Back to Journals » International Journal of General Medicine » Volume 16
Efficacy and Safety of Anlotinib-Containing Regimens in Advanced Non-Small Cell Lung Cancer: A Real-World Study
Authors Sun L, Zhao Q, Wang Y, Wang Y, Zheng M, Ding X, Miao L
Received 14 June 2023
Accepted for publication 31 August 2023
Published 12 September 2023 Volume 2023:16 Pages 4165—4179
DOI https://doi.org/10.2147/IJGM.S424777
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Lei Sun,1,* Qi Zhao,2,* Yanning Wang,3,* Yongsheng Wang,2 Ming Zheng,2 Xuansheng Ding,4 Liyun Miao2
1School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University Nanjing Drum Tower Hospital, Nanjing, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, People’s Republic of China; 3Clinical Stem Cell Center, The Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing, People’s Republic of China; 4School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liyun Miao, Department of Respiratory and Critical Care Medicine, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, Nanjing, 210008, People’s Republic of China, Tel +86 13813920964, Fax +86-25-68183333, Email [email protected]
Purpose: Anlotinib is widely used in the clinical treatment of non-small cell lung cancer (NSCLC), alone or in combination with other anticancer drugs. The aim of this study was to investigate the real-world efficacy and safety of anlotinib-containing regimens.
Patients and Methods: Confirmed advanced NSCLC patients who had received anlotinib alone or in combination were enrolled. An overall analysis of the efficacy and safety of anlotinib was performed in all patients, and then subgroup analysis was used to further compare the efficacy between anlotinib monotherapy and combination therapy. The primary endpoint was progression-free survival (PFS), and the secondary endpoints were ADR, ORR, and DCR.
Results: A total of 240 patients were included. The overall median PFS was 8.5 months (95% confidence interval [CI]: 7.1– 9.9 months). Anlotinib treatment regimens (monotherapy or combination therapy) and whether they received previous antiangiogenesis were associated with PFS. Anlotinib plus immunotherapy achieved longer PFS than anlotinib monotherapy (median PFS: 10.5 vs 6.5 months, p=0.007). Stratification analysis showed the PFS of anlotinib plus immunotherapy was significantly longer in male, adenocarcinoma, <=65 years old, patients stage IV, EGFR wild type, with extrathoracic metastasis, performance status scores ≥ 2, the first-line treatment, patients with a history of hypertension and no previous antiangiogenesis than anlotinib monotherapy. The median PFS of anlotinib plus chemotherapy, targeted therapy was slightly longer than anlotinib alone (respectively, 10.5 vs 6.5 months, p=0.095; 9.5 vs 6.5 months, p=0.177). Adverse reactions were mostly mild and acceptable, with hypertension being the most common.
Conclusion: Anlotinib is effective and tolerable in advanced NSCLC patients. Immunotherapy combination with anlotinib significantly improved PFS. The efficacy of anlotinib may be impaired by previous antiangiogenic therapy, which can be investigated in further studies.
Keywords: non-small cell lung cancer, anlotinib, combination therapy, immunotherapy, efficacy, safety
Introduction
Over the last decade, lung cancer has been the leading cause of cancer-related death among all malignancies. Lung cancer caused estimated 2.2 million new cases (11.7%) and 1.8 million deaths (18%) in 2020, as the second most commonly diagnosed cancer and the highest cancer-related mortality worldwide.1 Non-small cell lung cancer (NSCLC) accounts for approximately 83% of lung cancer cases and tends to be diagnosed at advanced stages, causing a huge mortality burden.2
The processes of angiogenesis play crucial roles in cancer development, including NSCLC.3,4 Antiangiogenic agents, such as multi-target tyrosine kinase inhibitors (TKIs), bevacizumab (Avastin) and recombinant human endostatin injection (endostar), can achieve therapeutic benefit in a wide range of solid tumors.5 Anlotinib, a novel TKI that inhibits tumor angiogenesis and proliferation, was approved for monotherapy as a third and further-line treatment for advanced or metastatic NSCLC in 2018.6–8 Anlotinib monotherapy in third and further-line NSCLC treatment showed significantly longer progression-free survival (PFS) (median PFS: 5.4 vs 1.4 months) than patients receiving placebo9,10 At the same time, with the rise of the concept of combination therapy in the field of tumors, anlotinib is being used more widely in clinical practice. Preclinical researches and case reports demonstrated that anlotinib may modulate the tumor microenvironment and act synergistically with immune checkpoint inhibitors.11–17 Current studies have shown encouraging efficacy of the clinical use of anlotinib combination therapy, and a case reported first-line pemetrexed and carboplatin plus anlotinib achieved CR.18–22 However, the sample size of current studies is small and more studies are still needed to confirm the efficacy and safety of anlotinib combination therapy in different patients with NSCLC.
Anlotinib is widely used in the field of NSCLC, including different treatment regimens and treatment timing, and there is still a lack of comprehensive and systematic description and analysis. Therefore, we conducted this retrospective study to describe the status, efficacy and safety of anlotinib in real-world clinical applications, and conducted subgroup analysis to compare the efficacy of anlotinib combination with immunotherapy/chemotherapy/targeted therapy and anlotinib alone. We performed comprehensive adjusted analysis and stratified analysis to explore factors associated with PFS and explore what kind of patients will benefit from anlotinib combination therapy.
Materials and Methods
Patients
Patients who visited the Department of Respiratory and Critical Care Medicine of Nanjing Drum Tower Hospital between June 2019 and March 2022 were retrospectively collected. The inclusion criteria were as follows: pathologically confirmed advanced NSCLC; with at least one measurable lesion; treated with anlotinib or anlotinib contained regimens. Patients who received anlotinib within two cycles or failed to obtain follow-up information were excluded. The clinical information system (Zesing software) was used to collect baseline characteristics, including gender, age, Eastern Cooperative Oncology Group performance status (ECOG PS) score, pathological type, stage, sites of metastasis, oncogenic driver mutations, history of hypertension, prior and subsequent treatment regimens.
Treatment
Anlotinib (Chia Tai Tianqing Pharmaceutical, China) was orally administered (12mg/day for 2 weeks on and 1 week off) in all patients, and the administration was adjusted by clinicians according to the patient’s response during treatment. The combination regimens of anlotinib included the combination of immunotherapy drugs (programmed death-ligand 1 and programmed cell death protein 1 inhibitors intravenously administered: pembrolizumab [200mg] or sintilimab [200mg] or atezolizumab [1200mg] or camrelizumab [200mg] or tislelizumab [200mg] on day 1 of a 3-week cycle, nivolumab 3mg/kg or 240mg on day 1 of a 2-week cycle), combination of conventional chemotherapy drugs intravenously administered (pemetrexed [500mg/m2] or docetaxel [75mg/m2] on day 1 or gemcitabine [1250mg/m2] on day 1 and day 8 of a 21-day cycle), and combination of targeted therapy drugs orally administered, including epidermal growth factor receptor (EGFR) inhibitors, and anaplastic lymphoma kinase (ALK) inhibitors (gefitinib once daily at 250mg/day, erlotinib once daily at 150mg/day, osimertinib once daily at 80mg/day, crizotinib twice daily at 250mg/once). Previous antiangiogenic therapy included bevacizumab (Avastin) 400mg or 500mg, d1 or recombinant human endostatin injection (endostar) 30mg, d1-d7.
Efficacy and Safety
Therapeutic response was assessed based on Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 every 2 cycles, defined as complete response (CR), partial response (PR), stable disease (SD) and progression disease (PD). PFS was defined as the time firstly received anlotinib to the date of disease progression. OS was defined as the time anlotinib beginning to death from any cause. The safety of the treatment was evaluated by the occurrence of adverse events, and the severity of the adverse events was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 5.0.
Statistical Analysis
The general analysis of efficacy and safety of anlotinib in all patients with advanced NSCLC were performed at first. The survival curves for OS and PFS were estimated with the Kaplan–Meier method and were compared between two groups using the Log rank test. Cox regression was used to analyze the statistically significant factors associated with PFS. Factors with p value less than 0.05 in univariate analysis were included in multivariate cox regression analysis. Adjusted or stratified analysis was conducted to reduce the risk of bias. Baseline characteristics, ORR and DCR between two groups were compared using chi-square test or Fisher's exact test when appropriate. All statistical tests were carried out on the basis of a 2-sided a=0.05 and 95% confidence interval (CI). All analyses were performed using SPSS 25.0 software (IBM, Armonk, NY, USA) and GraphPad Prism 9.0.0 software (La Jolla, CA, USA).
Ethical Approval and Informed Consent
The study was approved by Medical Ethics Committee of Nanjing Drum Tower Hospital Affiliated to Nanjing University School of Medicine (GYHT20200023) and conducted according to the principles of the Declaration of Helsinki. Written consent was waived due to the retrospective nature of the review. We confirmed that the data was maintained with confidentiality.
Results
Baseline Characteristics of All Patients
Total 240 patients were included in our study, with a median age of 67 years (range, 33 to 91 years). There were 184(76.6%) males and 56(23.3%) females. While 63(26.2%) patients staged III, 177(73.7%) patients staged IV. Pathological types included adenocarcinoma (128, 53.3%) squamous cell carcinoma (91, 37.9%), and other type NSCLC (21, 8.7%). A total of 104 patients had extrathoracic metastases, including brain metastases (29, 12.0%), bone metastases (59, 24.5%), liver metastases (17, 7.0%) and kidney metastases (7, 2.9%). Fifty-two (21.6%) patients were diagnosed with EGFR mutation, and ALK rearrangement in 4/240 (1.6%) patients, ROS1 mutation in 2(0.8%), KRAS in 2(0.8%) and met14 mutation in 3(1.2%) patient. While 57 (23.7%) patients received anlotinib in first-line treatment, 76(31.6%) received anlotinib in second-line treatment, and 107(44.6%) patients received anlotinib in third or further-line treatment. Among all 240 patients, 82(34.2%) patients received anlotinib monotherapy and 158(65.8%) patients received anlotinib combination therapy, including anlotinib plus immunotherapy (74 cases)/chemotherapy (42 cases)/targeted therapy (33 cases) and anlotinib plus immune-chemotherapy (9cases). The characteristics information is shown in Supplementary Table 1.
Overall Efficacy of Treatment
Of the 240 patients, CR was not achieved, PR was achieved in 58(24.1%) patients, SD was achieved in 147(61.2%) patients, and PD in 35(14.5%) patients. The ORR was 24.1% and DCR was 85.4%. The average follow-up duration was 10.7 months, and by the end of follow-up, 18 patients were declared dead. The overall median PFS was 8.5 months (95% CI: 7.1–9.9m) and the median OS was undefined (Figure 1). Univariate analysis revealed patients received anlotinib combination therapy, no previous antiangiogenic therapy and had hypertension history had significantly longer mPFS (Figure 2A–C and Supplementary Table 1). After adjusted for sex, age, histology, stage, ps scores, treatment line (first, second, third and further-line), EGFR gene state, previous antiangiogenic therapy (yes vs no HR:1.956, 95% CI: 1.257–3.044, p=0.003) and therapy regimen (combination vs monotherapy HR:0.675, 95% CI: 0.463–0.984, p=0.041) were associated with PFS (Table 1). The different combination treatment modalities (plus immunotherapy/chemotherapy/targeted therapy) were analyzed, which showed immunotherapy combination with anlotinib significantly improved PFS (after adjusted, HR:0.619, 95% CI:0.383–1.000, P=0.05), anlotinib plus chemotherapy and plus targeted therapy had a tendency to prolong survival, respectively (Figure 2D and Supplementary Table 2).
 |
Table 1 Cox Regression Analysis of Factors Associated with PFS in All Patients |
 |
Figure 1 Kaplan–Meier curves of PFS and OS in all patients. (A) PFS: progression-free survival; (B) OS: overall survival. |
Anlotinib Combination Therapy vs Monotherapy
Baseline characteristics were comparable between the anlotinib combination therapy (n=158) with anlotinib alone (n=82) group (Table 2).
 |
Table 2 Comparison of Baseline Characteristics Between Anlotinib Monotherapy and Combination Therapy |
Efficacy of Anlotinib Combined with Immunotherapy
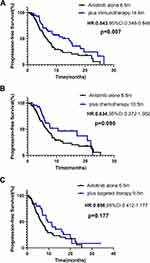
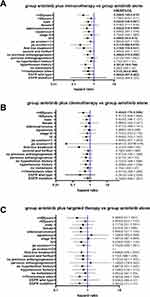
The basic characteristics were comparable between anlotinib combination and monotherapy group, except that patient EGFR-mutation status (Table 3). Patients receiving anlotinib plus immunotherapy (n=74) had a significantly longer median PFS than those receiving anlotinib alone (n=82), (14.4 vs 6.5 months; HR: 0.543, 95% CI: 0.348–0.846; p=0.007) (Figure 3A). The anlotinib alone group and plus immunotherapy group had similar ORR (14.6% vs 24.3%) and DCR (79.3% vs 90.5%) (Table 4). The median PFS was significantly longer with anlotinib plus immunotherapy than with anlotinib alone in male, adenocarcinoma, <=65 years old, patients stage IV, EGFR wild type, with or without extrathoracic metastasis, ps scores ≥2, the first-line treatment, patients with a history of hypertension and no previous antiangiogenesis (Figure 4 and Table 5). Forest plot of the stratification analysis is shown in Figure 5A
 |
Table 3 Comparison of Basic Characteristics of Patients Receiving Anlotinib Alone and Plus Immunotherapy, Chemotherapy, Targeted Therapy |
 |
Table 4 Response of All Patients and Subgroup Anlotinib Alone versus Combination Regimen |
 |
Table 5 Stratification Analysis of PFS Between Patients Receiving Anlotinib Alone and Plus Immunotherapy |
Efficacy of Anlotinib Combined with Chemotherapy
Patients receiving anlotinib plus chemotherapy (n=42) had a longer median PFS than those receiving anlotinib alone (n=82); however, the difference was not statistically significant (10.5 vs 6.5 months; HR: 0.634, 95% CI: 0.372–1.082; p=0.095) (Figure 3B). The ORR of anlotinib plus chemotherapy was higher than anlotinib monotherapy (31.0% vs 14.6%, p=0.032) (Table 4). The basic characteristics were well balanced in two groups, except that patient EGFR-mutation status and ps scores (Table 3). Median PFS was significantly longer in patients <=65 years (anlotinib plus chemotherapy vs anlotinib alone: 20.7 vs 3.7m, p=0.026) and first-line treatment (17.1 vs 6.9m, p=0.036). Although no statistically significant differences, median PFS of anlotinib plus chemotherapy tended to be longer in patients with adenocarcinoma, EGFR wild type, patients with a history of hypertension (Supplementary Table 3). Figure 5B present forest plot of the stratification analysis.
Efficacy of Anlotinib Combined with Targeted Therapy
Patients receiving anlotinib plus targeted therapy (n=33) had a slightly longer median PFS than those receiving anlotinib alone (9.5 vs 6.5 m; HR: 0.696, 95% CI: 0.412–1.177; p=0.177) (Figure 3C). The ORR of anlotinib plus targeted therapy was higher than monotherapy (33.3% vs 14.6%, p=0.023 (Table 4)). The basic characteristics were comparable between two groups, except patient histological type and status of EGFR mutation, sex and ps scores, which caused clinical differences in the choice of anlotinib therapy regimens (Table 3). Although not statistically significant, median PFS in patients receiving anlotinib plus targeted therapy tended to be longer in patients with adenocarcinoma, stage IV, ps scores<=1, first-line or second and further-line treatment, no previous antiangiogenic therapy, with EGFR mutation (Supplementary Table 4). Figure 5C present forest plot of the stratification analysis.
Safety Analysis
Anlotinib-related adverse reactions during the treatments with anlotinib alone or anlotinib combination therapy were well tolerable and there were no treatment-related deaths. The five most common adverse events were hypertension (64, 26.7%), fatigue (25, 10.4%), hand-foot syndrome (25, 10.4%), hemorrhinia (20, 8.3%) and cough (20, 8.3%), diarrhea (16, 6.7%), rash (16, 6.7%), oral mucositis (13, 5.4%). Grade 3–5 adverse events occurred in 34(14.2%) patients, including hypertension (21, 8.8%), hand-foot syndrome (6, 2.5%), hypothyroidism (3, 1.3%), hemorrhinia (2, 0.8%) and oral mucositis (1, 0.4%) (Table 6).
 |
Table 6 Anlotinib Treatment-Related Adverse Events in Patients Treated with Anlotinib Regimens |
Discussion
This retrospective study is conducted to investigate the safety and efficacy of anlotinib in real world and the first to comprehensively compare the efficacy between different combination regimens of anlotinib and anlotinb monotherapy, respectively. In our study, anlotinib had a promising efficacy (mPFS:8.5 months) and achieved mPFS 11.0 months in combination therapy. Anlotinb-related adverse reactions were mostly mild and could be well controlled.
Previous studies have shown that the combination of antiangiogenic agents and other anti-tumor drugs including immunotherapy, chemotherapy, and targeted drugs had synergistic effects for tumor treatment.23–26 Phase III IMpower150 study showed that the atezolizumab plus bevacizumab plus carboplatin plus paclitaxel (ABevCPac) group significantly improved PFS (8.4 months vs 6.8 months) and OS (19.8 months vs 14.9 months) compared with the BevCPac group in intention-to-treat population.27,28 The phase III BEYOND trial confirmed the efficacy of first-line bevacizumab plus carboplatin/paclitaxel (PacC) in patients with advanced or recurrent non-squamous NSCLC, and both PFS (9.2 months vs 6.5 months) and OS (24.3 months vs 17.7 months) was prolonged with PacC + bevacizumab versus PacC + placebo.29 Liu, H discovered that cisplatin-etoposide and bevacizumab combination, as the first-line treatment for ES-SCLC, can improve PFS.30 In an open-label, random, multicentre, Phase 2 study aimed to compare the efficacy and safety of the combination of erlotinib and bevacizumab compared with erlotinib alone, mPFS was 16.0 months with erlotinib plus bevacizumab and 9.7 months with erlotinib alone (HR 0.54, 95% CI 0.36–0.79; Log rank test p=0.0015).31
Preclinical research demonstrated that anlotinib can modulate the tumor microenvironment and act synergistically with immune checkpoint inhibitors.11–13,32 Our study showed that anlotinib combined with immunotherapy significantly improved PFS in male, adenocarcinoma, <=65 years old, patients stage IV, EGFR wild type, with metastasis, ps scores ≥2, the first-line treatment, patients with a history of hypertension and no previous antiangiogenesis. Thus, anlotinib in combination with immunotherapy may be a reliable treatment option. Meanwhile, the good efficacy of first-line anlotinib in combination with immunotherapy was a noteworthy point. Our patients were driver gene-negative, but not all of them had detected PD-L1 expression (only 1 patient was detected PD-L1 ≥1%, the other 18 patients were unknown). The reason for choosing this regimen was based on the patients’ own situation; some of them were unable to tolerate conventional first-line chemotherapy regimens because they had a lot of underlying disease, while others refused conventional chemotherapy because of fear of the adverse effects of chemotherapy or other reasons. Though in the context of evidence-based and precision medicine, it is not a conventional first-line treatment option, we made this choice of drug administration. Encouragingly, these patients benefited from first-line combination therapy even though they may not have PD-L1 indications for treatment, and we therefore recommend first-line immunotherapy combination with anlotinib as a treatment option to be chosen for patients who are driver gene-negative, have poor physical condition, are intolerable to chemo-regimen or do not want to receive chemotherapy.
Previous study indicated that anlotinib combined with chemotherapy had a significant longer median PFS than chemotherapy alone (5.0m vs 3.5m, p=0.002).33 A case reported that first-line pemetrexed and carboplatin plus anlotinib achieved CR for the treatment of EGFR wild-type NSCLC.22 In our study, anlotinib plus chemotherapy had a tendency to prolong survival and had a significantly longer mPFS in patients <=65 years and first-line treatment than anlotinib monotherapy group. These studies suggest that anlotinib combined with chemotherapy may be an effective treatment for advanced NSCLC.
Mechanistic study showed anlotinib may overcome acquired resistance to EGFR-TKIs in NSCLC via FGFR1 signaling or c-MET/MYC/AXL axis, which indicated a synergistic anti-tumor effect of anlotinib and targeted drugs.34,35 In our study, the efficacy in the anlotinib combination targeted therapy group tended to be better than in the anlotinib monotherapy group, which suggested anlotinib combined with targeted therapy may be a promising regimen.
Previous studies have shown that the adverse reactions to anlotinib in advanced NSCLC were mostly mild and tolerable, with a low incidence of serious adverse reactions (11–61.9%).9,36,37 The most common adverse reactions were hypertension, hypothyroidism, hand-foot syndrome, diarrhea, anemia and fatigue.36,38 Our findings were similar to previous reports, and the incidence of serious adverse reactions (≥3 grade) was lower, which may be due to the fact that clinicians paid close attention to patients during treatment and promptly managed patients’ adverse reactions, with management measures mainly being suspension of the drug and symptomatic treatment if necessary. Treatment-related adverse events could be controlled by early and appropriate intervention.
Bevacizumab and endostar are approved and undergoing clinical application in the systematic treatment of advanced NSCLC.5 A subgroup analysis of ALTER0303 trial reported previous antiangiogenic therapy had no impact on the efficacy of anlotinib (group yes vs group no, HR=1.588, 95% CI:0.802–3.145, p=0.185).39 However, the number of patients received previous antiangiogenic therapy was very low (only 27 patients); thus, the findings need to be verified in further studies. Interestingly, our study, which included 76 patients who received previous antiangiogenic therapy, came to a different conclusion. Median PFS was significantly longer in patients who received no prior antiangiogenic therapy than in those who received prior antiangiogenic therapy (9.4 vs 6.3m, p=0.004). Cox regression suggested previous antiangiogenic therapy was associated with PFS. We searched the literature in the relevant field and found that the efficacy of anlotinib may be impaired by previous antiangiogenic therapy. One plausible mechanism is tumor hypoxia. It is proposed that the more proficient an antiangiogenic agent is, it will more efficiently prune tumor vessels and cause hypoxia. Hypoxia-tolerant tumor cell clones are selected and escape antiangiogenic treatment in hypoxic niches. Meanwhile, metabolic reprogramming to glucose addiction allows tumor cells to generate energy in hypoxic conditions. Hypoxia thus selects for more malignant metastatic cells, showing less sensitive to antiangiogenic treatment.40,41 The hypoxic environment caused by previous antiangiogenic therapy may select hypoxia-tolerant tumor cell clones, thus diminishing the effect of anlotinib. A research about anlotinib resistance showed that protecting mitochondria contributes to the resistance of anlotinib.42 This may be related to energy generation and the hypoxic environment, while other signaling systems (such as p53, IL-8), stromal cells and cytokine response may be associated with the resistance.43,44 A research proposed that antiangiogenic agents (especially broad-spectrum VEGF RTKIs, which also inhibit PDGFRβ) or cytokine response they induce may inhibit the coverage of vessels by pericytes, which makes vessels more leaky and immature, facilitating intravasation of tumor cells and metastasis.45 In conclusion, considering the partial overlap of antiangiogenic mechanisms and the complexity of angiogenesis, whether the efficacy of anlotinib in patients who had previous antiangiogenic therapy would be affected is still need to be verified in clinical practice.
We made the attempt to provide a comprehensive analysis of the current clinical use of anlotinib and to compare the efficacy of different combination regimens of anlotinib with that of anlotinib monotherapy. However, our study had some limitations. First, observational studies almost always have bias because prognostic factors are unequally distributed between patients, so we did adjusted and stratified analysis to reduce the risk of bias. Second, this study was from a single center, and the sample size was not sufficient, which caused population bias and made it difficult to show statistical differences in the stratification analysis. Third, due to the corona virus disease 2019 (COVID-19), for some patients, regular visits to the hospital were not possible, resulting in a lack of follow-up information, which also limited the number of patients we included. Fourth, we did not conduct comparisons of efficacy between different combination regimens, which may limit physician selection of different combination regimens. Further studies with larger sample size and longer follow-up are needed.
Conclusion
In conclusion, anlotinib has reliable efficacy and tolerable toxicity. Anlotinib in combination with immunotherapy showed promising efficacy and can be considered as an option for NSCLC treatment. Anlotinib plus chemotherapy and targeted therapy had a tendency to prolong survival, respectively. Previous antiangiogenic therapy may be an independent factor for the efficacy of anlotinib in patients, but more studies are needed to verify this. Treatment-related adverse events could be better controlled by early and appropriate intervention.
Acknowledgments
We thank all the members of the Department of Respiratory and Critical Care Medicine of Nanjing Drum Tower Hospital for their discussions and suggestions.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA. 2019;69(5):363–385. doi:10.3322/caac.21565
3. Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273(2):114–127. doi:10.1111/joim.12019
4. Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8(4):299–309. doi:10.1016/j.ccr.2005.09.005
5. Qiang H, Chang Q, Xu J, et al. New advances in antiangiogenic combination therapeutic strategies for advanced non-small cell lung cancer. J Cancer Res Clin Oncol. 2020;146(3):631–645. doi:10.1007/s00432-020-03129-6
6. Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11(1):120. doi:10.1186/s13045-018-0664-7
7. Syed YY. Anlotinib: first Global Approval. Drugs. 2018;78(10):1057–1062. doi:10.1007/s40265-018-0939-x
8. Lin B, Song X, Yang D, Bai D, Yao Y, Lu N. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene. 2018;654:77–86. doi:10.1016/j.gene.2018.02.026
9. Han B, Li K, Wang Q, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA oncol. 2018;4(11):1569–1575. doi:10.1001/jamaoncol.2018.3039
10. Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised Phase II trial (ALTER0302). Br J Cancer. 2018;118(5):654–661. doi:10.1038/bjc.2017.478
11. Liu S, Qin T, Liu Z, et al. anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis. 2020;11(5):309. doi:10.1038/s41419-020-2511-3
12. Yang Y, Li L, Jiang Z, Wang B, Pan Z. Anlotinib optimizes anti-tumor innate immunity to potentiate the therapeutic effect of PD-1 blockade in lung cancer. Cancer Immunol Immunother. 2020;69(12):2523–2532. doi:10.1007/s00262-020-02641-5
13. Su Y, Luo B, Lu Y, et al. Anlotinib induces a T cell-inflamed tumor microenvironment by facilitating vessel normalization and enhances the efficacy of PD-1 checkpoint blockade in neuroblastoma. Clin Cancer Res. 2021;2021:1.
14. Liu Y, Long L, Liu J, Zhu L, Luo F. Case report: anlotinib reverses nivolumab resistance in advanced primary pulmonary lymphoepithelioma-like carcinoma with FGFR3 gene amplification. Front Oncol. 2021;11:749682. doi:10.3389/fonc.2021.749682
15. Luo Y, Wei J, Zhang J, Zeng L, Zeng Z, Liu A. Two different patients with pulmonary pleomorphic carcinoma response to PD-1 inhibitor plus anlotinib. Lung Cancer. 2021;155:170–174. doi:10.1016/j.lungcan.2021.03.019
16. Su H, Yu C, Ma X, Song Q. Combined immunotherapy and targeted treatment for primary alveolar soft part sarcoma of the lung: case report and literature review. Invest New Drugs. 2021;39(5):1411–1418. doi:10.1007/s10637-021-01105-6
17. Jin C, Yang B. Dramatic response of pulmonary sarcomatoid carcinoma to nivolumab combined with anlotinib: a case report. Case Rep Oncol. 2020;13(2):601–605. doi:10.1159/000507568
18. Wang P, Fang X, Yin T, Tian H, Yu J, Teng F. Efficacy and safety of anti-PD-1 plus anlotinib in patients with advanced non-small-cell lung cancer after previous systemic treatment failure-A retrospective study. Front Oncol. 2021;11:628124. doi:10.3389/fonc.2021.628124
19. Wang Y, Shi X, Qi Q, Ye B, Zou Z. Safety of anlotinib capsules combined with PD-1 inhibitor camrelizumab in the third-line treatment of advanced non-small-cell lung cancer and their effect on serum tumor markers. J Healthc Eng. 2021;2021:2338800. doi:10.1155/2021/2338800
20. Xiong Q, Qin B, Xin L, et al. Real-world efficacy and safety of anlotinib with and without immunotherapy in advanced non-small cell lung cancer. Front Oncol. 2021;11:659380. doi:10.3389/fonc.2021.659380
21. Yuan M, Zhu Z, Mao W, et al. Anlotinib combined with anti-PD-1 antibodies therapy in patients with advanced refractory solid tumors: a single-center, observational, prospective study. Front Oncol. 2021;11:683502. doi:10.3389/fonc.2021.683502
22. Zhang C, Kong FW, Wu WB, et al. First-line pemetrexed and carboplatin plus anlotinib for epidermal growth factor receptor wild-type and anaplastic lymphoma kinase-negative lung adenocarcinoma with brain metastasis: a case report and review of the literature. Medicine. 2020;99(36):e22128. doi:10.1097/MD.0000000000022128
23. Manegold C, Dingemans AC, Gray JE, et al. The potential of combined immunotherapy and antiangiogenesis for the synergistic treatment of advanced NSCLC. J Thorac Oncol. 2017;12(2):194–207. doi:10.1016/j.jtho.2016.10.003
24. Tabchi S, Blais N. Antiangiogenesis for advanced non-small-cell lung cancer in the era of immunotherapy and personalized medicine. Front Oncol. 2017;7:52. doi:10.3389/fonc.2017.00052
25. Attili I, Passaro A, Pavan A, Conte P, De Marinis F, Bonanno L. Combination immunotherapy strategies in advanced non-small cell lung cancer (NSCLC): does biological rationale meet clinical needs? Crit Rev Oncol Hematol. 2017;119:30–39. doi:10.1016/j.critrevonc.2017.09.007
26. Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18(1):60. doi:10.1186/s12943-019-0974-6
27. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi:10.1056/NEJMoa1716948
28. Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7(5):387–401. doi:10.1016/S2213-2600(19)30084-0
29. Zhou C, Wu YL, Chen G, et al. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase iii study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33(19):2197–2204. doi:10.1200/JCO.2014.59.4424
30. Lu H, Jiang Z. Advances in antiangiogenic treatment of small-cell lung cancer. Onco Targets Ther. 2017;10:353–359. doi:10.2147/OTT.S119714
31. Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15(11):1236–1244. doi:10.1016/S1470-2045(14)70381-X
32. Rahma OE, Hodi FS. The intersection between tumor angiogenesis and immune suppression. Clin Cancer Res. 2019;25(18):5449–5457. doi:10.1158/1078-0432.CCR-18-1543
33. Wang HY, Chu JF, Zhao Y, et al. A trial of the safety and efficacy of chemotherapy plus anlotinib vs chemotherapy alone as second- or third-line salvage treatment for advanced non-small cell lung cancer. Cancer Manag Res. 2020;12:3827–3834. doi:10.2147/CMAR.S249678
34. Lian Z, Du W, Zhang Y, et al. Anlotinib can overcome acquired resistance to EGFR-TKIs via FGFR1 signaling in non-small cell lung cancer without harboring EGFR T790M mutation. Thorac Cancer. 2020;11(7):1934–1943. doi:10.1111/1759-7714.13485
35. Lei T, Xu T, Zhang N, et al. Anlotinib combined with osimertinib reverses acquired osimertinib resistance in NSCLC by targeting the c-MET/MYC/AXL axis. Pharm Res. 2023;188:106668. doi:10.1016/j.phrs.2023.106668
36. Si X, Zhang L, Wang H, et al. Management of anlotinib-related adverse events in patients with advanced non-small cell lung cancer: experiences in ALTER-0303. Thorac Cancer. 2019;10(3):551–556. doi:10.1111/1759-7714.12977
37. Zhang K, Ma X, Gao H, et al. Efficacy and safety of anlotinib in advanced non-small cell lung cancer: a real-world study. Cancer Manag Res. 2020;12:3409–3417. doi:10.2147/CMAR.S246000
38. Lv B, Chen J, Liu XL. Anlotinib-induced hypertension: current concepts and future prospects. Curr Pharm Des. 2021;28:216–224.
39. Wang L, He Z, Yang S, et al. The impact of previous therapy strategy on the efficiency of anlotinib hydrochloride as a third-line treatment on patients with advanced non-small cell lung cancer (NSCLC): a subgroup analysis of ALTER0303 trial. Transl Lung Cancer Res. 2019;8(5):575–583. doi:10.21037/tlcr.2019.09.21
40. Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15(3):167–170. doi:10.1016/j.ccr.2009.02.007
41. Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–622. doi:10.1016/j.ccell.2014.10.006
42. Li S, Cao C, Huang Z, et al. SOD2 confers anlotinib resistance via regulation of mitochondrial damage in OSCC. Oral Dis. 2022. doi:10.1111/odi.14404
43. Bottsford-Miller JN, Coleman RL, Sood AK. Resistance and escape from antiangiogenesis therapy: clinical implications and future strategies. J Clin Oncol. 2012;30(32):4026–4034. doi:10.1200/JCO.2012.41.9242
44. Huijbers EJ, van Beijnum JR, Thijssen VL, Sabrkhany S, Nowak-Sliwinska P, Griffioen AW. Role of the tumor stroma in resistance to anti-angiogenic therapy. Drug Resist Updat. 2016;25:26–37. doi:10.1016/j.drup.2016.02.002
45. Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi:10.1038/nrc2442
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.