β1-blockers, in particular bisoprolol as first choice, should be preferred in case of clinical and/or radiological signs of lung fluid accumulation.18 Despite clear evidence of the efficiency of BBs, there is confusion due to contraindications and fear of inducing adverse effects and bronchospasm, as well as the difficulty in determining whether the benefits of the drug are related to coexisting cardiovascular disease.19,20 There is a general hesitation to use BBs in patients with COPD,21 but if not, they may lead to an increase in cardiovascular events in high-risk patients.22 The results of a recent meta-analysis suggest that the use of cardioselective BBs in patients with COPD not only reduces their all-cause and in-hospital mortality rates, but even reduces the exacerbation of COPD.23 The use of BBs and the optimal treatment regimen for BBs remains controversial despite the overwhelming evidence of their benefit in patients with COPD. And to the best of our knowledge, no review has been conducted to separately investigate the clinical efficacy and safety of bisoprolol in patients with COPD. In order to provide new evidence-based medical evidence for clinical treatment, the present study was the first to apply Meta-analysis to analyze the efficacy and safety of bisoprolol in COPD patients in combination.
Methods
This systematic review and meta-analysis was registered at PROSPERO (http://www.crd.york.ac.uk/prospero; CRD: 42023411032).The study was designed as per the Cochrane Handbook for Systematic Reviews of Interventions24 and reported according to the PRISMA guidelines.25
Search databases: PubMed, Web of science, Cochrane, CNKI and Wan Fang library databases. The date of searching the database was from the date of construction to June 10, 2023. The search combined subject and free terms: Pulmonary Disease, Chronic Obstructive, Bisoprolol. See Supplementary Data S1 for detailed search strategy.
Literature Inclusion Exclusion Criteria
Inclusion criteria were as follows: (1) adults ≥40 years of age (2) meeting the diagnostic criteria for COPD (3) bisoprolol in the experimental group and placebo or conventional treatment in the control group (4) RCT studies (5) at least one data outcome available for extraction, (6) Chinese or English literature.
Exclusion criteria were as follows: (1) studies that researched animals or cells (2) studies that are conference abstracts, letters, editorials, reviews, and meta-analyses (3) incomplete or unavailable data for extraction.
Data Extraction and Quality Assessment
Screening literature, We imported the retrieved literature into the ENDNOTE software, and two researchers independently reviewed the retrieved documents. Based on the inclusion and exclusion criteria, the documents were initially screened by reading the titles and abstracts, and then further screened by reading the full text. For trials that met the inclusion criteria, we extracted basic information from the articles, such as last name of the first author, year of publication, type of participant, sample size, drug dosage, control, outcome and duration of follow-up. Quality assessment was performed using the Cochrane risk of bias tool to assess the quality of the RCTs, including randomized sequence generation, allocation concealment, blinding of patients and interveners, blinding of outcome measures, incomplete outcome data, selective reporting, and other potential biases. Each item was rated as “low risk”, “high risk” or “unclear”. Any disagreements were resolved through discussion and arbitration in consultation with a third author.
Data Analysis
Statistical analyses were performed using REVMAN 4.5 and STATA MP 17.0, binary variables were represented by risks ratio (RR), and continuous variables were represented by mean difference (MD), and all effect sizes were expressed as 95% confidence intervals (CI). Heterogeneity between the results of the included studies was analyzed using the I2 test. When the test of heterogeneity was P ≥ 0.05 and I2 < 50%, it was considered that multiple similar studies were homogeneous, and a fixed-effects model was used. Otherwise, a random-effects model was used. If the number of studies for quantitative analysis was more than 10, potential publication bias was assessed using funnel plots, Begg’s test and Egger’s test. Sensitivity analyses were performed by conducting each meta-analysis, excluding each study in turn. Sensitivity indicated the stability of the results.
Results
Study Retrieved and Characteristics
Initially, 299 related studies were examined, and after reading the titles and abstracts of the studies, they were screened according to the inclusion and exclusion criteria, and finally 35 qualified clinical studies were included,26–60 with a total of 3269 subjects, including 1650 subjects in the bisoprolol group and 1619 subjects in the control group. The screening process is shown in Figure 1, and the basic characteristics of the included studies are shown in Table 1.
 |
Table 1 Characteristics of All Studies Included in Meta-Analysis |
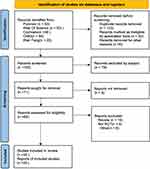 |
Figure 1 PRISMA (preferred reporting items for systematic reviews and meta-analysis) flow diagram. Notes: PRISMA figure adapted from Page MJ, Moher D, Bossuyt PM et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372. Creative Commons.27 |
Methodological Quality Evaluation
Of the 35 included RCT studies, 3 were in English and the rest were in Chinese.The method of generating randomized sequences was explicitly mentioned in 12 studies, of which 2 were blinded. Conventional treatment was used as the control group in 24 studies, phlegm chemotherapy tablets were given to the control group in 9 studies, placebo was given to the control group in 2 studies, and only conventional oxygen treatment was given to the control group in 1 study.No other risk of bias was identified in the results of any of the studies.Details are shown in Supplementary Figure S1.
Primary Outcome
FEV1
Twenty-two studies26,27,29–32,34,37–39,42,43,46,47,49,51,52,55,57–60 reported FEV1, with a heterogeneity test result of I2=78.2%, P=0.000, and using a random-effects model, the results showed a statistically significant difference in MD (0.46 [95% CI, 0.27 to 0.65], P=0.000). Nine of the studies26,29,31,38,39,47,52,59,60 included patients with COPD with heart failure, and 13 studies27,30,32,34,37,42,43,46,49,51,55,57,58 included only patients with COPD alone, with which we performed subgroup analyses, and the results showed a statistically significant difference in MD (0.32 [95% CI, 0.07 to 0.56], P=0.011) in the COPD with heart failure group. MD in the COPD alone group (0.57 [95% CI, 0.29 to 0.85], P=0.000), a statistically significant result (Figure 2). It suggests that bisoprolol significantly improved patients’ FEV1 in patients with COPD, and this change was independent of the presence or absence of heart failure. Despite the large heterogeneity of the results, we performed a sensitivity analysis and excluding any of the studies, the experimental results were unchanged, suggesting that the results were stable (Supplementary Figure S2.1). Meanwhile, we analyzed the results by qualitatively analyzing the funnel plot (Figure 3) and quantitatively analyzing Begg’s test (p=0.430, detailed figure in Supplementary Figure S2.2) and Egger’s test (p=0.264, detailed figure in Supplementary Figure S2.3), the results showed no publication bias.
 |
Figure 2 Forest plot of FEV1, subgroup analysis was performed according to variable of heart failure and no heart failure. |
 |
Figure 3 Funnel plot of FEV1. |
Secondary Outcomes
FEV1%
Fourteen studies28,33–36,40,42–45,47–49,54 reported FEV1%, with a heterogeneity test result of I2=72.6%, P=0.000, and using a random-effects model, the results showed an MD (−0.64 [95% CI, 0.42 to 0.86], P=0.000), which showed a statistically significant difference. Nine of the studies28,33,35,36,44,45,47,48,54 included patients with COPD with heart failure, and 5 studies34,40,42,43,49 included only patients with COPD alone, and we used this as a subgroup, which showed a statistically significant result for the MD (−0.70 [95% CI, 0.33 to 1.06], P=0.000) in the COPD with heart failure group. MD (0.54 [95% CI, 0.37 to 0.70], P=0.000) in the COPD alone group, with a statistically significant result (Figure 4). It suggests that bisoprolol significantly improved patients’ FEV1% in patients with COPD, and this change was independent of the presence or absence of heart failure. However, there was a great deal of heterogeneity in the results, and according to the sensitivity analysis, excluding any of the studies, the experimental results were unchanged, suggesting that the results were stable (Supplementary Figure S3.1). Meanwhile, we analyzed the results by qualitatively analyzing the funnel plot (Figure 5) and quantitatively analyzing Begg’s test (p=0.661, detailed figure in Supplementary Figure S3.2) and Egger’s test (p=0.917, detailed figure in Supplementary Figure S3.3), which showed no publication bias.
 |
Figure 4 Forest plot of FEV1%, subgroup analysis was performed according to variable of heart failure and no heart failure. |
 |
Figure 5 Funnel plot of FEV1%. |
FVC
Ten studies26,27,29–31,34,38,39,52,58 reported FVC, with a heterogeneity test result of I2=84.4%, P=0.000, and using a random-effects model, the results showed a statistically significant difference in MD (0.20 [95% CI, 0.05 to 0.34], P=0.008). Six of the studies26,29,31,38,39,52 included patients with COPD with heart failure, and 4 studies27,30,34,58 included only patients with COPD alone, we used this as a subgroup for our statistics, which showed a statistically significant result for the MD (0.10 [95% CI, 0.02 to 0.19], P=0.018) in the COPD with heart failure group. MD (0.46 [95% CI, 0.29 to 0.64], P=0.000) in the COPD alone group, with a statistically significant result (Figure 6). It suggests that bisoprolol improved patients’ FVC in COPD patients, and the change was not related to the presence or absence of heart failure. According to the sensitivity analysis, excluding any of the studies, the experimental results were unchanged, suggesting that the results were stable (Supplementary Figure S4.1). Meanwhile, we analyzed the results by qualitatively analyzing the funnel plot (Figure 7) and quantitatively analyzing Begg’s test (p=0.721, detailed figure in Supplementary Figure S4.2)) and Egger’s test (p=0.740, detailed figure in Supplementary Figure S4.3), the results indicate that there is no publication bias.
 |
Figure 6 Forest plot of FVC, subgroup analysis was performed according to variable of heart failure and no heart failure. |
 |
Figure 7 Funnel plot of FVC. |
6MWD
Six studies33,40,41,43,52,54 reported the results of 6MWD, with a test of heterogeneity of I2=66.0%, P=0.012, and using a random-effects model, the results showed a statistically significant result of MD (1.37 [95% CI, 1.08 to 1.66], P=0.000), the result was statistically significant. Three of the studies33,52,54 included patients with COPD with heart failure, and three studies40,41,43 included only patients with COPD alone, and we performed statistics with this subgroup, which showed a statistically significant result with MD (1.05 [95% CI, 0.80 to 1.30], P=0.000) in the COPD with heart failure group. MD (1.65 [95% CI, 1.43 to 1.87], P=0.000) in the COPD alone group, with statistically significant results (Figure 8). It suggests that bisoprolol significantly improves motor function in patients with COPD, and this change is independent of the presence or absence of heart failure. According to the sensitivity analysis, excluding any of the studies, the experimental results were unchanged, suggesting that the results were stable (Supplementary Figure S5).
 |
Figure 8 Forest plot of 6-minute walking distance (6MWD), subgroup analysis was performed according to variable of heart failure and no heart failure. |
Adverse Events
Fourteen studies26–28,30–32,35,36,43,44,49,50,53,56 reported adverse events, with a heterogeneity test result of I2=24.9%, P=0.207, and using a fixed-effects model, the results showed an RR (0.83 [95% CI, 0.54 to 1.26], P=0.382), which showed no statistically significant difference. Eight of the studies26,28,31,35,36,44,53,56 included patients with COPD with heart failure, and 6 studies27,30,32,43,49,50 included only patients with COPD alone, and we modeled this as a subgroup, which showed RR (0.77 [95% CI, 0.47 to 1.27], P=0.315) in the COPD with heart failure group, which showed no statistically significant results. RR in the COPD alone group (0.97 [95% CI, 0.44 to 2.13], P=0.944), the result was not statistically significant (Figure 9). It suggests that the use of bisoprolol to treat COPD patients is safe and reliable. Sensitivity analysis suggested that the results were stable (Supplementary Figure S6).
 |
Figure 9 Forest plot of adverse events, subgroup analysis was performed according to variable of heart failure and no heart failure. |
IL-6
Six studies29,36,37,42,43,49 reported results for IL-6, with a heterogeneity test result of I2=90.0%, P=0.000, and using a random-effects model, the results showed a statistically significant result for MD (−1.16 [95% CI, −1.67 to −0.65], P=0.000), results. Two of the studies29,36 included patients with COPD with heart failure, and 4 studies37,42,43,49 included only patients with COPD alone, and we used this as a subgroup for our statistics, which showed a statistically significant result for the MD (−1.93 [95% CI, −3.31 to −0.56], P=0.006) in the COPD with heart failure group. MD (−0.78 [95% CI, −0.96 to −0.60], P=0.000) in the COPD alone group, with statistically significant results (Figure 10). It suggests that bisoprolol significantly reduced the inflammatory marker IL-6 in COPD patients, and this change was not related to the presence or absence of heart failure. According to the sensitivity analysis, there was a large heterogeneity in the results of Yang2017, and the exclusion of that study left the experimental results unchanged, suggesting that the study might be a source of heterogeneity but had little effect on the final experimental results (Supplementary Figure S7).
 |
Figure 10 Forest plot of IL-6, subgroup analysis was performed according to variable of heart failure and no heart failure. |
IL-8
Six studies29,36,37,42,43,49 reported the results of IL-8, with a heterogeneity test result of I2=83.1%, P=0.000, and using a random-effects model, the results showed a statistically significant result of MD (−0.94 [95% CI, −1.32 to −0.56], P=0.000), results. Two of the studies29,36 included patients with COPD with heart failure, and 4 studies37,42,43,49 included only patients with COPD alone, and we used this as a subgroup for our statistics, which showed a statistically significant result for the MD (−1.43 [95% CI, −1.74 to −1.13], P=0.000) in the COPD with heart failure group, MD (−0.71 [95% CI, −1.10 to −0.32], P=0.000) in the COPD alone group, with statistically significant results (Figure 11). It suggests that bisoprolol significantly reduced the inflammatory marker IL-8 in patients with COPD, and this change was not associated with the presence or absence of heart failure. According to the sensitivity analysis, excluding any of the studies, the experimental results were unchanged, suggesting that the results were stable (Supplementary Figure S8).
 |
Figure 11 Forest plot of IL-8, subgroup analysis was performed according to variable of heart failure and no heart failure. |
CRP
Ten studies27,29,36,37,41,43,46,50,51,55 reported the results of CRP, and the test of heterogeneity resulted in a statistically significant result of I2=95.5%, P=0.000, using a random-effects model, which showed an MD (−1.74 [95% CI, −2.40 to −1.09], P=0.000), result. Two of the studies27,36 included patients with COPD with heart failure, and 8 studies27,37,41,43,46,50,51,55 included only patients with COPD alone, and we used this as a subgroup for our statistics, which showed a statistically significant result for the MD (−1.53 [95% CI, −1.84 to −1.22], P=0.000) in the COPD with heart failure group, MD (−1.80 [95% CI, −2.63 to −0.97], P=0.000) in the COPD alone group, with statistically significant results (Figure 12). It indicated that bisoprolol significantly reduced CRP, an indicator of inflammation, in patients with COPD, and this change was not related to the presence or absence of heart failure. According to the sensitivity analysis, there was a large heterogeneity in the results of Yang2016, and the experimental results were unchanged by excluding that study, suggesting that the study might be a source of heterogeneity but had little effect on the final experimental results (Supplementary Figure S9.1). Meanwhile, we analyzed the results by qualitatively analyzing the funnel plot (Figure 13) and quantitatively analyzing the Begg’s test (p=0.107, see Supplementary Figure S9.2 for detailed figure)) and Egger’s test (p=0.114, see Supplementary Figure S9.3), the results indicate that there is no publication bias.
 |
Figure 12 Forest plot of CRP, subgroup analysis was performed according to variable of heart failure and no heart failure. |
 |
Figure 13 Funnel plot of CRP. |
Discussion
The mechanistic link between chronic obstructive pulmonary disease (COPD) and cardiovascular disease (CVD) is complex, multifactorial, and not fully understood. Patients with COPD often suffer from hypoxemia and hypercapnia, and when the patient’s blood carbon dioxide concentration is too high, it stimulates the chemoreceptors in the body, causing sympathetic excitation, accelerated heart rate, and increased oxygen consumption of the myocardium, which aggravates the patient’s symptoms of chest tightness and shortness of breath.61,62 COPD patients with limited motor function and reduced cardiac functional reserve significantly increase the risk of cardiovascular events in patients. It has been shown that cardiovascular mortality is reduced in COPD patients with heart rate <75 bpm compared to those with heart rate ≥75, and that elevated resting heart rate severely affects their prognosis and has emerged as an independent risk factor for their cardiovascular events.63 Bisoprolol is a highly selective β1-AR blocker with a selectivity for β1/β2 receptors of approximately 14:1,64 preventing the effects of the endogenous catecholamines epinephrine and norepinephrine. It can have an inhibitory effect on the sympathetic nerves of the body, which can effectively reduce the heart rate of the patients, thus improving their left ventricular compliance, decreasing myocardial contractility and cardiac afterload, decreasing myocardial oxygen consumption and preventing ventricular remodeling, which leads to an improvement in the patients’ blood rheology. At the same time, the regulation of cellular immunity is enhanced, and studies have shown that long-term administration of BBs can reduce inflammation and pulmonary mucus secretion, can inhibit neutrophil chemotaxis and oxygen free radical production, reduce the cuprocyte and mucin content of airway epithelium, reduce cytokine levels, and reduce the release of endothelin-1 in human endothelial cells with bronchoconstrictor effects to slow down the deterioration of COPD.65–68 At the same time, BBs reduce airway hyperresponsiveness, and long-term use of BBs also modulates β2-AR levels in the lungs, thereby improving the effects of bronchodilators in mice, probably because β1 blockers can play a complementary role by sensitizing β2 receptors to β2 agonists.69,70 In conclusion, treatment of COPD patients with BBs not only benefits from improved blood rheology, but may also produce beneficial effects through anti-inflammatory activity and bronchoprotection.
Our meta-analysis showed that bisoprolol significantly improved lung function (FEV1, FEV1%, FVC) and exercise performance (6WMD) in patients with COPD, and the improvement was independent of whether or not there was concomitant cardiac disease, suggesting that the efficacy of bisoprolol in the treatment of patients with COPD not only benefited from the improvement in cardiac function, but also was beneficial for COPD itself. This may benefit from the multiple actions of bisoprolol, which not only improves cardiac function and reduces cardiac load and oxygen consumption in COPD patients, but also reduces airway hyperresponsiveness in COPD patients and improves the efficacy of bronchodilators, which relieves airflow obstruction. In the analysis of inflammatory factors, bisoprolol can significantly reduce IL-6, IL-8, CRP in COPD patients, and this efficacy is also independent of whether or not it is accompanied by cardiac disease, probably because bisoprolol itself has certain anti-inflammatory effects, reduces the secretion of pulmonary mucus, and can inhibit the neutrophil chemotaxis and the production of oxygen free radicals, and reduces the content of cuprocytes and mucins in the airway epithelium and lowers the level of cytokine levels.65–68 In the safety analysis, there was no significant difference between the use of bisoprolol and no bisoprolol, suggesting that bisoprolol is safe and reliable for patients with COPD. Despite the great heterogeneity of most of the outcome indicators, through our analysis of the data, we still believe that the results have a certain stability and reliability. At the same time, by carefully reading and comparing the original studies and reviewing the relevant literature, we found that there are still some difficulties in the diagnosis and treatment of COPD. According to the GOLD reporting criteria, a total of 22.7% of patients hospitalized and treated for COPD had no evidence of COPD. Some studies recommend the use of the individual lower limit of normal (LLN), instead of the GOLD classification, to define COPD and prevent overdiagnosis of COPD.71,72 Heart failure and chronic obstructive lung disease share common risk factors and pathophysiologic mechanisms, and HF itself causes a reduction in FEV1 and FVC of about 20%, therefore, sometimes, it is impossible to determine whether these are COPD, HF, or both.73 The studies we included were all conducted with the addition of bisoprolol to conventional therapy, which is administered as needed and has a great deal of heterogeneity, including treatments such as cardiotonic, diuretic, vasodilator, phlegmolytic, and bronchodilator agents, and even the use of antibiotics. And the use of these drugs and their interactions may have an unexpected impact on the experimental results.
In conclusion, the available evidence suggests that bisoprolol is safe and effective for the treatment of patients with COPD, and that this efficacy is not only effective in patients with COPD with heart failure, but also in patients without heart failure, and that the early use of bisoprolol may have a positive prognostic impact in patients with COPD. Two RCTs evaluating the effect of bisoprolol on the rate of deterioration and prevention of cardiovascular events in patients with COPD are ongoing (ISRCTN10497306, NCT03917914), and more evidence of bisoprolol for COPD will be provided in the future.
Limitations
This study provides a comprehensive meta-analysis of bisoprolol in the treatment of COPD, which is clinically instructive, but there are still some limitations, including: 1. Although we only included RCT studies, the quality of all the included literature varied, and the overall quality was not very high. 2. All the literature was not blinded, probably because the pharmacological effects of bisoprolol significantly reduced the heart rate of the patients. There is some difficulty in adopting the blinding method. 3. Most of the included literature was from China, and there may be regional differences in the population.
Conclusion
Based on the available evidence, the results of our meta-analysis showed that bisoprolol significantly improved pulmonary function, motor function, and inflammatory factors in COPD patients, and the improvement was independent of the presence or absence of concomitant cardiac disease, suggesting that bisoprolol is effective and safe for COPD patients.
Acknowledgments
The authors gratefully acknowledge all the authors who shared the results of the included studies, the support of the Lanzhou University First Affiliated Hospital, (Lanzhou, China), and the First Clinical Medical College of Lanzhou University.
Disclosure
There are no other conflicts of interest in this work.
References
1. Vogelmeier CF, Criner GJ, Martinez FJ. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
2. Augusti A, Beasley R, Celli BR, et al. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnoses, management, and prevention of COPD. 2020. Available from: http://www.goldcopd.org.
3. Kovacs G, Agusti A, Barberà JA, et al. Pulmonary Vascular Involvement in Chronic Obstructive Pulmonary Disease. Is There a Pulmonary Vascular Phenotype? Am J Respir Crit Care Med. 2018;198(8):1000–1011. doi:10.1164/rccm.201801-0095PP
4. Voelkel NF, Natarajan R, Drake JI, Bogaard HJ. Right ventricle in pulmonary hypertension. Compr Physiol. 2011;1(1):525–540. doi:10.1002/cphy.c090008
5. Andrea R, López-Giraldo A, Falces C, et al. Lung function abnormalities are highly frequent in patients with heart failure and preserved ejection fraction. Heart Lung Circ. 2014;23(3):273–279. doi:10.1016/j.hlc.2013.08.003
6. Beghé B, Verduri A, Roca M, Fabbri LM. Exacerbation of respiratory symptoms in COPD patients may not be exacerbations of COPD. Eur Respir J. 2013;41(4):993–995. doi:10.1183/09031936.00180812
7. Roca M, Verduri A, Corbetta L, Clini E, Fabbri LM, Beghé B. Mechanisms of acute exacerbation of respiratory symptoms in chronic obstructive pulmonary disease. Eur J Clin Invest. 2013;43(5):510–521. doi:10.1111/eci.12064
8. Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–969. doi:10.1183/09031936.00012408
9. Sharma S, Saluja M, Sharma P. Effectiveness of Convalescent Plasma Therapy in Moderate to Severely Ill Covid-19 Patients. J Assoc Physicians India. 2022;70(4):11–12.
10. Bazroon AA, Alrashidi NF. Bisoprolol. Treasure Island (FL): StatPearls Publishing; 2023.
11. Tsutsui H, Momomura S-I, Masuyama T, et al.; CIBIS-J Investigators. Tolerability, Efficacy, and Safety of Bisoprolol vs. Carvedilol in Japanese Patients With Heart Failure and Reduced Ejection Fraction ― The CIBIS-J Trial ―. Circ J. 2019;83(6):1269–1277. doi:10.1253/circj.CJ-18-1199
12. Salpeter SR, Ormiston TM, Salpeter EE. Cardioselective β-Blockers in Patients with Reactive Airway Disease. Ann Intern Med. 2002;137(9):715–725. doi:10.7326/0003-4819-137-9-200211050-00035
13. Etminan M, Jafari S, Carleton B, FitzGerald JM. Beta-blocker use and COPD mortality: a systematic review and meta-analysis. BMC Pulm Med. 2012;12(1):48. doi:10.1186/1471-2466-12-48
14. Du Q, Sun Y, Ding N, Lu L, Chen Y, Chalmers JD. Beta-blockers reduced the risk of mortality and exacerbation in patients with COPD: a meta-analysis of observational studies. PLoS One. 2014;9(11):e113048. doi:10.1371/journal.pone.0113048
15. Kubota Y, Asai K, Furuse E, et al. Impact of β-blocker selectivity on long-term outcomes in congestive heart failure patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:515–523. doi:10.2147/COPD.S79942
16. Zvizdic F, Begic E, Mujakovic A, et al.Beta-blocker use in moderate and severe chronic obstructive pulmonary Disease. Med arch. 2019;73(2):72–75. doi:10.5455/medarh.2019.73.72-75.
17. Salpeter S, Ormiston T, Salpeter E. Cardioselective beta-blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;2005(4):Cd003566. doi:10.1002/14651858.CD003566.pub2
18. Paolillo S, Dell’Aversana S, Esposito I, Poccia A, Perrone Filardi P. The use of β-blockers in patients with heart failure and comorbidities: doubts, certainties and unsolved issues. Eur J Intern Med. 2021;88:9–14. doi:10.1016/j.ejim.2021.03.035
19. Rutten FH. β-Blockers May Reduce Mortality and Risk of Exacerbations in Patients With Chronic Obstructive Pulmonary Disease. Arch Intern Med. 2010;170(10):880–887. doi:10.1001/archinternmed.2010.112
20. Lim KP, Loughrey S, Musk M, Lavender M and Wrobel J. (2017). Beta-blocker under-use in COPD patients. COPD. 2017;12:3041–3046. doi:10.2147/COPD.S144333
21. Albouaini K, Andron M, Alahmar A, Egred M. Beta-blockers use in patients with chronic obstructive pulmonary disease and concomitant cardiovascular conditions. Int J Chron Obstruct Pulmon Dis. 2007;2(4):535–540.
22. Chatila WM, Thomashow BM, Minai OA, Criner GJ, Make BJ. Comorbidities in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):549–555. doi:10.1513/pats.200709-148ET
23. Yang YL, Xiang ZJ, Yang JH, Wang WJ, Xu ZC, Xiang RL. Association of β-blocker use with survival and pulmonary function in patients with chronic obstructive pulmonary and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J. 2020;41(46):4415–4422. doi:10.1093/eurheartj/ehaa793
24. Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions.
25. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:160. doi:10.1136/bmj.n160
26. Lv Z, Chen F. The efficacy and safety of bisoprolol in the treatment of chronic obstructive pulmonary disease, pulmonary heart disease, and heart failure patients. Chinese J Clin Ration Drug Use. 2022;15(25):9–12+7.
27. Wu Y. Observation on the efficacy of bisoprolol in the treatment of chronic obstructive pulmonary disease with pulmonary infection. Chinese J Clin Ration Drug Use. 2020;13(05):49–50.
28. Liu G, Wang L. Effect of Bisuoluoer on cardiopulmonary function in patients with heart failure combined with COPD after AMI. Med J Liaoning. 2019;33(06):13–15.
29. Lv C. The effect of bisoprolol on the efficacy and expression of inflammatory factors in patients with chronic obstructive pulmonary disease and heart failure. Clin Res. 2019;27(10):84–85.
30. Zeng J. The effect of bisoprolol combined with home oxygen therapy on lung function and prognosis in COPD patients. Chronic Pathematol J. 2019;20(08):1260–1262.
31. Yang L, Yang X, Chen J. The effect of bisoprolol on lung function in elderly patients with coronary heart disease and COPD. Prevention and Treatment of Cardiovascular Disease. 2019;9(23):27–28.
32. Jin Y. The Effect of Bisoprolol Combined with Carboxymethylestan on Pulmonary Function and Blood Gas Analysis in Patients with Chronic Obstructive Pulmonary Disease. Chinese J Clin Ration Drug Use. 2019;12(11):56.
33. Zhao M, Wang L. Effects of bisoprolol on cardiopulmonary function and exercise tolerance in patients with acute exacerbation of chronic obstructive pulmonary disease complicated with pulmonary heart disease. Chinese Journal of Heart and Heart Rhythm(Electronic Edition. 2019;7(1):32–34.
34. Yi S, Yuan C, Yi X. The effect of bisoprolol on resting heart rate and lung function in COPD patients with pulmonary infection. The Medical Forum. 2018;22(05):648–649.
35. Yuan W, Sun H, Zhang N, Wang D. Effect of Bisoprolol on cardiopulmonary function and quality of life in patients with moderate and severe chronic obstructive pulmonary disease combined with chronic heart failure. Herald Med. 2017;36(10):1161–1164.
36. Yang H. Effect of Bissolol on cardiopulmonary function and inflammatory factors in patients with chronic obstructive pulmonary disease and heart failure. Chine J Rural Med Pharm. 2017;24(16):20–21.
37. Wu B. Study on the effect of bisoprolol on the efficacy and expression of related inflammatory media in patients with chronic obstructive pulmonary disease. China Continuing Medical Education. 2017;9(19):159–161.
38. Ma L, Liu D, Xie Y. Effect of Bisoprolol on Cardiopulmonary Function in Patients with Hypertension and Heart Failure Complicated with Chronic Obstructive Pulmonary Disease. Hebei Med. 2017;23(06):1002–1005.
39. Zhou H, Liu L, Luo C, Zong K. Efficacy and pulmonary function analysis of pesolo in the treatment of chronic obstructive pulmonary disease with chronic heart failure. Systems Med. 2017;2(01):50–52.
40. Cai B, Xu S. Effects of Bisorol on exercise tolerance and lung function in simple stable chronic obstructive pulmonary disease. Qingdao Med J. 2017;49(01):50–52.
41. Hu M, Zhang W. Clinical efficacy of bisoprolol in the treatment of chronic obstructive pulmonary disease and its impact on serum inflammatory factor levels and exercise tolerance. Practical J Cardiac Cerebral Pneumal Vascular Dis. 2016;24(12):138–139.
42. Wang Y, Jia X, Wang N, Chen L. The effect of bisoprolol on lung function and related inflammatory mediators in patients with chronic obstructive pulmonary disease. J Community Med. 2016;14(20):27–28.
43. Yang D, Chen B, Lin J, Dai M. The effect of bisoprolol on lung function and expression of related inflammatory neurotransmitters in patients with simple stable chronic obstructive pulmonary disease. Chinese J Clin Ration Drug Use. 2016;9(22):27–28.
44. Hong S. Clinical analysis of bisoprolol in elderly patients with chronic obstructive pulmonary disease and chronic heart failure. CHINA MED Pharm. 2016;6(11):94–96.
45. Zhou Z. Clinical study of bisoprolol on elderly patients with chronic obstructive pulmonary disease (COPD) and chronic heart failure (CHF). World Latest Med Information. 2016;16(20):66–67.
46. Hu X. Effect of Bisoprolol on the Lung Function of Patients with Chronic Obstructive Pulmonary Disease and the Expression of Inflammatory Medium. Med Recapitulate. 2016;22(03):563–565.
47. Jiang X. Effect of bisoprolol in treatment of chronic obstructive pulmonary disease and pulmonary heart disease in heart failure. Chin Health Stand Manag. 2016;7(03):95–97.
48. Fang Y. Curative effect and safety requirement of bisoprolol for patients with elderly chronic obstructive pulmonary disease complicated with chronic heart failure. World Latest Med Information. 2015;15(99):22–23.
49. Xiong J. The effect of bisoprolol on lung function and expression of related inflammatory mediators in patients with chronic obstructive pulmonary disease. Chine J Rural Med Pharm. 2015;22(22):7–8.
50. Du J. Effects of bisoprolol on lung function and expression of inflammatory mediators in patients with chronic obstructive pulmonary disease.The Chinese. J Clin Pharmacol. 2015.
51. Li X, Wang G. Evaluation of Bisoprolol in the Treatment of 61 Cases of Stable Chronic Obstructive Pulmonary Disease. China Pharmaceuticals. 2015;24(17):50–51.
52. Peng H, Xu T, Chang B, Sun Y, Zhong J. The Effect of Bisoprolol on Cardiopulmonary Function in Elderly COPD Patients with CHF. Med J National Defending Forces Southwest China. 2015;25(06):646–648.
53. Zhang G. Analysis of the application effect of bisoprolol in chronic obstructive pulmonary disease with pulmonary heart disease and heart failure. Modern Diagnosis Treatment. 2014;25(07):1590–1591.
54. Yuan W. Effect of Bisoprolol on Cardiopulmonary Function in Patients with Chronic Obstructive Pulmonary Disease and Chronic Heart Failure. J Pra Med. 2012;28(19):3280–3282.
55. Zhang L, Gao P, Fang Y. The prognostic impact of bisoprolol on COPD patients. China Practical Med. 2012;7(18):176–178.
56. Yu Y. Observation of the Curative Effect and Safety of Bisoprolol on Heart Failure in COPD Cor Pulmonale. J Military Surgeon Southwest China. 2010;12(06):1042–1044.
57. Pei Y. The application of bisoprolol in chronic obstructive pulmonary disease. Proce Clin Med. 2007;6:415–417.
58. Mainguy V, Girard D, Maltais F, et al. Effect of bisoprolol on respiratory function and exercise capacity in chronic obstructive pulmonary disease. Am J Cardiol. 2012;110(2):258–263. doi:10.1016/j.amjcard.2012.03.019
59. Hawkins NM, MacDonald MR, Petrie MC, et al. Bisoprolol in patients with heart failure and moderate to severe chronic obstructive pulmonary disease: a randomized controlled trial. Eur J Heart Fail. 2009;11(7):684–690. doi:10.1093/eurjhf/hfp066
60. Ke Y, Xu D, Li M, Wu Z, Huang Y. Effects of bisoprolol in combination with trimetazidine on the treatment of heart failure and concomitant chronic obstructive pulmonary disease. Pak J Med Sci. 2016;32(5):1208–1212. doi:10.12669/pjms.325.10850
61. Chhabra SK, De S. Cardiovascular autonomic neuropathy in chronic obstructive pulmonary disease. Respir Med. 2005;99(1):126–133. doi:10.1016/j.rmed.2004.06.003
62. Rasheedy D, Taha HM. Cardiac autonomic neuropathy: the hidden cardiovascular comorbidity in elderly patients with chronic obstructive pulmonary disease attending primary care settings. Geriatr Gerontol Int. 2016;16(3):329–335. doi:10.1111/ggi.12473
63. Jensen MT, Marott JL, Lange P, et al. Resting heart rate is a predictor of mortality in COPD. Eur Respir J. 2013;42(2):341–349. doi:10.1183/09031936.00072212
64. Schnabel P, Maack C, Mies F, Tyroller S, Scheer A, Böhm M. Binding properties of beta-blockers at recombinant beta1-, beta2-, and beta3-adrenoceptors. J Cardiovasc Pharmacol. 2000;36(4):466–471. doi:10.1097/00005344-200010000-00008
65. Nguyen LP, Omoluabi O, Parra S, et al. Chronic exposure to beta-blockers attenuates inflammation and mucin content in a murine asthma model. Am J Respir Cell Mol Biol. 2008;38(3):256–262. doi:10.1165/rcmb.2007-0279RC
66. Lipworth B, Wedzicha J, Devereux G, Vestbo J, Dransfield MT. Beta-blockers in COPD: time for reappraisal. Eur Respir J. 2016;48(3):880–888. doi:10.1183/13993003.01847-2015
67. Dunzendorfer S, Wiedermann CJ. Modulation of neutrophil migration and superoxide anion release by metoprolol. J Mol Cell Cardiol. 2000;32(6):915–924. doi:10.1006/jmcc.2000.1148
68. Roland M, Bhowmik A, Sapsford RJ, et al. Sputum and plasma endothelin-1 levels in exacerbations of chronic obstructive pulmonary disease. Thorax. 2001;56(1):30–35. doi:10.1136/thorax.56.1.30
69. Lin R, Peng H, Nguyen LP, et al. Changes in beta 2-adrenoceptor and other signaling proteins produced by chronic administration of ‘beta-blockers’ in a murine asthma model. Pulm Pharmacol Ther. 2008;21(1):115–124. doi:10.1016/j.pupt.2007.06.003
70. Hanania NA, Singh S, El-Wali R, et al. The safety and effects of the beta-blocker, nadolol, in mild asthma: an open-label pilot study. Pulm Pharmacol Ther. 2008;21(1):134–141. doi:10.1016/j.pupt.2007.07.002
71. GOLD Report Global Initiative for Chronic Obstructive Lung Disease 2020 Report. Global Initiative for Chronic Obstructive Lung Disease. 2020. Available from: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf.
72. Darawshy F, Abu Rmeileh A, Kuint R, Goychmann-Cohen P, Fridlender ZG, Berkman N. How Accurate Is the Diagnosis of “Chronic Obstructive Pulmonary Disease” in Patients Hospitalized with an Acute Exacerbation? Medicina (Kaunas). 2023;59(3):632. doi:10.3390/medicina59030632
73. Cuthbert JJ, Pellicori P, Clark AL. Optimal Management of Heart Failure and Chronic Obstructive Pulmonary Disease: clinical Challenges. Int J Gen Med. 2022;15:7961–7975. doi:10.2147/IJGM.S295467
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
