Back to Journals » Drug Design, Development and Therapy » Volume 17
Efficacy and Safety of Anlotinib in Overall and Disease-Specific Advanced Gynecological Cancer: A Real-World Study
Authors Hong X, Qiu S, Wu X, Chen S, Chen X , Zhang B, He A, Xu Y, Wang J, Gao Y, Xu X, Sun L, Zhang Y, Xiang L, Zhou J, Guan Q, Zhu Y, Liu H , Xu H, Zhou Y, Chen B , Shen Y
Received 12 February 2023
Accepted for publication 3 June 2023
Published 6 July 2023 Volume 2023:17 Pages 2025—2033
DOI https://doi.org/10.2147/DDDT.S408304
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yan Zhu
Xinyi Hong,1,* Shanhu Qiu,2,* Xia Wu,3 Sizhen Chen,4 Xiaoxiang Chen,5 Bei Zhang,6 Aiqin He,3 Yun Xu,7 Jianqing Wang,8 Yingchun Gao,9 Xizhong Xu,10 Li Sun,11 Yang Zhang,12 Libing Xiang,13 Jundong Zhou,14 Qun Guan,15 Yanling Zhu,16 Haiyan Liu,17 Hao Xu,18 Ying Zhou,19 Bingwei Chen,4 Yang Shen1
1Department of Obstetrics and Gynecology, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, Jiangsu, 210009, People’s Republic of China; 2Department of General Practice, Zhongda Hospital, Institute of Diabetes, School of Medicine, Southeast University, Nanjing, People’s Republic of China; 3Department of Gynecological Oncology, Tumor Hospital Affiliated to Nantong University, Nantong, Jiangsu, People’s Republic of China; 4Department of Epidemiology and Biostatistics, School of Public Health, Southeast University, Nanjing, People’s Republic of China; 5Department of Gynecological Oncology, Jiangsu Cancer Hospital, The Affiliated Cancer Hospital of Nanjing Medical University, Nanjing, People’s Republic of China; 6Department of Obstetrics and Gynecology, Xuzhou Central Hospital, Xuzhou, People’s Republic of China; 7Department of Obstetrics and Gynecology, The First People’s Hospital of Changzhou, Changzhou, People’s Republic of China; 8Department of Obstetrics and Gynecology, The Yancheng Clinical College of Xuzhou Medical University, The First People’s Hospital of Yancheng, Yancheng, People’s Republic of China; 9Department of Obstetrics and Gynecology, Huai’an First People’s Hospital, The Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University, Huai’an, People’s Republic of China; 10Department of Obstetrics and Gynecology, The Affiliated Hospital of Jiangnan University, Wuxi, People’s Republic of China; 11Department of Gynecological Oncology, Qingdao Central Hospital, The Second Affiliated Hospital of Medical College of Qingdao University, Qingdao, People’s Republic of China; 12Gynecology Department, The First People’s Hospital of Lianyungang, Lianyungang, People’s Republic of China; 13Ovarian Cancer Program, Department of Gynecologic Oncology, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 14Radio-oncology Department, Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou, People’s Republic of China; 15Gynecology Department, Jiangsu Province Hospital of Chinese Medicine, Nanjing, People’s Republic of China; 16Gynecology Department, Xuzhou Cancer Hospital, Xuzhou, People’s Republic of China; 17Department of Obstetrics and Gynecology, Affiliated Hospital of Yangzhou University, Yangzhou, People’s Republic of China; 18Gynecology Department, Huangshi Love & Health Hospital Affiliated to Hubei Polytechnic University, Huangshi, People’s Republic of China; 19The First Affiliated Hospital of USTC, University of Science and Technology of China, Hefei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yang Shen, Department of Obstetrics and Gynecology, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, Jiangsu, 210009, People’s Republic of China, Tel +86 15366166769, Fax +86 +25 83272111, Email [email protected] Bingwei Chen, Department of Epidemiology and Biostatistics, School of Public Health, Southeast University, Nanjing, 210009, People’s Republic of China, Email [email protected]
Purpose: Anlotinib is a novel oral small-molecule multi-target tyrosine kinase inhibitor that has been approved for treating non-small cell lung cancer. However, its efficacy and safety among patients with advanced gynecological cancer have not been comprehensively evaluated. We conducted this study to address this issue in the real-world setting.
Patients and Methods: Data from patients treated with Anlotinib for persistent, recurrent or metastatic gynecological cancer were collected from 17 centers from August 2018. The database lock-time was on March 2022. Anlotinib was administered orally on days 1– 14 every 3 weeks until disease progression, severe toxicity occurred, or death. In this study, disease-specific advanced gynecological cancer was mainly referred to cervical, endometrial, and ovarian cancer. The outcomes included objective response rate (ORR), disease control rate (DCR), and progression-free survival (PFS).
Results: A total of 249 patients were analyzed, with a median follow-up of 14.5 months. The overall ORR and DCR were 28.1% [95% confidence interval (CI) 22.6% to 34.1%] and 80.7% (95% CI 75.3% to 85.4%), respectively. Specifically, the ORR varied from 19.7% to 34.4% and the DCR differed from 81.7% to 90.0% in disease-specific advanced gynecological cancer. The median PFS was 6.1 months and ranged from 5.6 to 10.0 months in the overall and disease-specific advanced gynecological cancer, respectively. Larger cumulative dosage of Anlotinib (> 700 mg) was in general associated with longer PFS in the overall and disease-specific advanced gynecological cancer. The most common adverse event related to Anlotinib treatment was pain/arthralgia (18.3%).
Conclusion: In conclusion, Anlotinib holds promise in treating patients with advanced gynecological cancer including its disease-specific types, with reasonable efficacy and tolerable safety.
Keywords: molecular-targeting therapy, angiogenesis inhibitors, real-world study
Introduction
Gynecological cancer, majorly comprised of cervical, endometrial, and ovarian cancer, is one of the leading causes of death among women.1 Despite the outcomes related to advanced gynecological cancer have been substantially improved in the past decades, resistance or nonresponse to standard treatment, in particular the first-line treatment, challenges the overall survival of patients with advanced gynecological cancer.2 Chemotherapy, immunotherapy, and targeted therapy have been recommended to treat advanced gynecological cancer. However, their efficacy remains concerned. For instance, in the GOG-0218 randomized Phase III trial, bevacizumab combined with chemotherapy showed longer progression-free survival (PFS) than chemotherapy alone for patients with stage III and IV epithelial ovarian and fallopian tube cancer following surgical resection.3 However, no further improvement was observed for the overall survival during the subsequent follow-ups. Similar outcomes were also reported in the AURELIA, OCEANS, and GOG-240 studies.4,5 These collectively point to the urgent need of efficacious treatment options for advanced gynecological cancer including its disease-specific types such as cervical, endometrial, and ovarian cancer.
Several randomized controlled trials suggest that tyrosine kinase inhibitors (TKIs), such as pazopanib, nintedanib, and cediranib,6 are effective in prolonging PFS and might outperform bevacizumab in improving overall survival in patients with ovarian cancer.7 Anlotinib, a novel oral small-molecule multi-target TKI, was marketed in China in May 2018 through the priority review procedure by the National Medical Products Administration for the treatment of malignant tumors including non-small cell lung cancer and soft tissue sarcoma,8 but unfortunately, not for gynecologic cancer. However, a search on the Clinical Trials.gov yielded more than 50 registered Clinical Trials on Anlotinib for gynecologic cancer, highlighting its therapeutic potential. Indeed, there have been several studies assessing the efficacy and safety of Anlotinib in patients with gynecological cancer (such as cervical or ovarian cancer).9 For instance, a Phase II single-arm prospective study of 41 patients with recurrent or metastatic cervical cancer showed that Anlotinib monotherapy was associated with an objective response rate (ORR) of 24.4%, a disease control rate (DCR) of 58.5%, and a median PFS of 3.2 months. Another study, which enrolled 15 patients with advanced ovarian cancer who had experienced disease progression after chemotherapy, found that Anlotinib resulted in an ORR of 20.0% and a median PFS of 3.5 months, with fatigue and anorexia as the major adverse events (AEs). However, interpretation from their findings is largely limited to the very small sample sizes (ranged from 20 to 42).8–14 Furthermore, it remains unclear whether there is any factor affecting the effectiveness of Anlotinib treatment.
Considering these, and given that the real-world study better accounts for the real clinical environment and shows higher external validity in developing clinical evidence-base than randomized controlled trials,15 we conducted this multi-center analysis to assess the efficacy and safety of Anlotinib treatment in the overall and disease-specific advanced gynecological cancer in the real-world setting.
Materials and Methods
Study Design
This study was a multi-center retrospective analysis of medical records and image data based on the hospital information system and picture archiving and communication system from 17 medical centers located in Nanjing, Changzhou, Wuxi and other places in China. Information from patients with advanced gynecological cancer (eg, cervical, endometrial, and ovarian cancer et al), who received Anlotinib treatment from August 2018 and, thereafter, was collected via the online system, generating a gynecological cancer database.
In this database, we collected variables including sociodemographic status, tumor type, Eastern Cooperative Oncology Group performance status (ECOG PS), history of chronic disease, lifestyle factors (drinking and smoking), history of previous surgery and medication therapy, laboratory examination before and after treatment, and imaging evaluation, when available. This study did not interfere with any clinical medication in all the patients. The database lock-time for this study was on March 31, 2022. This study was approved by the Ethics Committee of Zhongda Hospital, Southeast University, and adhered to the Declaration of Helsinki. Informed consent was waived due to the retrospective nature of the analysis, which involved no human tissue collection and storage process. All patient data involved in this study were kept confidential and used solely for relevant academic analysis.
Patients
Participants aged ≥18 years were included if they met the following criteria: being diagnosed with any persistent, recurrent or metastatic gynecologic cancer based on histopathology and/or cytology; being administrated with Anlotinib due to the nonresponse or resistance to standard treatment; and providing available information at baseline and follow-up. Participants were excluded if they: were concomitant with another primary cancer, had a follow-up time-length <1 month prior to the database lock-time (unless disease progression or death occurred), or did not provide adequate follow-up information on the efficacy assessment.
Anlotinib Treatment
Anlotinib was used alone or in combination with other treatments (eg, chemotherapy). Anlotinib capsules were available in 8, 10 and 12 mg, respectively. The initial dose for treatment was determined according to the health condition of the patient and the experience from clinicians, which was set at 10 or 12 mg in general. Patients were administered with Anlotinib orally on days 1–14 every 3 weeks for different cycles until disease progression, severe toxicity occurred, or death. The cumulative dosage for Anlotinib was calculated as the number of days (number of cycles × 14 days) × daily dose (that is, 8, 10, or 12 mg). In this study, we used the median cumulative dosage (that is, 700 mg) as the cut-off value to define the smaller and larger cumulative dosages.
Outcomes
The outcomes assessed in this study included the ORR, DCR, and PFS. ORR was defined as the proportion of patients with a complete or partial response who had the best overall response based on Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. DCR was calculated as (CR+PR+SD)/n × 100%, where CR referred to complete response, PR to partial response, and SD to stable disease. PFS was defined as the time from the first medication to the first recording of disease progression or death from any cause. For this, at least one record of imaging (eg, computed tomography or magnetic resonance imaging) had to be provided within 6 months following Anlotinib treatment.
Tumor response was assessed according to the RECIST version 1.1. If disease progression occurred in the context of the imaging assessment, the study treatment follow-up was ended then. Safety was assessed throughout the study and 30 days following the treatment discontinuation based on the Common Terminology Criteria for Adverse Events version 4.03.
Statistical Analyses
Categorical variables were presented as frequencies and percentages, and continuous variables were described as medians (ranges). Clopper-Pearson method was used to 95% confidence interval (CI) for the binomial proportion. Kaplan–Meier method was used to estimate the survival curve of PFS with 95% confidence intervals (CIs). For patients who survived or did not experience disease progression at the time of analysis, their follow-up time was calculated to the latest date of the periodic assessment. Subgroup analyses for PFS were conducted, based on age (<65 vs ≥65 years), history of hypertension or diabetes (yes vs no), history of antiangiogenic drug use (yes vs no), Anlotinib treatment time-point (as second- vs third- vs further-line treatment), ECOG/PS (0–1 vs ≥2), FIGO stage at diagnosis (I–II vs III–IV), history of previous therapy (had or did not have surgery, platinum-based chemotherapy, radiotherapy history), Anlotinib treatment regimens (monotherapy vs combination therapy), and cumulative dosage for Anlotinib (≤700 mg vs >700 mg), with their differences being compared using a Log rank test, in the overall or disease-specific advanced gynecological cancer. Specifically, in this study disease-specific advanced gynecological cancer was mainly referred to advanced cervical, endometrial, and ovarian cancer. All statistical analyses were performed using the R software version 4.1.0 (R Project for Statistical Computing), with a 2-sided P < 0.05 considered statistically significant.
Results
Baseline Characteristics
A total of 320 patients treated with Anlotinib for advanced gynecologic cancer were included. Among them, one patient was excluded due to the lack of biopsy to ascertain tumor type, 11 were due to the incomplete data at baseline, 58 were due to the missing information on follow-up evaluation, and one was due to irregular use of Anlotinib, leaving 249 patients eligible for the final analysis (Figure 1).
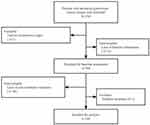 |
Figure 1 Study flowchart. |
The baseline characteristics of included patients are shown in Table 1. Their median age was 57 years (range: 33 to 87 years). No patients reported a history of smoking and alcohol abuse. Most patients were diagnosed as ovarian cancer (50.2%), followed by cervical cancer (28.5%), and endometrial cancer (12.0%). A total of 166 patients (66.7%) had the ECOG/PS score of 0–1 when starting Anlotinib treatment, and about one-half were with FIGO stage III–IV at diagnosis. Prior to the use of Anlotinib, 81.1% of the patients received platinum-based chemotherapy, 63.5% received surgery, and 26.9% received adjuvant radiotherapy (P < 0.01).
 |
Table 1 Baseline Characteristics |
The proportions of treatment lines prior to the use of Anlotinib are shown in Figure 2, with 53.8% having three- or further-line treatment. Specifically, more than 50% of the patients with ovarian or cervical cancer received third-line treatments, while only about 33.3% and 33.0% of the patients with endometrial cancer received second- and third-line treatment, respectively. In addition, 35.3% of the patients received prior antiangiogenic drug therapy (mainly bevacizumab). About 42.0% patients received Anlotinib as monotherapy, while the remaining was in combination with other therapies. Patients with endometrial cancer were more likely to receive Anlotinib monotherapy.
Effectiveness
The median follow-up period for included patients was 14.5 months (95% CI, 12.2 to 16.8 months). Based on the RECIST version 1.1, their overall ORR and DCR were 28.1% (95% CI 22.6% to 34.1%) and 80.7% (95% CI 75.3% to 85.4%), respectively (Table 2). Complete response was achieved in 21 cases (8.4%, 95% CI 5.3% to 12.6%). The overall median PFS was 6.1 months (95% CI 5.1 to 7.0 months), and the 6-month and 12-month PFS rates were 49.5% (43.1% to 56.9%) and 24.2% (95% CI 18.5% to 31.7%), respectively. The Log rank test suggested that age, history of hypertension or diabetes, history of antiangiogenic drug use, and history of previous therapy were not related to PFS (all P > 0.05). However, FIGO stage of I–II at diagnosis, ECOG score of 0–1, Anlotinib as second-line treatment, used as combination therapy, and larger cumulative dosage of Anlotinib (>700 mg) were associated with longer PFS (all P ≤ 0.02) in the overall advanced gynecologic cancer.
 |
Table 2 Analysis of the Efficacy of Anlotinib |
In terms of disease-specific advanced gynecological cancer (that is, cervical, endometrial, and ovarian cancer), the ORR varied from 19.7% to 34.4%, the DCR differed from 81.7% to 90.0%, and the median PFS ranged from 5.6 to 10.0 months (Table 2). Moreover, longer PFS was associated with ECOG score of 0–1, FIGO stage of I–II and larger cumulative dosage of Anlotinib in patients with advanced cervical cancer (all P ≤ 0.01), and with ECOG score of 0–1, combination therapy with Anlotinib, and larger cumulative dosage of Anlotinib in patients with advanced ovarian cancer (all P ≤ 0.01) (Supplementary Figures 1–3).
Safety
Of the 246 patients with available information, 43.9% reported AEs related to Anlotinib treatment during follow-up. The most common AEs were pain/arthralgia (18.3%), followed by nausea and vomiting (8.5%), neutropenia (5.7%), bleeding (4.0%, including 2 cases of epistaxis, 4 cases of vaginal bleeding, and 4 cases of gastrointestinal bleeding) in the overall advanced gynecological cancer (Table 3).
 |
Table 3 Treatment-Related Adverse Events of Any Grade and Grade 3/4 AEs in All Patients |
The reported AEs were mild (grade 1/2) in general. There were 28 patients reported grade 3/4 AEs, which resulted in dose reduction in 2 patients and discontinuation in 11 patients. Specifically, grade 3 AEs included neutropenia (3.2%), pain/arthralgia (2.0%), deep vein thrombosis (1.2%), bleeding (0.8%), ulcer (0.8%), hypertension (0.8%), hypoproteinemia (0.4%) and atrial fibrillation (0.4%), and grade 4 AEs included hypertension (0.8%), gastrointestinal bleeding (0.4%), and bradycardia (0.4%). No death events were reported. The information on the AEs in patients with disease-specific advanced gynecological cancer is shown in Supplementary Table 1, which was in general consistent with the overall population.
Discussion
Main Findings
To the best of our knowledge, this study was the first analysis in the real-world setting enrolling patients with advanced gynecologic cancer from 17 medical centers in China, who were treated with Anlotinib mostly as third- or further-line treatment. We showed in overall and disease-specific advanced gynecologic cancer that (i) Anlotinib had favorable antitumor activity and may help to control disease progression; (ii) larger cumulative dosage of Anlotinib use seemed to be associated with longer PFS; and (iii) Anlotinib exhibited a tolerable safety in clinical application.
Interpretation and Implications
The latest NCCN guideline issued in 2021 recommends bevacizumab and other antiangiogenic drugs as the first-line treatment for recurrent ovarian cancer and cervical cancer. However, no further instructions were provided on the following treatment approaches for patients who are resistant or nonresponsive to the standard treatment.16–18 Anlotinib is a novel oral small-molecule multi-target TKIs, which acts on VEGFR, PDGFR and FGFR targets comprehensively in the angiogenesis pathway. It inhibits the targets like c-Kit, Ret, and c-Met, and exerts functionalities such as anti-angiogenesis and inhibiting tumor cell proliferation and metastasis.19 Although there has been an increasing interest in exploring the efficacy of Anlotinib in advanced gynecologic cancer, which is evidenced by the ongoing number of registered trials on Clinical Trials.gov website, Anlotinib has only been approved for treatment of cancers such as non-small cell lung cancer.
As an attempt to support the “off-label” use of Anlotinib for patients with advanced gynecological cancer, our study, which included patients from 17 centers, showed that the ORR associated with Anlotinib treatment was about 30%, with a DCR up to 80% and the median PFS around 6 months in the overall advanced gynecological cancer under the real-world setting. Our findings appear to be more encouraging than prior studies. In the study of recurrent or metastatic cervical cancer, the median PFS from Anlotinib treatment was only reported to be 3.2 months, and the ORR and DCR were 24.4% and 58.5%, respectively.10 Another study in patients with platinum-resistant advanced ovarian cancer, the authors observed only an ORR of 20.0% and the median PFS of 3.5 months related to Anlotinib treatment.14
Interestingly, we found that patients who had the FIGO stage of I–II or the ECOG score of 0–1 at diagnosis might benefit more from the use of Anlotinib in terms of longer PFS. This, to some extent, indicates that baseline health condition might affect the outcomes from Anlotinib treatment. This outcome was also partly observed in patients with advanced ovarian or cervical cancer. Our analysis detected a significant difference on the PFS in relation to the Anlotinib treatment time-point, showing that patients who started using Anlotinib earlier after their nonresponsive or resistance to the standard first-line treatment might have longer PFS. Moreover, Anlotinib treatment in combination with other therapies led to a longer PFS than using Anlotinib as monotherapy, in particular in patients with advanced ovarian cancer. In addition to these, we also found that Anlotinib, which was administrated at a higher cumulative dosage, was associated with longer PFS in advanced gynecological cancer including its disease-specific types such as cervical and ovarian cancer. This suggests that treatment with Anlotinib for a longer time period might bring larger benefits. However, it could be also explained by the fact that some patients responded well to the Anlotinib treatment, resulting in a higher cumulative dosage because of the prolonged treatment periods, and subsequently longer PFS. However, we did not show that the presence of diabetes or hypertension, which might worsen the outcomes of advanced gynecologic cancer such as advanced ovarian or cervical cancer, affected the PFS.
For the safety of Anlotinib, we did not detect any death events, and the outcomes on the AEs are consistent with the results from other antiangiogenic agents. Overall, the incidence of grade 3/4 AEs in our study (11.38%) was lower than that in the previous trial of sintilimab and Anlotinib for advanced non-small cell lung cancer (54.5%).12 However, it is worth noting that the most common AEs observed in our study for Anlotinib in the overall population were hand/foot pain and arthralgia, while AEs like hypertension, hypoproteinemia, and atrial fibrillation were relatively rare. In disease-specific advanced gynecological cancer, we found that patients with advanced ovarian cancer appeared to exhibit a higher incidence of adverse effects, although this might be largely attributable to differences in sample sizes compared with other advanced gynecological cancer types.
Our study also provided some insights into the preferences of the clinicians in prescribing Anlotinib for patients with advanced gynecologic cancer. It seems likely that clinicians were more in favor of the use of Anlotinib as monotherapy or to be combined with a class of drugs related to chemotherapy. Moreover, approximately 54.0% of the patients were prescribed with Anlotinib as the third-line or further treatment. This might be probably due to the very limited evidence regarding the efficacy and safety of Anlotinib as an earlier treatment-line.
Strengths and Limitations
A strength of our study was the largest sample size from 17 medical centers in China than any other prior studies that investigated the efficacy and safety of Anlotinib in overall or disease-specific advanced gynecologic cancer patients. Another strength was the employment of a series of subgroup analyses that enriched our understanding of the clinical use for Anlotinib. However, our study has some limitations. First, as a retrospective observational study, our data were derived from medical records and imaging data of HIS and PACS systems, which may lead to increased risk of selection bias. Second, some information at baseline, such as the immunohistochemistry, gene sequencing datum, ECOG PS, was not obtained or not fully available, which might confound our findings. Finally, the adherence to Anlotinib treatment was not collected and there was a lack of standardized imaging evaluation during follow-up in our study. These may further affect the robustness of our main findings. Moreover, due to the very small sample sizes in other tumor types, such as vaginal carcinoma, we were not able to provide sufficient analyses on the efficacy and safety of Anlotinib treatment in these patients.
Conclusion
In conclusion, our study shows that Anlotinib was effective in treating patients with advanced gynecological cancer including its disease-specific types, with favorable efficacy and tolerable safety in the real-world setting. However, well-designed prospective studies (eg, randomized controlled trials) are required to confirm our findings.
Acknowledgments
ShanHu Qiu has been supported by the “Best Young Scholars” Fellowship from Southeast University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yetkin-Arik B, Kastelein AW, Klaassen I, et al. Angiogenesis in gynecological cancers and the options for anti-angiogenesis therapy. Biochim Biophys Acta. 2021;1875:188446. doi:10.1016/j.bbcan.2020.188446
2. Inayama Y, Hamanishi J, Matsumura N, et al. Antitumor effect of nivolumab on subsequent chemotherapy for platinum-resistant ovarian cancer. Oncologist. 2018;23:1382–1384. doi:10.1634/theoncologist.2018-0167
3. Tewari KS, Burger RA, Enserro D, et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol. 2019;37:2317–2328. doi:10.1200/JCO.19.01009
4. Bamias A, Gibbs E, Khoon Lee C, et al. Bevacizumab with or after chemotherapy for platinum-resistant recurrent ovarian cancer: exploratory analyses of the AURELIA trial. Ann Oncol. 2017;28:1842–1848. doi:10.1093/annonc/mdx228
5. Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–2045. doi:10.1200/JCO.2012.42.0505
6. Lee C-H, Shah AY, Rasco D, et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): a phase 1b/2 study. Lancet Oncol. 2021;22:946–958. doi:10.1016/S1470-2045(21)00241-2
7. Jin C, Yuan M, Bu H, et al. Antiangiogenic strategies in epithelial ovarian cancer: mechanism, resistance, and combination therapy. J Oncol. 2022;2022:4880355. doi:10.1155/2022/4880355
8. Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. 2016;9:105. doi:10.1186/s13045-016-0332-8
9. Yang H, Sun S, Mei Z, et al. A retrospective cohort study evaluates clinical value of anlotinib in persistent, metastatic, or recurrent cervical cancer after failure of first-line therapy. Drug Des Devel Ther. 2021;15:4665–4674. doi:10.2147/DDDT.S335870
10. Zhu J, Song CY, Zheng Z, et al. Anlotinib in Chinese patients with recurrent advanced cervical cancer: a prospective single-arm, open-label phase II trial. Front Oncol. 2021;11:8. doi:10.3389/fonc.2021.720343
11. Xu Q, Wang J, Sun Y, et al. Efficacy and safety of sintilimab plus anlotinib for PD-L1-positive recurrent or metastatic cervical cancer: a multicenter, single-arm, prospective phase II trial. J Clin Oncol. 2022;40:1795–1805. doi:10.1200/JCO.21.02091
12. Wei W, Ban X, Yang F, et al. Phase II trial of efficacy, safety and biomarker analysis of sintilimab plus anlotinib for patients with recurrent or advanced endometrial cancer. J Immunother Cancer. 2022;10:e004338. doi:10.1136/jitc-2021-004338
13. Lan C-Y, Zhao J, Yang F, et al. Anlotinib combined with TQB2450 in patients with platinum-resistant or -refractory ovarian cancer: a multi-center, single-arm, phase 1b trial. Cell Rep Med. 2022;3:100689. doi:10.1016/j.xcrm.2022.100689
14. Cui Q, Hu Y, Ma D, et al. A retrospective observational study of anlotinib in patients with platinum-resistant or platinum-refractory epithelial ovarian cancer. Drug Des Devel Ther. 2021;15:339–347. doi:10.2147/DDDT.S286529
15. Eisenhauer EA. Real-world evidence in the treatment of ovarian cancer. J Eur Soc Med Oncol. 2017;28:viii61–viii65. doi:10.1093/annonc/mdx443
16. Abu-Rustum NR, Yashar CM, Bean S, et al. NCCN guidelines insights: cervical cancer, version 1.2020. JNCCN. 2020;18:660–666. doi:10.6004/jnccn.2020.0027
17. Abu-Rustum NR, Yashar CM, Bradley K, et al. NCCN guidelines® insights: uterine neoplasms, version 3.2021. JNCCN. 2021;19:888–895. doi:10.6004/jnccn.2021.0038
18. Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. Ovarian cancer, version 2.2020, NCCN clinical practice guidelines in oncology. JNCCN. 2021;19:191–226. doi:10.6004/jnccn.2021.0007
19. Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11:120. doi:10.1186/s13045-018-0664-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

