Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
Efficacy and Safety of Artemisinin-Based Combination Therapy for the Treatment of Uncomplicated Malaria in Pregnant Women: A Systematic Review and Meta-Analysis
Authors Shibeshi W , Baye AM , Alemkere G, Engidawork E
Received 30 August 2021
Accepted for publication 12 December 2021
Published 22 December 2021 Volume 2021:17 Pages 1353—1370
DOI https://doi.org/10.2147/TCRM.S336771
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Workineh Shibeshi, Assefa Mulu Baye, Getachew Alemkere, Ephrem Engidawork
Department of Pharmacology and Clinical Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Workineh Shibeshi Email [email protected]
Introduction: Malaria is one of the infectious diseases with substantial risks for pregnant women, the fetus and the newborn child. Thus, prevention and treatment of malaria with safe and effective drugs is of paramount importance. Pregnant women are mostly excluded from clinical trials, and systematic approaches of pharmacovigilance in pregnancy are limited. This means the safety and efficacy of antimalarial agents during pregnancy are unclear.
Purpose: This study was designed to carry out a systematic review and aggregate data meta-analysis of literature published on efficacy and safety of artemisinin-based combination therapy (ACT) for uncomplicated malaria in pregnant women.
Methods: A search of literature published between 1998 to 2020 on efficacy and safety of artemisinin-based combination therapy (ACT) in pregnant women was made using Cochrane Library, Medline and the Malaria in Pregnancy Consortium Library. Data were extracted independently by two reviewers, and any discrepancies were resolved by consensus. Meta-analysis was carried out using Open Meta-Analyst software. Random effects model was applied, and the heterogeneity of studies was evaluated using Higgins I2.
Results: Twenty-four studies that fulfilled the inclusion criteria were included in the final assessment. Overall, days 28 to 63 malaria treatment success rate was 96.1%. Overall days 28 to 63 cure rates for AL, AS+AQ, AS+MQ, DHA+PQ, AS+ATQ+PG and AS+SP were 95.1%, 92.2%, 97.0%,94.3%, 96.5% and 97.4%, respectively. Comparison of ACTs with non-ACTs revealed that the risk of treatment failure was substantially lower in patients treated with ACTs than with non-ACTs (risk ratio 0.20, 95% C.I. 0.09– 0.43). The overall prevalences of miscarriage, stillbirth and congenital anomalies were 0.3%, 2.1% and 1.0%, respectively, and found to be comparable among various ACTs. There was comparable tolerability across ACTs during pregnancy.
Conclusion: ACTs demonstrated a high cure rate, safety and tolerability against Plasmodium falciparum infection in pregnant women. The higher treatment success and comparable tolerability could be used as an input for decision makers to support the continued usage of ACTs for treatment of uncomplicated falciparum malaria in pregnant women.
Keywords: efficacy, safety, artemisinin-based combination therapy, uncomplicated malaria, pregnant women, systematic review and meta-analysis
Introduction
Although the global malaria burden has tended to decline with time (238 million in 2000, 231 million in 2017, 229 million cases in 2019); it still remains a significant health problem, particularly in Africa, which is home for the large majority of cases (with an estimated 215 million cases in 2019, accounting for about 94% of cases).1 Malaria is one of the infectious diseases with significant mortality and morbidity in pregnancy, posing substantial risks for the pregnant woman, the fetus and the newborn child.2 Analysis of the prevalence of malaria in pregnancy by sub-region of Africa showed that it was highest in West Africa and Central Africa, each with 35%, followed by East and Southern Africa (20%). Thus, prevention and treatment of malaria with safe and effective drugs are required.1,2 However, clinical trials are scarce in pregnant women and systematic approaches of pharmacovigilance in pregnancy are limited, indicating a lack of enough evidence on the safety and efficacy of antimalarial agents during pregnancy.3,4
Artemisinin-based combination therapy (ACT) is a combination of artemisinin family of drugs and other non-artemisinin partners. Even though both the artemisinin and non-artemisinin components are essential for the antimalarial efficacy, the artemisinin component is vital to decrease parasite density in the early days of treatment. The remaining parasites are cleared by the partner drug.5 Therefore, the two drugs achieve effective clinical and parasitological cures and it is also believed that using this combination protects each other from development of resistance.6 The most important challenge in the global fight against malaria is Plasmodium resistance to antimalarial medicines. Regular monitoring of efficacy of antimalarial agents supports early identification of changes in how well the recommended treatments work so as to mitigate any impact of resistance and prevent its spread.1
Currently, the World Health Organization (WHO) recommends ACTs for the treatment of uncomplicated malaria in pregnant women in the 2nd or 3rd trimester.7 Seven days of quinine with clindamycin is recommended for the management of malaria in the first trimester.8 However, the use of ACTs in the first trimester of pregnancy is recommended if: (i) ACTs are the only treatment option immediately available; (ii) failure occurs after treatment with 7-day quinine plus clindamycin; or (iii) adherence is a challenge with the 7-day treatment.7
As evidenced by animal studies, ACT derivatives were found to be embryotoxic and teratogenic.3 The studies suggested that the critical time period for exposure in humans is in the first trimester. Various organs including uterus, ears, brain and eyes are still in development and growth phase in the second trimester, and are therefore sensitive to exogenous substances that can result in malformations.9 However, data from animal studies do not always predict the effects observed in humans. Indeed, observational studies and randomized controlled clinical trials (RCTs)10–16 did not report increased risk of adverse pregnancy outcomes such as stillbirth or miscarriage after taking artemisinins during pregnancy compared with those taking non-artemisinins as well as those with no antimalarial agents. Periodic evidence synthesis from several clinical and observational studies on therapeutic efficacy and safety of ACTs in pregnant population is required, which may indicate any evolving changes or trends in the clinical use of ACTs. Hence, our study aimed to conduct a systematic review and meta-analysis, and to generate comprehensive evidence on the efficacy and risk of adverse pregnancy outcomes associated with the use of ACTs during pregnancy.
Methods
Eligibility Criteria
Eligible studies for this review were interventional, observational cohort and pharmacokinetic studies enrolling pregnant women at first, second and third trimesters of pregnancy and receiving an ACT with or without comparative arm interventions for the treatment of uncomplicated P. falciparum or P. vivax mono-infections. Only published articles in English were included. Studies on non-pregnant adults and pediatrics, not involving artemisinins treatment or using artemisinin monotherapy, on malaria prevention or prophylaxis, and that did not report either efficacy or pregnancy safety outcomes were excluded.
Search Strategy
A literature search was performed to identify articles that included safety and efficacy outcomes of ACT exposure during pregnancy. The electronic search was carried out on February 28, 2020 through the Cochrane Library, Medline and Malaria in Pregnancy Consortium Library using keywords and MeSH terms with no restriction for time of publication. The search followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines (S1 Table).17 The search terms consisted of (Pregnant women OR pregnant* AND malaria) AND (Artemisinin* OR artemisinin combination therapy OR ACT OR artemether OR artesunate OR dihydroartemisinin OR arteflene* OR artemotil OR arteether OR dihydroarte* OR treatment) AND (safety OR serious adverse event OR miscarriage OR stillbirth OR pregnancy loss OR spontaneous abortion OR birth defect OR congenital abnormalities OR congenital malformations OR congenital anomalies).
Data Extraction
Decision to select a retrieved article was made by two reviewers (AM and GA) who worked independently through screening of titles and abstracts followed by full text assessment. A data abstraction format was used for screening purposes and an article is selected when there is an agreement between the two reviewers. Any disagreements were resolved by discussion and consensus. Data related to author (s) of the articles, year of publication, year the studies were conducted, geographic location of the study, duration of the study, trimester of pregnancy, age, weight, parity and gravida of participants, type of malaria species, baseline parasite density and temperature, and type of study design (observational, interventional) were extracted from each article. Data regarding the types of malaria treatment agents, treatment duration, treatment outcome measures, pregnancy outcome (miscarriage, still birth, birth weight, intrauterine growth retardation rate, gestational age and prematurity) and other adverse events including laboratory abnormalities were also extracted and included in the systematic review and meta-analysis.
Quality Assessment
The two reviewers independently assessed methodological quality of studies using revised tool for assessing risk of bias in randomized trials (ROB 2)18 for RCTs and Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for observational studies.19 The observational studies were categorized as high quality (over 75% of the STROBE checklist) and low quality (under 75% of the STROBE checklist). The quality of the included clinical trials was assessed and quality was categorized as “low risk of bias”, “some concerns” or “high risk of bias”.
Outcome Definitions
Days 28 to 63 (whichever was reported latest) PCR-corrected adequate clinical and parasitological response (ACPR) was taken as primary outcome of efficacy for the meta-analysis. ACPR was defined as absence of parasitemia on day 28 irrespective of axillary temperature without previously meeting any of the criteria of early treatment failure or late clinical failure or late parasitological failure.20 The primary outcomes for safety included mean birth weight and gestational age as well as prevalence of low birth weight, prematurity, congenital abnormalities, stillbirth, and miscarriage. To evaluate tolerability, we assessed the prevalence of adverse effects, including tinnitus, aesthesia, dizziness, poor appetite, nausea, and vomiting.
Statistical Methods
The meta-analysis of malaria treatment efficacy was performed by OpenMeta Analyst software for Windows.21 Cochrane Q and the I2 statistic were employed to evaluate heterogeneity of the included studies. Cochrane Q with P < 0.10 and I2> 50 were taken as standard to indicate the presence of heterogeneity of the included studies.22 P < 0.05 was used to declare statistical significance.
Results
Study Characteristics
Out of 1108 studies identified, 89 were included for review of the full text. Of these, 24 studies met the inclusion criteria (Figure 1). Studies based on the same cohort were considered as a single study. As indicated in Table 1, 18 studies were from sub-Saharan Africa, including 3 studies from Nigeria,23–25 2 studies from Tanzania,26,27 2 studies each from Sudan28,29 and Uganda,10,30 1 study each from Burkina Faso,31 Congo,32 Ghana,33 Malawi,34 and Rwanda,11. One study was reported from four African countries (Burkina Faso, Ghana, Malawi, Zambia, Congo).35 The outcome of pregnancy and infant mortality from this study was reported separately.36 As part of the study from the four African countries,35 the output from Zambia was reported separately.37 The other 8 studies were from Asia; one from India38 and one from the Thai-Myanmar border39 and six studies from Thailand.13–15,40–42
 |  |  |
Table 1 Description of the Studies Identified in the Systematic Review and Meta-Analysis of Safety and Efficacy of Artemisinin-Based Therapy in Pregnant Women |
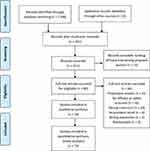 |
Figure 1 PRISMA flow diagram for search results February 28, 2020. Notes: PRISMA figure adapted from Liberati A, Altman D, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). Creative Commons.17. |
The studies were conducted between May 1986 and April 2015 but published between 1998 and February 2020. Sixteen of the studies were clinical trials (one double blind and 15 open-label, all were RCTs but one), 7 were prospective observational studies, two studies one of which is part of a clinical trial,43 were pharmacokinetic studies and the remaining was a single arm study29 (Table 2). By type of malaria infection, 25 studies involved falciparum malaria, but two observational studies involved both falciparum and vivax malaria infections.13,39
 |  |  |
Table 2 Characteristics of Participants at Enrollment in the Studies Evaluating Efficacy and Safety of Artemisinin-Based Therapies in Pregnancy |
ACTs used in the trials include artemether-lumefantrine (15 studies), amodiaquine-artesunate (7 studies), mefloquine-artesunate (8 studies), sulfadoxine-pyrimethamine (SP-artesunate (4 studies), atovaquone-proguanil-artesunate (2 studies), artemether-mefloquine (1 study), artemether-lumefantrine with quinine (1 study), dihydroartemisinin + piperaquine (4 studies) and unspecified artemisinin derivative (1 study).
In most of the studies, durations of ACTs were 3 days. But there were exceptions, where 5 days of artemether injection and artemether-lumefantrine combination therapy was used in Nigeria44 and Congo,32 respectively, as well as 7 days of artesunate therapy in Thailand.15,41
Other antimalarial drugs used in the studies included quinine (5 studies), SP (3 studies), mefloquine (2 studies), SP in combination with azithromycin (1 study), SP with amodiaquine (1 study), amodiaquine (1 study), chloroquine (1 study), atovaquone with proguanil (1 study) and chlorproguanil and dapsone (CD) (2 studies). Detailed description of studies included in this systematic review and meta-analysis is indicated in a supplementary file (S2 Table).
Characteristics of the Study Participants
A total of 9482 pregnant women were exposed to antimalarial agents, with the majority (6860, 72.3%) of them exposed in the 2nd or 3rd trimester, and 2622 (27.7%) in the first trimester. In addition, 2482 pregnant women were unexposed controls in the 24 studies but included for comparison purpose. In one of these studies, 30 healthy non-pregnant women were included as comparators.24 Twenty-four non-pregnant women on AS+MQ were also compared with pregnant women.31
A total of 6600 women in the 24 studies received ACT during the first, second and third trimesters of pregnancy (Tables 1 and 2). The most common ACT exposures were: artemether-lumefantrine (AL) (n= 2624), artesunate-amodiaquine (AS+AQ) (n = 1295), artesunate-mefloquine (AS+MQ) (n = 1158), dihydroartemisinin-piperaquine (DHA+PQ) (n= 1068), artesunate -sulfadoxine-pyrimethamine (AS+SP) (n = 215), artesunate atovaquone-proguanil (AS+ATQ+PG) (n = 65), artesunate-clindamycin (n = 50), artemether-mefloquine (AM+MQ) (n = 25) and AL and quinine (n = 8) and unspecified artemisinin derivatives were provided for 92 pregnant women.
Documented fever ranged from 2–74% at enrollment. Among the documented mean or median body weights, participants from Sudan28 and Nigeria23,24,44 had the highest body weight compared with those from Thailand.15,40 Detailed description of study participants included in this systematic review and meta-analysis is indicated in a supplementary file (S3 Table).
Efficacy of ACTs
Among the 24 studies, 16 reported the efficacy of ACTs in the management of malaria in pregnant women (S2 Table). Among these efficacy studies, PCR was corrected in 12 studies. Studies with PCR correction of day-28 or later cure rate were included for meta-analysis. In addition, two studies29,30 with no late treatment failure were also included in the meta-analysis (Figure 2). The detailed results observed in individual studies are given in the supplementary materials (S2 Table and S3 Table).
Artemether-Lumefantrine (AL)
There were eight studies that included AL as intervention group. The Ugandan study compared AL with quinine and reported high efficacy and the non-inferiority of AL relative to quinine.10 Mean fever clearance as well as parasite clearance was also comparable in a similar study in Uganda.30 However, a Thai study comparing AL and artesunate treatment in women with positive blood smears (AL, 125 women; AS, 128 women) reported a cure rate of less than 95% for AL at day 42 and for both treatments at delivery (or day 42 if later).15
The PREGACT study in four African countries (Burkina Faso, Ghana, Malawi and Zambia) compared the efficacy of AL, AS+AQ, DHA+PQ and AS+MQ. In this study, there was no significant difference among the AS+AQ, DHA+PQ, and AS+MQ groups. However, the cure rate in the AL group was significantly lower than the rate in the other three arms.35
The Congolese study compared the efficacy of a 5-day regimen of AL with the standard 3-day treatment in 48 pregnant women with uncomplicated falciparum malaria in an open-label RCT. The PCR-corrected clinical and parasitological response was 100% in both treatment arms, indicating that both the current and extended regimens of AL are highly efficacious.32
A comparative clinical study in Nigeria was conducted on two commonly used fixed dose ACTs, AS+AQ and AL, for the treatment of uncomplicated P. falciparum malaria in the second and third trimester of pregnancy. The late parasitological failure observed on day 14 was 2.63% for AS+AQ and 5.41% for AL. The failure observed may be due to reinfection because there was no PCR-corrected result to rule out recrudescence.23 Another study from Nigeria, a double-blind randomized trial, was conducted to compare AS+AQ and AL combinations in the treatment of acute uncomplicated P. falciparum malaria during pregnancy. Unlike the other studies, the efficacy of the two treatment arms did not differ significantly in this study. High cure rates of both day-3 parasite clearance and day-28 ACPR were reported in this study.25
Artesunate-Amodiaquine (AS+AQ)
Six studies evaluated the efficacy of AS+AQ with other antimalarial agents. As stated above in the PREGACT study, the PCR-corrected cure rate in the AS+AQ treatment group was significantly higher than the AL treatment group.35
According to an open-label RCT study in Tanzania, pregnant women with P. falciparum malaria were randomized to one of the 4 regimens: SP, CD, SP+AQ or AS+AQ. PCR-corrected parasitological outcomes for AQ+AS were significantly better than for CD (AS+AQ versus CD: AOR for parasitological failure rates was 0.36, 95% CI: 0.14–0.94).27
In an open-label, randomized, non-inferiority trial in Ghana, 212 and 205 second and third trimester pregnant women, respectively, with confirmed asymptomatic P. falciparum parasitemia were randomized to receive DHA+PQ or AS+AQ. After controlling for age, gestational age, hemoglobin, gravidity and parasite density at enrollment, efficacy was 3.5 (95% CI: −1.5–8.5) and 3.9 (95% CI: −2.7–10.4) -fold higher by days 28 and 42, respectively, for DHA+PQ than AS+AQ. This finding brought out that DHA+PQ was non-inferior to AS+AQ for treatment of malaria infection during pregnancy, although the efficacy was not PCR corrected.33 The Nigerian studies did not also reveal a significant difference in the clinical and parasitological response between AS+AQ and AL, although PCR adjustment is lacking in both studies.23,25
Artesunate-Mefloquine (AS+MQ)
Five studies compared AS+MQ with other ACTs and quinine. An Indian study on pregnant women in the second or third trimester with P. falciparum mono-infection randomized to receive either AS+SP or AS+MQ did not show detectable difference in the probability of treatment failure between the two arms following Kaplan–Meier survival analysis.38 The PREGACT study did not also reveal significant difference in PCR-corrected cure rate among AS+AQ, DHA+PQ and AS+MQ groups.35
An open-label randomized comparison of AS+MQ versus quinine was conducted from 1995 to 1997 in 108 pregnant Thai women with acute uncomplicated P. falciparum malaria in the second or third trimesters of pregnancy. AS+MQ treatment arm was more effective than the quinine treatment arm as revealed by the day 63 PCR-corrected cure rates.14
Dihydroartemisinin-Piperaquine (DHA+PQ)
The study in four African countries (Burkina Faso, Ghana, Malawi and Zambia) showed that the cure rate in the AL group was significantly lower than the rate in the DHA+PQ treatment groups.35
The Ghanaian study indicated that the differences in efficacy (DHA+PQ versus AS+AQ) after controlling for other variables were 3.5 (95% CI: −1.5–8.5) and 3.9 (95% CI: −2.7–10.4) by days 28 and 42, respectively.33
Meta-Analysis of Efficacy of ACTs
Out of 16 clinical trials identified, only 13 studies were included in the meta-analysis for efficacy of ACTs in the management of uncomplicated malaria in pregnant women. Three studies were excluded (no PCR correction for “Iribhogbe 2017” and “Ukah 2015”, and no efficacy outcome for “Iribhogbe 2017a” as they do not report PCR-corrected efficacy). Overall, PCR-corrected day 28 to day 63 malaria treatment success rate for ACTs was 96.1%. However, we found high heterogeneity (overall I2 = 83.41%; p = 0.000, 13 studies, 18 ACTs treatment groups).
Subgroup analysis was carried out to assess the heterogeneity. There were five RCTs with efficacy report of AL.10,15,25,32,35 Overall PCR-corrected cure rate was 95.1% for AL (95% CI: 91.3–98.9%; I2 = 89.41%, p <0.001, 5 studies) (Figure 2). AL therapy was associated with ≥95% cure rate in 3 of the 5 studies;10,30,32 the exceptions were studies from the four African countries (Burkina Faso, Ghana, Malawi and Zambia)35 and Thailand.15
There were three RCTs with efficacy report for AS+AQ.27,33,35 Overall day 28 to 63 PCR-corrected ACPR for AS+AQ was 92.2% (95% CI: 83.6–100%; I2 = 92.33%, p = 000, 3 studies) (Figure 2). AS+AQ was associated with ≥95% cure rate in 1 of the 3 studies;35 but cure rates were <95% in studies from Tanzania27 and Ghana.33 There were four RCTs with efficacy report for AS+MQ.14,31,35,38 Overall day 28 to 63 PCR-corrected ACPR for AS+MQ was 97.0% (95% CI: 95.9–98.2%; I2 = 2.1%, p = 0.382, 4 studies) (Figure 2). AS+MQ combination therapies were associated with ≥95% cure rates in all these studies.
There were two RCTs with efficacy report of DHA+PQ.33,35 Overall day 28 to 63 PCR-corrected ACPR for DHA+PQ was 94.92% (95% CI: 84.3–104.3%; I2 = 94.92%, p<0.000, 2 studies) (Figure 2). DHA+PQ combination therapy was associated with ≥95% cure rate in 1 of the 2 studies;35 the exception was the study from Ghana.33
There were two RCTs with efficacy report of AS+ATQ+PG.40,42 Overall day 28 to 63 PCR-corrected ACPR for AS+ATQ+PG was 96.5% (95% CI: 91.8–101.1%; I2 = 0.0%, p<0.548, 2 studies) (Figure 2). AS+ATQ+PG combination therapies were associated with ≥95% cure rates in both of these studies.
There were two RCTs with efficacy report of AS+SP.29,38 Overall day 28 to 63 PCR-corrected ACPR for AS+SP was 97.4% (95% CI: 94.3–102.7%; I2 = 0.0%, p<0.524, 2 studies) (Figure 2). AS+SP combination therapies were associated with ≥95% cure rates in both of these studies. The limited number of available studies with PCR-corrected outcome comparing different ACTs precluded aggregate data meta-analysis.
PCR-Corrected ACPR Comparison of Different Treatments
In the five RCTs that were included for meta-analysis to compare ACTs and non-ACTs, the risk of treatment failure was substantially lower in patients treated with ACTs than in patients treated with non-ACTs (risk ratio 0.20, 95% CI: 0.09–0.43) (Figure 3), although the compared treatments and methodologies differed.
Safety of ACTs
The safety of AL and quinine was recently compared in an open-label randomized non-inferiority trial in Uganda on second and third trimester pregnant women with uncomplicated P. falciparum malaria treated with either AL (152) or quinine (152). No significant differences were observed between the treatment arms in the frequency of miscarriages, stillbirths, early neonatal deaths, malformations, prematurity and low birth weight.10
Between 2006 and 2008, an observational study investigated the safety of artemisinins including artemether injection, AS+SP and AL in the first trimester of pregnancy in Sudan.28 There was no congenital abnormality documented, but miscarriage occurred in two women who were treated with artemether injection in the first trimester while quinine infusion was administered for a subsequent malaria attack.28
Between April 2004 and August 2006, the safety of AL and artesunate was compared in an open-label RCT in the second and third trimesters of pregnancy in Thailand.15 Pregnant women with acute P. falciparum malaria were allocated to AL and artesunate treatment groups. There was no significant birth outcome difference in the two treatment groups. But death rates were significantly more in the artesunate group (6.7%) than the AL group (0.9%), although these deaths were unrelated to the treatments received.15
In an observational cohort study conducted in Tanzania in pregnant women in the first trimester, 1783 (82.3%) women completed the study until delivery. Out of them, 319 (17.9%) used antimalarial drugs in the first trimester, of whom 172 were on AL, 78 on QN, 66 on SP and 11 on AQ. Analysis showed that first trimester exposure of quinine was associated with a two-fold increased risk of miscarriage, stillbirth and preterm birth compared with the other treatments.26
In the PREGACT study, there were no significant difference in the proportion of live births among the treatment arms. The average birth weight and the incidence of miscarriages, stillbirths, preterm deliveries, and malformations were similar among the treatment arms.35
In the Rwandan prospective cohort study from June 2007–July 2009, exposed group (pregnant women with malaria given AL), and a matched non-exposed group (pregnant women without malaria and not exposed to AL) were followed until delivery. The authors noted that the rates of abortion, perinatal mortality, stillbirth and premature delivery were slightly increased in the treatment group and argued that this was probably due to the acute complications of malaria itself instead of the interventions given.11
The Indian study that compared AS+SP and AS+MQ reported that low birth weight (< 2.5 kg) was significantly higher in the AS+SP than in the AS+MQ treatment group, however, there was no statistically significant difference in miscarriages, preterm delivery or stillbirths between the treatment arms.38
In the Malawian randomized clinical trial, pregnant women with uncomplicated P. falciparum malaria were allocated in three treatment arms: 47 women in each of the treatment arms (SP only, SP + Azithromycin and AS+SP). The authors indicated that the observed abortions, stillbirths and neonatal deaths potentially had other proximal causes than directly related to the interventions provided.34
The observational study in the Thai–Burmese border analyzed antenatal register of pregnant women exposed to chloroquine-based, quinine-based or artemisinin-based treatments in the first trimester. The risk of miscarriage and stillbirth was similar in all groups. Congenital anomaly also similarly occurred among babies born from women treated with chloroquine, quinine or artesunate.13
In the Tanzanian study from January 2004–September 2006, pregnant women with uncomplicated P. falciparum malaria were allocated in four treatment arms: SP, CD, SP+AQ or AQ+AS. In this trial there was no report of apparent excess stillbirths or adverse birth outcomes in any of the arms.27
Low birth weight was significantly higher in the DHA+PQ arm as compared with AS+AQ in the Ghanaian study. Furthermore, two cases each of neonatal deaths and stillbirths were recorded in the DHA+PQ arm, while one case of stillbirth was observed in the AS+AQ arm. The authors argued that the higher prevalence of low birth weight in the DHA+PQ arm could be attributed to a chance finding not attributable to DHA+PQ.33
In a study from Thailand comparing the safety of QN and AS+ATQ+PG, there was no significant difference in mean birth weight, baby growth parameters and estimated gestational age (EGA) or in the proportion of low birth weight, premature, or intrauterine growth–retarded infants. Identified congenital abnormalities were considered unlikely to be drug related.40
Meta-Analysis of Pregnancy Outcomes
Miscariage
Seven studies reported a total of 20 miscarriages among 3996 women who received ACTs during pregnancy. The overall prevalence was 0.3% and found to be similar among various ACTs (0.1% for AL, 0.5% for AS+AQ, DHA+PQ and AS+MQ) (S1 Figure).
Stillbirth
Six studies reported a total of 90 stillbirths among 3927 women who received ACTs during pregnancy. The overall prevalence of stillbirth was 2.1% and found to be similar among various ACTs (1.7% for AL, 1.9% for AS+AQ, 2.8% for AS+MQ and 2.5% for DHA+PQ) (S2 Figure).
Low Birth Weight
Nine studies reported a total of 603 low birthweight newborns among 3891 women who received ACTs during pregnancy. The overall prevalence of low birthweight was 14.2% and found to be higher for AS+SP (23.6%) but it was relatively similar among the other ACTs (16.3% for AS+MQ, 14.4% for DHA+PQ, 12.6% for AL and 10.2% for AS+AQ) (S3 Figure).
Congenital Anomalies
Six studies reported a total of 43 congenital anomalies among 3973 women who received ACTs during pregnancy. The overall prevalence of congenital anomalies was 1.0% and found to be similar among the ACTs (1.5% for DHA+PQ, 0.9% for AS+AQ and 1.2% for AL) (S4 Figure).
Tolerability of ACTs
According to the Thailand study,15 tolerability of AL and AS treatments was similar. Hematology, blood chemistry and ECG evaluations detected no difference in the occurrence of abnormal values. However, tinnitus was significantly different in the two treatment groups (AL, 0%; AS, 8.5%).
The PREGACT study in four African countries indicated that there was no significant difference in the occurrence of serious adverse events among the treatment arms.35 Among these severe adverse events, severe vomiting associated with the AS+MQ arm was considered to be related to the study treatment.37 The Ghanaian trial of AS+AQ and DHA+PQ documented severe diarrhea associated with AS+AQ, which was thought to be related to the treatment.33 Other trials, including RCTs, comparing ACT with other ACTs or other antimalarial agents reported no treatment-related severe adverse events in India,38 Malawi,34 Nigeria24 and Thailand.14,40
Tinnitus was one of the common adverse events reported in these trials. It was largely noted with treatment of QN as revealed by the Ugandan (QN versus AL),10 and Thailand studies (AS+MQ versus QN)14 and (QN versus AS+ATQ+PG).40 In a comparative study of AL and AS7 conducted in Thailand, tinnitus was once again the only symptom that significantly differed between the treatment arms, which was more frequent in the AS7 arm (8.5%).15
Headache, dizziness, nausea, vomiting, poor appetite and asthenia were the other common treatment-related adverse events reported by these trials. In the PREGACT trial conducted in four African countries, significantly higher rate of treatment-related adverse events occurred in the AS+MQ group and the AS+AQ group than in the DHA+PQ group and the AL group.35 Other studies also demonstrated that a higher rate of dizziness was noted in the QN than the AS+MQ arm.14 Vomiting was significantly higher in the AS+MQ than the AS+SP treatment arm in the Indian study.38 Whilst women in the quinine arm had more frequent adverse events, such as nausea, vomiting, and anorexia than AL group, headache was higher in AL group in the Ugandan study.10
Risk of Bias Across Studies
Fifteen clinical trials were evaluated using a revised tool for assessing risk of bias in randomized trials (ROB 2),18 while the other eight studies were assessed with the STROBE statement.19 Two clinical trials were found to have low risk of bias and all observational studies were judged to have high quality (S4 Table).
Discussion
Systematic review and meta-analysis was conducted to compile and synthesize comprehensive evidence on efficacy and safety of ACT use for treatment of uncomplicated malaria during pregnancy. The overall ACPR of the ACTs was more than 95%, which is in line with WHO recommendations. Except for three treatment arms: AL,15 AS+AQ33 and DHA+PQ;33 all the ACT treatment arms had an ACPR of greater than 90% at day 28 or greater follow-up. The WHO recommends a change in the treatment regimen if the treatment failure of an ACT is greater than or equal to 10%.20
In the five RCTs that were included for meta-analysis to compare ACTs and non-ACTs, the risk of treatment failure was substantially lower in patients treated with ACTs than in patients treated with non-ACTs (risk ratio 0.20, 95% C.I. 0.09–0.43). Similar findings were reported in a meta-analysis that compared ACTs with quinine-based therapies (risk ratio 0.22, 95% C.I. 0.07–0.63).45
This review revealed that the overall efficacy of AL in pregnant women was greater than 95% with meta-analysis of five trials. Despite this, it was reported that the cure rate in AL treatment group was significantly lower compared with AS+AQ, AS+MQ and DHA+PQ.35 In one trial a less than 90% cure rate of AL was reported in pregnant patients.15 This could have resulted from the lower drug concentrations achieved by both artemether and lumefantrine.46,47 Indeed, an Ugandan study demonstrated that pregnant women had a 27% lower plasma concentration of lumefantrine than non-pregnant women.48
According to this review, the overall efficacy of AS+AQ was greater than 90% with the meta-analysis of three of the studies included in this review. One of the trials indicated that AS+AQ had significantly greater cure rate than CD.27 Previous pharmacokinetic studies on pregnant women in Thailand showed that increasing the usual dose of proguanil was mandatory to achieve comparable plasma drug concentrations.49,50 Findings of a study indicated the equivalence of AS+AQ efficacy with DHA+PQ.33
The overall efficacy of AS+MQ was shown to be 97.0% (95% C.I. 95.9–98.2%), which is greater than 95% with the meta-analysis of four studies included in this review. Studies included in this trial indicated that the efficacy of AS+MQ is comparable with AS+SP,38 DHA+PQ,35 AS+AQ35 but significantly greater than the cure rate of AL35 and quinine.14
The overall ACPR for DHA+PQ was 94.3% (95% C.I. 84.3–104.3%). Studies included in this trial indicated that the efficacy of DHA+PQ is comparable with AS+AQ,33,35 AS+MQ,35 but significantly greater than the cure rate of AL.35 The overall day-28 or greater ACPR of AS+ATQ+PG and AS+SP was greater than 95%.
Trials showed that AL could grant the shortest-range post-treatment preventive activity against malaria as compared with the other ACTs. In the PREGACT study, re-infection rate was the highest in patients managed with AL and time to reinfection was the shortest in this treatment group.35 One of the crucial factors in the choice of drugs for malaria management is the duration of post-treatment prophylaxis, particularly in regions with a high probability of infection. It was found that lumefantrine has a rapid clearance,51 followed by amodiaquine,52 mefloquine,31 and then piperaquine.53
In this review, there were 607 pregnant women exposed to artemisinin treatment in the first trimester of pregnancy, as reported by observational studies in Sudan,28 Tanzania,26 Thailand,13,42 Thai-Myanmar39 and Rwanda.11 Data on cure rates in the first trimester were only available from observational studies, which posed difficulty to compare the outcomes using aggregated evidences. On top of this, the findings were rarely sorted by trimester, and the assessment of gestational age varied among the studies.
With the available observational studies, the risk of birth outcomes was not found to be higher in women taking ACTs in the first trimester. There was no significant birth outcome difference including miscarriage, stillbirth, early neonatal deaths, malformations, prematurity, low birth weight in various trials comparing combination of the various treatment groups. Observational studies in the antenatal registers of pregnant women in the first trimester could not also demonstrate the risk of adverse birth outcomes associated with ACTs. From 62 women who received ACTs (including artemether injection, AS+SP and AL) in the first trimester of pregnancy, there was no maternal mortality reported and all newborns were delivered at full term, with no congenital abnormalities.28 By contrast, first trimester exposure of quinine was associated with an increased risk of miscarriage and stillbirth in the Tanzanian observational cohort study26 and abortive effect of quinine was linked to induction of uterine contractions.54 Moreover, the Ugandan study also revealed that oral quinine had nearly two-fold increase in intrauterine fetal deaths than the AL group among women in the second and third trimesters of pregnancy.10 But, first trimester exposure of pregnant women with chloroquine, quinine or artesunate resulted in similar risk of miscarriage and stillbirth in the Thai–Burmese study based on analysis made using the antenatal registers of pregnant women.13 Likewise, in an observational study in Thai–Myanmar border, the risk of miscarriage and major congenital anomaly were similar among patients receiving ACT and quinine in the first trimester of pregnancy.39
The Indian study that compared AS+SP and AS+MQ reported that miscarriages, preterm delivery or stillbirths were similar in treatment arms but low birth weight (< 2.5 kg) was reported to be higher in the AS+SP treatment group (28.1%) than in the AS+MQ treatment group (16.7%).38 Higher proportion of low birth weight was not reported in other studies.28,34 In the Ghanaian trial comparing DHA+PQ and AS+AQ in pregnant women, low birth weight was observed in 9.3% of babies and was higher in the DHA+PQ arm compared with AS+AQ (13.2% versus 4.2%).33 This finding was not consistent with other studies using DHA+PQ for treatment or prevention of malaria in pregnancy.35,55–57
Trials comparing ACT with other ACTs or other antimalarial agents reported no treatment-related severe adverse events including RCTs in India,38 Malawi, Nigeria24 and Thailand.14,40 Although 10 severe adverse events were reported in the PREGACT study, there was no significant difference in the occurrence of severe adverse events among the treatment arms.35 Tinnitus was dominantly reported with pregnant women who took quinine than ACTs or other antimalarial drugs.10,13,14,40 Other adverse events including dizziness, headache, nausea, vomiting, poor appetite and asthenia occurred significantly at higher rates in the AS+MQ and AS+MQ groups in the PREGACT study.35
Even though this review generated comprehensive evidence on efficacy and safety of ACTs in pregnant women, it was subject to some limitations. The studies included were heterogeneous in design. Each study used a different ACT, and the timing of the measurement of the primary efficacy endpoint, the PCR-corrected failure rate varied from days 28 to 63. Indicators for tolerability differed among studies as well as the methods and timing of assessment of many of the secondary efficacy endpoints varied, which made it difficult to pool the information. The number of studies and study participants were limited, and data for studies with first trimester exposure of ACTs were compiled only from observational studies (not RCTs). This may limit the generalizability of the findings.
Conclusion
Meta-analysis of available evidence indicated that ACTs demonstrated high overall efficacy for treatment of uncomplicated falciparum malaria (96.1%). Overall days 28 to 63 cure rates for AL, AS+AQ, AS+MQ, DHA+PQ, AS+ATQ+PG and AS+SP were 95.1%, 92.2%, 97.0%, 94.3%, 96.5% and 97.4%, respectively. Analysis of treatment failure among ACTs and non-ACTs indicated that risk of treatment failure at days 28 to 63 cure rate was significantly lower in patients treated with ACTs than in patients treated with non-ACTs. Adverse birth outcomes were comparable among ACTs and the risks of pregnancy and neonatal outcomes, and other adverse events were not found to be higher in women taking ACT, supporting the use of ACTs during pregnancy. As evidenced by data from observational studies, first trimester exposure of ACTs was not found to be associated with an increased risk of adverse birth outcomes.
Abbreviations
ACPR, Adequate clinical and parasitological response; AL, Artemether-lumefantrine; AS+AQ, Artesunate + amodiaquine; AS+ATQ+PG, Artesunate + atovaquone + proguanil; AS+MQ, Artesunate + mefloquine; AS+SP, Artesunate + sulfadoxine-pyrimethamine; DHA+PQ, Dihydroartemisinin + piperaquine; MeSH, Medical subject heading; PICOS, Population, intervention, comparison, outcome, study; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Data Sharing Statement
The datasets used during the current study are presented within the manuscript and/or additional supporting files, and also available from the corresponding author on reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work is supported by a grant from European and Developing Countries Clinical Trials Partnership (EDCTP) and European Union (Grant number:TMA2016IF-1778).
Disclosure
The authors declare that there is not any real or perceived conflict of interest.
References
1. World Health Organization. World Malaria Report 2020: 20 Years of Global Progress and Challenges. Geneva: World Health Organization; 2020.
2. Desai M, Ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7(2):93–104. doi:10.1016/S1473-3099(07)70021-X
3. Nosten F, McGready R, d’Alessandro U, et al. Antimalarial drugs in pregnancy: a review. Curr Drug Saf. 2006;1(1):1–15. doi:10.2174/157488606775252584
4. White NJ, McGready RM, Nosten FH. New medicines for tropical diseases in pregnancy: catch-22. PLoS Med. 2008;5(6):e133. doi:10.1371/journal.pmed.0050133
5. World Health Organization. Artemisinin Resistance and Artemisinin-Based Combination Therapy Efficacy: Status Report. World Health Organization; 2018.
6. World Health Organization. 2001. Antimalarial drug combination therapy. Report of a WHO technical consultation: 1–35.
7. World Health Organization. Guidelines for the Treatment of Malaria. World Health Organization; 2015.
8. WHO Malaria Policy Advisory Committee, Secretariat. Malaria policy advisory committee to the WHO: conclusions and recommendations of September 2012 meeting. Malar J. 2012;11(1):424. doi:10.1186/1475-2875-11-424
9. Chaabane S, Bérard A. Epidemiology of major congenital malformations with specific focus on teratogens. Curr Drug Saf. 2013;8(2):128–140. doi:10.2174/15748863112079990011
10. Piola P, Nabasumba C, Turyakira E, et al. Efficacy and safety of artemether–lumefantrine compared with quinine in pregnant women with uncomplicated Plasmodium falciparum malaria: an open-label, randomised, non-inferiority trial. Lancet Infect Dis. 2010;10(11):762–769. doi:10.1016/S1473-3099(10)70202-4
11. Rulisa S, Kaligirwa N, Agaba S, Karema C, Mens PF, de Vries PJ. Pharmacovigilance of artemether-lumefantrine in pregnant women followed until delivery in Rwanda. Malar J. 2012;11(1):225. doi:10.1186/1475-2875-11-225
12. Manyando C, Mkandawire R, Puma L, et al. Safety of artemether-lumefantrine in pregnant women with malaria: results of a prospective cohort study in Zambia. Malar J. 2010;9(1):249. doi:10.1186/1475-2875-9-249
13. McGready R, Lee S, Wiladphaingern J, et al. Adverse effects of falciparum and vivax malaria and the safety of antimalarial treatment in early pregnancy: a population-based study. Lancet Infect Dis. 2012;12(5):388–396. doi:10.1016/S1473-3099(11)70339-5
14. McGready R, Brockman A, Cho T, et al. Randomized comparison of mefloquine-artesunate versus quinine in the treatment of multidrug-resistant falciparum malaria in pregnancy. Trans R Soc Trop Med Hyg. 2000;94(6):689–693. doi:10.1016/S0035-9203(00)90235-9
15. McGready R, Tan SO, Ashley EA, et al. A randomised controlled trial of artemether-lumefantrine versus artesunate for uncomplicated Plasmodium falciparum treatment in pregnancy. PLoS Med. 2008;5(12):e253. doi:10.1371/journal.pmed.0050253
16. McGready R, Cho T, Keo NK, et al. Artemisinin antimalarials in pregnancy: a prospective treatment study of 539 episodes of multidrug-resistant Plasmodium falciparum. Clin Infect Dis. 2001;33(12):2009–2016. doi:10.1086/324349
17. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. doi:10.1016/j.jclinepi.2009.06.006
18. Sterne J, Savović J, Page M, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. 2019;366:l4898. doi:10.1136/bmj.l4898
19. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi:10.1016/j.ijsu.2014.07.013
20. World Health Organization. Methods for Surveillance of Antimalarial Drug Efficacy. Geneva 27, Switzerland: World Health Organization; 2009.
21. Brodley C, Lau J, Schmid C. Open meta-analyst; 2012. Available from: http://www.cebm.brown.edu/openmeta/downloads/open_meta_analyst_win8.zip(11.05.2018).
22. Sedgwick P. Meta-analyses: heterogeneity and subgroup analysis. Br Med J. 2013;346:f4040. doi:10.1136/bmj.f4040
23. Iribhogbe OI, Emmanuel I, Odianosen M. Comparative analysis of the safety and tolerability of fixed-dose artesunate/amodiaquine versus artemether/lumefantrine combinations for uncomplicated falciparum malaria in pregnancy: a randomized open label study. Clin Pharmacol. 2017;9:45. doi:10.2147/CPAA.S131351
24. Iribhogbe OI, Igue EO, Odianosen M. Assessment of the safety of non-fixed-dose combination of artesunate and amodiaquine for uncomplicated falciparum malaria in pregnancy: a nonrandomized open-label study. J Pharm Health Serv Res. 2017;8(1):31–38. doi:10.1111/jphs.12166
25. Ukah M, Badejoko O, Ogunniyi S, Loto O, Aboderin O, Fatusi A. A randomized trial of artesunate-amodiaquine versus artemether-lumefantrine for the treatment of acute uncomplicated malaria in pregnancy. Int J Gynaecol Obstet. 2015;131(1):41–44. doi:10.1016/j.ijgo.2015.05.009
26. Mosha D, Mazuguni F, Mrema S, Sevene E, Abdulla S, Genton B. Safety of artemether-lumefantrine exposure in first trimester of pregnancy: an observational cohort. Malar J. 2014;13(1):197. doi:10.1186/1475-2875-13-197
27. Mutabingwa TK, Muze K, Ord R, et al. Randomized trial of artesunate+ amodiaquine, sulfadoxine-pyrimethamine+ amodiaquine, chlorproguanal-dapsone and SP for malaria in pregnancy in Tanzania. PLoS One. 2009;4(4):e5138. doi:10.1371/journal.pone.0005138
28. Adam I, Elhassan E. O263 safety of artemisinins during early pregnancy, assessed in 62 Sudanese women. Int J Gynecol Obstet. 2009;107:S168–S168. doi:10.1016/S0020-7292(09)60635-4
29. Adam I, Ali DM, Abdalla MA. Artesunate plus sulfadoxine—pyrimethamine in the treatment of uncomplicated Plasmodium falciparum malaria during pregnancy in eastern Sudan. Trans R Soc Trop Med Hyg. 2006;100(7):632–635. doi:10.1016/j.trstmh.2005.09.019
30. Kaye DK, Nshemerirwe R, Mutyaba TS, Ndeezi G. A randomized clinical trial comparing safety, clinical and parasitological response to artemether-lumefantrine and chlorproguanil-dapsone in treatment of uncomplicated malaria in pregnancy in Mulago hospital, Uganda. J Infect Dev Ctries. 2008;2(2):135–139. doi:10.3855/T2.2.135
31. Valea I, Tinto H, Traore/Coulibaly M, et al. Pharmacokinetics of co-formulated mefloquine and artesunate in pregnant and non-pregnant women with uncomplicated Plasmodium falciparum infection in Burkina Faso. J Antimicrob Chemother. 2014;69(9):2499–2507. doi:10.1093/jac/dku154
32. Onyamboko MA, Hoglund RM, Lee SJ, et al. A randomized controlled trial of three-versus five-day artemether-lumefantrine regimens for treatment of uncomplicated Plasmodium falciparum malaria in pregnancy in Africa. Antimicrob Agents Chemother. 2020;64:e01140–19.
33. Osarfo J, Tagbor H, Cairns M, Alifrangis M, Magnussen P. Dihydroartemisinin‐piperaquine versus artesunate‐amodiaquine for treatment of malaria infection in pregnancy in Ghana: an open‐label, randomised, non‐inferiority trial. Trop Med Int Health. 2017;22(8):1043–1052. doi:10.1111/tmi.12905
34. Kalilani L, Mofolo I, Chaponda M, et al. A randomized controlled pilot trial of azithromycin or artesunate added to sulfadoxine-pyrimethamine as treatment for malaria in pregnant women. PLoS One. 2007;2(11):e1166. doi:10.1371/journal.pone.0001166
35. Pekyi D, Ampromfi AA, Tinto H, et al.; PREGACT Study Group. Four artemisinin-based treatments in African pregnant women with malaria. N Engl J Med. 2016;374:913–927.
36. Nambozi M, Tinto H, Mwapasa V, et al. Artemisinin-based combination therapy during pregnancy: outcome of pregnancy and infant mortality: a cohort study. Malar J. 2019;18(1):105. doi:10.1186/s12936-019-2737-7
37. Nambozi M, Kabuya J-B-B, Hachizovu S, et al. Artemisinin-based combination therapy in pregnant women in Zambia: efficacy, safety and risk of recurrent malaria. Malar J. 2017;16(1):199. doi:10.1186/s12936-017-1851-7
38. Anvikar AR, Kuepfer I, Mishra V, et al. Efficacy of two artemisinin-based combinations for the treatment of malaria in pregnancy in India: a randomized controlled trial. Malar J. 2018;17(1):246. doi:10.1186/s12936-018-2393-3
39. Moore KA, Simpson JA, Paw MK, et al. Safety of artemisinins in first trimester of prospectively followed pregnancies: an observational study. Lancet Infect Dis. 2016;16(5):576–583. doi:10.1016/S1473-3099(15)00547-2
40. McGready R, Ashley EA, Moo E, et al. A randomized comparison of artesunate-atovaquone-proguanil versus quinine in treatment for uncomplicated falciparum malaria during pregnancy. J Infect Dis. 2005;192(5):846–853. doi:10.1086/432551
41. McGready R, Cho T, Cho JJ, et al. Artemisinin derivatives in the treatment of falciparum malaria in pregnancy. Trans R Soc Trop Med Hyg. 1998;92(4):430–433. doi:10.1016/S0035-9203(98)91081-1
42. McGready R, Keo NK, Villegas L, White NJ, Looareesuwan S, Nosten F. Artesunate-atovaquone-proguanil rescue treatment of multidrug-resistantPlasmodium falciparum malaria in pregnancy: a preliminary report. Trans R Soc Trop Med Hyg. 2003;97(5):592–594. doi:10.1016/S0035-9203(03)80040-8
43. Tarning J, McGready R, Lindegardh N, et al. Population pharmacokinetics of lumefantrine in pregnant women treated with artemether-lumefantrine for uncomplicated Plasmodium falciparum malaria. Antimicrob Agents Chemother. 2009;53(9):3837–3846. doi:10.1128/AAC.00195-09
44. Sowunmi A, Oduuola A, Ogundahunsi O, et al. Randomised trial of artemether versus artemether and mefloquine for the treatment of chloroquine/sufadoxine-pyrimethamine-resistant falciparum malaria during pregnancy. J Obstet Gynaecol. 1998;18(4):322–327. doi:10.1080/01443619867038
45. Saito M, Gilder ME, Nosten F, McGready R, Guérin PJ. Systematic literature review and meta-analysis of the efficacy of artemisinin-based and quinine-based treatments for uncomplicated falciparum malaria in pregnancy: methodological challenges. Malar J. 2017;16:1–17. doi:10.1186/s12936-016-1650-6
46. McGready R, Stepniewska K, Lindegardh N, et al. The pharmacokinetics of artemether and lumefantrine in pregnant women with uncomplicated falciparum malaria. Eur J Clin Pharmacol. 2006;62(12):1021. doi:10.1007/s00228-006-0199-7
47. McGready R, Stepniewska K, Ward S, et al. Pharmacokinetics of dihydroartemisinin following oral artesunate treatment of pregnant women with acute uncomplicated falciparum malaria. Eur J Clin Pharmacol. 2006;62(5):367–371. doi:10.1007/s00228-006-0118-y
48. Kloprogge F, Piola P, Dhorda M, et al. Population pharmacokinetics of lumefantrine in pregnant and nonpregnant women with uncomplicated Plasmodium falciparum malaria in Uganda. CPT. 2013;2:1–10.
49. Wangboonskul J, White N, Nosten F, Ter Kuile F, Moody R, Taylor R. Single dose pharmacokinetics of proguanil and its metabolites in pregnancy. Eur J Clin Pharmacol. 1993;44(3):247–251. doi:10.1007/BF00271366
50. Vallely A, Vallely L, Changalucha J, Greenwood B, Chandramohan D. Intermittent preventive treatment for malaria in pregnancy in Africa: what’s new, what’s needed? Malar J. 2007;6(1):16. doi:10.1186/1475-2875-6-16
51. Douglas NM, Anstey NM, Angus BJ, Nosten F, Price RN. Artemisinin combination therapy for vivax malaria. Lancet Infect Dis. 2010;10(6):405–416. doi:10.1016/S1473-3099(10)70079-7
52. Rijken MJ, McGready R, Jullien V, et al. Pharmacokinetics of amodiaquine and desethylamodiaquine in pregnant and postpartum women with Plasmodium vivax malaria. Antimicrob Agents Chemother. 2011;55(9):4338–4342. doi:10.1128/AAC.00154-11
53. Hoglund RM, Adam I, Hanpithakpong W, et al. A population pharmacokinetic model of piperaquine in pregnant and non-pregnant women with uncomplicated Plasmodium falciparum malaria in Sudan. Malar J. 2012;11(1):398. doi:10.1186/1475-2875-11-398
54. Preston Maxwell DJ. The use of quinine during pregnancy, labour, and the puerperium. Trans R Soc Trop Med Hyg. 1908;1:229–235. doi:10.1016/S0035-9203(07)90031-0
55. Desai M, Gutman J, L’lanziva A, et al. Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin–piperaquine versus intermittent preventive treatment with sulfadoxine–pyrimethamine for the control of malaria during pregnancy in western Kenya: an open-label, three-group, randomised controlled superiority trial. The Lancet. 2015;386:2507–2519.
56. Kakuru A, Jagannathan P, Muhindo MK, et al. Dihydroartemisinin–piperaquine for the prevention of malaria in pregnancy. N Engl J Med. 2016;374(10):928–939. doi:10.1056/NEJMoa1509150
57. Rijken MJ, McGready R, Boel ME, et al. Dihydroartemisinin—Piperaquine rescue treatment of multidrug-resistant Plasmodium falciparum malaria in pregnancy: a preliminary report. Am J Trop Med Hyg. 2008;78:543–545. doi:10.4269/ajtmh.2008.78.543
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


