Back to Journals » International Journal of General Medicine » Volume 15
Efficacy and Safety of Anlotinib Combined with PD-1 Blockades for Patients with Previously Treated Epithelial Ovarian Cancer: A Retrospective Study
Authors Li XY, Rao Y, Sun B , Mao XM
Received 4 December 2021
Accepted for publication 23 February 2022
Published 12 April 2022 Volume 2022:15 Pages 3977—3989
DOI https://doi.org/10.2147/IJGM.S352536
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Xiao-Yuan Li,1,* Yang Rao,2,* Bing Sun,3 Xue-Mei Mao4
1Department of Medical Oncology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, 100730, People’s Republic of China; 2Department of Gynecological Oncology, Tianjin Central Obstetrics and Gynecology Hospital, Tianjin, 300199, People’s Republic of China; 3Department of Radiation Oncology, The Fifth Medical Center, Chinese PLA General Hospital, Beijing, 100071, People’s Republic of China; 4Department of Obstetrics and Gynecology, Tianjin Integrated Traditional Chinese and Western Medicine Hospital (Nankai Hospital), Tianjin, 300102, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xue-Mei Mao; Bing Sun, Tel +86 13820312420 ; +86 13810193881, Email [email protected]; [email protected]
Purpose: This study was to investigate the efficacy and safety of anlotinib combined with programmed cell death protein 1 (PD-1) blockades for patients with previously treated advanced epithelial ovarian cancer (EOC).
Patients and Methods: Present study was designed as a retrospective study, a total of 32 patients with advanced EOC who progressed after at least two lines previously available standard therapy were included in this study. All the patients were administered with anlotinib combined with PD-1 blockades administration. Clinical activity was implemented and analyzed, which was assessed according to the change of target lesion by imaging evidence and all the subjects were followed up regularly. Safety profile were collected and documented during the treatment. Univariate analysis was carried out using log rank test and multivariate analysis were adjusted by Cox regression analysis.
Results: The best overall response suggested that partial response was noted in 12 patients, stable disease was observed in 14 patients, progressive disease was found in 6 patients. Therefore, the objective response rate (ORR) of the 32 patients was 37.5% (95% CI: 21.1– 56.3%), disease control rate (DCR) of the patients was 81.3% (95% CI: 63.6– 92.8%). The median follow-up duration of this study was 17.5 months (follow-up range: 0.9– 33.5 months). And the median PFS and OS of the 32-patient cohort was 6.8 months (95% CI: 2.64– 10.96) and 18.5 months (95% CI: 14.08– 22.92), respectively. The most common treatment-related adverse reactions were fatigue (68.8%), nausea and vomiting (56.3%), hypertension (50.0%) and diarrhea (40.6%). Multivariate Cox regression analysis for PFS indicated that ECOG performance status and FIGO stage were independent factors to predict PFS of patients with previously treated EOC.
Conclusion: Anlotinib combined with PD-1 blockades demonstrated promising efficacy and tolerable safety profile for patients with previously treated advanced EOC preliminarily. The conclusion should be confirmed in more patients with advanced EOC subsequently.
Keywords: epithelial ovarian cancer, anlotinib, PD-1 blockade, efficacy, safety
Introduction
Ovarian cancer was reported to be the seventh most common cancer in female and the leading cause of death among patients with gynecological malignancy,1 which was estimated that there were 295,414 new cases and 184,799 death globally.2 Currently, there were approximately 52,100 new cases and 22,500 deaths of ovarian cancer in China annually.3 It was reported that approximately 90% of patients with ovarian cancer were epithelial ovarian cancer (EOC).4 Owing to the vague and nonspecific symptoms of the disease, more than 70% of the patients were diagnosed with advanced stage.5 In spite of the dramatically therapeutic progress over the past two decades, prognosis of patients with EOC remained dismal with the 5-year overall survival rates <30% in advanced stage.6 The standard chemotherapy treatment among EOC patients with II-IV stage consisted of cytoreductive surgery followed by carboplatin and paclitaxel regimens for 6–8 cycles.7 Despite the fact that the majority of the patients exhibited superior therapeutic response after the initial chemotherapy, almost all patients with EOC might develop platinum resistance ultimately, which resulted in the subsequent therapy with the non-platinum-based drugs.8 The mainstay of treatment for recurrent, platinum-resistant disease was nonplatinum-based monotherapy: liposomal doxorubicin, weekly paclitaxel, topotecan, gemcitabine and etoposide. The response varied based on the interval between completion of first-line therapy and disease recurrence, which yielded an objective response rate (ORR) ranging from 19% to 31% and median progression free survival (PFS) of 3–4 months.9 Therefore, effectively therapeutic regimens with tolerable safety profile in subsequent-line therapy for patients with EOC were needed urgently.
Recent years had witnessed that angiogenesis played an important role both in normal ovarian physiology and the development of ovarian cancer.10 To our knowledge, increasing evidence had demonstrated that multi-targets tyrosine kinase inhibitors (TKIs) such as pazopanib, sorafenib and anlotinib exhibited potential efficacy for patients with previously treated EOC to some extent currently.11 However, the efficacy of antiangiogenic targeted TKIs was also modest and most treated patients eventually developed acquired resistance, highlighting the need for new combination strategies.
Immunotherapy with PD-1/PD-L1 blockades revolutionized the therapeutic landscape of different types of tumors and unprecedented long-term survivorship was observed accordingly.12 Previous study indicated that the immune system played a vital role in prognosis of ovarian cancer, which suggested that intratumor T cell was positively associated with prolonged PFS of patients with ovarian cancer who received traditional regimens.13 Furthermore, study performed in archival EOC specimens supported the notion that the immune system was involved in ovarian cancer therapy and increased PD-L1/PD-L2 expression was correlated with worse overall survival (OS) in ovarian cancer.14 Consequently, previous studies elucidated that PD-1 blockades (pembrolizumab and nivolumab monotherapy) exhibited durable antitumor activity and acceptable safety profile for patients with treatment-refractory ovarian cancer according to the Keynote-100 and Checkmate trials, respectively.15,16 Unfortunately, it should be noted that an obvious limitation of both PD-1 and PD-L1 blockade as monotherapy in ovarian cancer was the relatively low ORR (<15%), which highlighted the urgency for appropriate combination therapy strategy as well.
Interestingly, previous study exhibited that antiangiogenic drugs reprogrammed the tumor milieu from an immunosuppressive to an immune permissive microenvironment, and the activated immunity by PD-1/PD-L1 blockades also facilitated anti-angiogenesis by downregulating the expression of vascular endothelial growth factor and alleviating hypoxia condition.17 Furthermore, a recent study found that anlotinib increased infiltration of innate immune cells, including natural killer (NK) cells and antigen-presenting cell (APC). These results consolidated a role for anlotinib in the innate immune cell in the tumor microenvironment and a potentially synergistic action combination with immune checkpoint inhibitor.18 Consequently, we noticed that atezolizumab plus bevacizumab showed promising clinical efficacy in unresectable hepatocellular carcinoma, thus becoming the standard of care as first-line therapy for unresectable hepatocellular carcinoma.19 Additionally, recent studies had demonstrated that anlotinib combined with PD-1 blockades exhibited potentially synergistic effect in patients with non-small cell lung cancer and small cell lung cancer.20,21 However, the clinical activity and safety of anlotinib combined with PD-1 blockades for patients with previously treated EOC was still scanty.
Therefore, this study was to investigate the efficacy and safety of anlotinib combined with PD-1 blockades for patients with previously treated EOC retrospectively.
Materials and Methods
Study Design
To our knowledge, anlotinib and PD-1 blockades were licensed in China more than three years, a part of patients with ovarian cancer were treated with anlotinib combined with PD-1 blockades administration clinically. Therefore, our study was designed retrospectively. Patients with ovarian cancer who failed the previous systemic chemotherapy regimens in the department of medical oncology of Peking union medical college hospital from August 2018 to June 2021 was included in this study consecutively. The main inclusion criteria were: (a) histological diagnosis of EOC, fallopian tube cancer and peritoneal cancer; (b) pathological staging of III and IV according to International Federation of Gynecology Obstetrics (FIGO) staging system (the initial stage at diagnosis); (c) aged ≥18 years; (d) Eastern cooperative oncology group (ECOG) performance status of 0–2 score; (e) patients progressed after at least two lines previously available standard therapy, including those who relapsed >6 months after completion of platinum-based chemotherapy (platinum-sensitive patients), and those who relapsed <6 months after completion of platinum-based chemotherapy (platinum-resistant patients), which was based on the initial platinum response status; (f) patients were administered with anlotinib plus PD-1 blockades (any PD-1 checkpoint inhibitors that approved in China) administration; (g) at least one measurable target lesion according to response evaluation criteria in solid tumors (RECIST 1.1). The major exclusion criteria included: (a) previous exposure to PD-1, PD-L1 and CTLA-4 blockades, or anlotinib was administered previously. However, previous bevacizumab administration was permitted; (b) patients were diagnosed with squamous cell skin cancer or in situ cancer of cervix uteri; (c) active or uncontrolled autoimmune disease; (d) concomitant with another cancer or serious diseases; (e) efficacy assessment data were not available. The study profile of this study is illustrated in Figure 1. Eventually, a total of 32 patients with advanced EOC was enrolled. This study was approved by the ethics committee of Peking union medical college hospital. Written informed consent was obtained by each enrolled patient according to the recommendations of the Declaration of Helsinki.
 |
Figure 1 Flow chart of the retrospective study of anlotinib combined with PD-1 blockades in the treatment for patients with previously treated epithelial ovarian cancer. |
Therapeutic Regimens
All the patients with advanced EOC participated in present study were administered with anlotinib combined with PD-1 blockades treatment. Anlotinib was administered orally at an initial dosage of 12mg or 10mg per day (continued for 14 days and discontinued for 7 days, every 21 days as one cycle). And the PD-1 blockades in this study included camrelizumab (200mg), sintilimab (200mg) and pembrolizumab (200mg). All the PD-1 blockades were 21-day regimen as well (intravenously administered on day 1, every 21 days as one cycle). The combination regimen continued until disease progression or intolerable adverse reactions of the patients. Overall response of the therapy was assessed based on RECIST version 1.1 criteria in the opinion of the investigators.22 Target lesions were assessed radiologically using computed tomography (CT) or magnetic resonance imaging (MRI) for each patient before and during the combination treatment. Overall response of the target lesions was assessed using CT or MRI scans every two cycles or depended on the actual situation when the clinical symptoms of the patients were worsened. And the primary endpoint of this study was PFS, secondary endpoints were OS, ORR, disease control rate (DCR) and safety profile of the treatment.
Assessment of Adverse Reactions of the Combination Regimen
Adverse reactions during the treatment were assessed using Common Terminology Criteria for Adverse Events (CTCAE) 4.03 version to present toxicity profile that might be drug-related.23 Safety profile of the patients who received anlotinib combined with PD-1 blockades was exhibited and the maximum grade of the adverse reactions of the patients was recorded as detailed as possible to present the safety profile of the combination regimen.
Statistical Analysis
All the statistical analysis in this study was performed using the statistical analysis software SPSS version 25.0 (IBM, USA). ORR was defined as the percentage of patients with the best overall response of CR or PR in total patients. DCR was calculated as the percentage of patients with the best overall response of CR or PR or SD in total patients. PFS was calculated from the date of the onset of anlotinib combined with PD-1 blockades therapy to the date of disease progression or death, whichever occurred first. OS was calculated from the date of the onset of anlotinib combined with PD-1 blockades treatment to the date of death from any cause.24 When no prognostic events were observed, survival end points were censored at the date of last follow-up. Kaplan–Meier survival curves were drawn using Stata version 14.0 to present the PFS and OS data. Survival difference according to baseline characteristic subgroup was calculated using Log rank test. Cox regression analysis was introduced for PFS in multivariable analysis. P<0.05 was accepted as statistically significant.
Results
Baseline Characteristics of the 32 Patients with EOC
Baseline characteristics of the 32 patients with EOC is exhibited in Table 1. Median age of the 32 patients was 56 years (range: 22–73 years), patients with ≥56 and <56 years were observed in 17 and 15 cases, respectively. ECOG performance status of 0–1 and 2 score was noted in 18 and 14 patients, respectively. FIGO stage of III and IV was reported in 21 and 11 patients, respectively. Furthermore, serous and mixed histology of EOC was observed in 23 and 9 patients, respectively. Regarding the type of first-line platinum response, patients with platinum refractory, platinum-sensitive and platinum-resistant were observed in 7, 15 and 10 cases, respectively. And patients with well differentiated, intermediate differentiated and poorly differentiated were noted in 5, 11 and 16 patients. It should be noted that lines of previous treatment regimens of 2 and ≥3 lines were observed in 5 and 27 patients, respectively. Besides, a total of 12 patients (37.5%) had been treated with targeted drugs previously. Interestingly, the initial dosage of anlotinib with 12mg and 10mg was found in 26 and 6 patients, respectively. Furthermore, PD-1 blockades of camrelizumab, sintilimab and pembrolizumab were noted in 18, 9 and 5 patients, respectively. Additionally, given that this study was designed as retrospective study, the adherence of some patients was poor. A total of 5 patients received the combination therapy in first cycle and then they refused the treatment and were lost to follow-up. Therefore, these 5 patients were absent from the data for efficacy assessment and were excluded from the analysis ultimately.
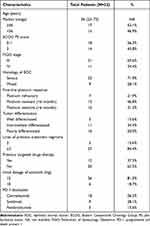 |
Table 1 Baseline Characteristics of the 32 Patients with EOC |
Efficacy of Anlotinib Plus PD-1 Blockades
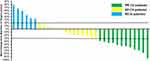
All the 32 patients with treatment-refractory EOC were able to evaluate the efficacy data. The best overall response suggested that no complete response was observed, partial response was noted in 12 patients, stable disease was observed in 14 patients, progressive disease was found in 6 patients. As a result, ORR of the 32 patients with treatment-refractory EOC who received anlotinib plus PD-1 blockades was 37.5% [95% confidence interval (CI): 21.1–56.3%], DCR was 81.3% (95% CI: 63.6–92.8%). Specifically, the waterfall plot for the best percentage change in target lesion of the 32 patients with treatment refractory EOC is presented in Figure 2. Most of the target lesions among the 32 patients with EOC reduced significantly. Furthermore, one patient with EOC exhibited a PR after the therapy of anlotinib plus sintilimab regimen. The CT scan of the target lesion in lymph node site before and after the therapy is illustrated in Figure 3. This patient had the longest response to the treatment with a PFS of 25.5 months among the 12 patients with PR. The target lesion shrank dramatically after the combination therapy. This patient benefited significantly from the combination therapy.
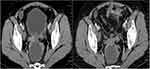 |
Figure 3 CT scan results of the changes for target lesions in one patient with epithelial ovarian cancer before and after anlotinib combined with PD-1 blockades administration. |
Prognosis of Anlotinib Plus PD-1 Blockades
Follow-up was conducted in this study monthly and regularly. The last follow-up date of this study was September 30 2021. Regarding the prognostic data, the median follow-up duration for the 32 patients with EOC from the date of the treatment to the date of data cut-off was 17.5 months (follow-up range: 0.9–33.5 months). As illustrated in Figure 4, the median PFS of the 32 patients with EOC who received anlotinib combined with PD-1 blockades was 6.8 months (95% CI: 2.64–10.96). And the 6-month and 12-month PFS rate was 56.3% (95% CI: 37.6–71.3%) and 40.4% (95% CI: 23.6–56.6%), respectively. Besides, given that the follow-up duration of this study was accurate and enough, OS analysis was performed simultaneously. As exhibited in Figure 5, the median OS of the 32 patients with EOC who received anlotinib combined with PD-1 blockades was 18.5 months (95% CI: 14.08–22.92). And the 12-month and 24-month OS rate was 75.0% (95% CI: 56.2–86.6%) and 38.8% (95% CI: 21.8–55.5%), respectively.
 |
Figure 4 Progression-free survival the 32 patients with previously treated epithelial ovarian cancer who were treated with anlotinib combined with PD-1 blockades administration. |
 |
Figure 5 Overall survival the 32 patients with previously treated epithelial ovarian cancer who were treated with anlotinib combined with PD-1 blockades administration. |
Additionally, it should be noted that the univariate analysis according to baseline characteristics was carried out in this study subsequently. As shown in Table 2, ECOG performance status score, FIGO stage and first-line platinum response were significantly associated with PFS in univariate analysis, which suggested that the prognosis of anlotinib plus PD-1 blockades regimen might be different according to these characteristics. Obviously, the median PFS of patients with performance status of 0–1 score was dramatically longer than that of patients with the 2 score (median PFS: 8.5 vs 5.5 months, P=0.027), and the median PFS of patients with FIGO stage of III was better than that of patients with stage IV (median PFS: 9.7 vs 5.5 months, P=0.016). Besides, patients with platinum refractory and platinum resistant conferred a worse PFS compared with those with platinum sensitive (median PFS: 5.5 vs 8.5 months, P=0.046). Interestingly, it should be noted that patients received three PD-1 blockades shared a similar and comparable PFS when analyzed separately (median PFS: 6.1 vs 6.8 vs 7.6 months, P=0.572).
 |
Table 2 Univariate Analysis for PFS of the 32 Patients with EOC According to Baseline Characteristics |
Furthermore, multivariate Cox regression analysis was constructed for PFS including the baseline characteristics that were significant in the univariate analysis. The multivariate analysis results are illustrated in Table 3. Obviously, significantly statistical difference was still observed regarding ECOG performance status and FIGO stage for PFS, which demonstrated that both ECOG PS score and FIGO stage were independent factors for PFS [hazard ratio (HR)=0.66, P=0.041 and HR = 0.57, P=0.026)]. Nevertheless, as illustrated in Table 3, after adjusted in the Cox regression analysis, first-line platinum response conferred a trend to correlate with PFS (HR = 1.44, P=0.069).
 |
Table 3 Multivariate Cox Regression Analysis for PFS According to Baseline Characteristics |
Safety Profile of Anlotinib Plus PD-1 Blockades
The maximum grade of each adverse reaction among the 32 patients with EOC who received anlotinib plus PD-1 blockades administration was collected and analyzed in this study. On the whole, treatment related adverse reactions (TRARs) were observed in 30 patients among the 32 patients included (93.8%). And the grade 3–4 TRARs were noted in 12 patients (37.5%), no grade 5 adverse reactions were detected. During the combination administration, a total of 6 patients (18.7%) experienced dose reduction of anlotinib. Of the 26 patients who were initially administered with anlotinib dosage of 12mg, 5 patients required a dosage reduction to 10mg. Of the 6 patients who were administered with anlotinib dosage of 10mg, 1 patient required a dosage reduction to 8mg. And 2 patients (6.3%) experienced a dose discontinuity of anlotinib and 3 patients (9.4%) experienced a dose discontinuity of PD-1 blockades. The common reasons of dosage reduction or discontinuity in these patients included hypertension, hand-foot syndrome, fatigue, diarrhea and pneumonia.
Detailly, as shown in Table 4, the common TRARs were fatigue (68.8%), nausea and vomiting (56.3%), hypertension (50.0%), diarrhea (40.6%), rash (31.3%), pain (28.1%), hand and foot syndrome (28.1%), weight loss (21.9%), hepatotoxicity (15.6%), reactive cutaneous capillary endothelial proliferation (RCCEP) (12.5%), proteinuria (12.5%), pneumonia (8.3%) and bleeding (6.3%). Furthermore, the grade 3–4 adverse reactions were observed with nausea and vomiting (15.6%), hypertension (15.6%), fatigue (9.4%), diarrhea (6.3%), hand and foot syndrome (6.3%), rash (3.1%) and proteinuria (3.1%).
 |
Table 4 Safety Profile Among the 32 Patients with EOC Who Were Treated with Anlotinib Combined with PD-1 Blockades |
Discussion
To our knowledge, this study was the first retrospective study that provided real-world evidence regarding the efficacy and safety of anlotinib combined with PD-1 blockades for female patients with treatment-refractory EOC. Anlotinib combined with PD-1 blockades might be a promising effective and safety regimen for patients with treatment-refractory EOC clinically.
Although EOC was usually sensitive to platinum-based regimen in first-line setting initially, majority of patients might relapse and develop to chemotherapy resistance eventually, especially platinum resistance.25 Unfortunately, therapeutic options among patients with EOC who had underwent second-line or more line therapy were still scanty currently.26 Nevertheless, there were considerable patients in superior physical condition that might continue the salvage treatment in order to prolong the survival. And a relatively small number of patients were able to obtain the available third-line of such treatments.27 For those with previously treated platinum-resistant ovarian cancer, a non-platinum-based drug or clinical trials were recommended. However, the objective response of these regimens using the single drugs was approximately 10–30%.28 New drugs or treatment regimens were needed urgently to achieve higher response and longer survival. It was proved that angiogenesis was a hallmark process in EOC and was responsible for tumor spread and metastasis.29 Consequently, antiangiogenic TKIs also demonstrated potential therapeutic activity for patients with EOC in third-line treatment as monotherapy. Apatinib and sunitinib monotherapy both showed promising efficacy and safety for patients with treatment-refractory EOC previously.30 Furthermore, recent years had witnessed that immunotherapy with PD-1/PD-L1 blockades also exhibited potential activity and tolerable safety for patients with previously treated EOC. As a result, we noticed that pembrolizumab and nivolumab monotherapy both exhibited durable antitumor activity and acceptable safety profile for patients with treatment-refractory ovarian cancer as third-line according to the Keynote-100 and Checkmate trials, respectively.15,16 However, it should be noted that an obvious limitation of both pembrolizumab and nivolumab as monotherapy in ovarian cancer was the low ORR (<15%), highlighting the combination study of PD-1 blockades plus other agents were of growing importance in this disease.24
Patients with EOC included in this study was those who failed the previous standard therapeutic regimens, which suggested that the combination regimen was reasonable in view of the fact that no indication of both anlotinib and PD-1 blockades were available among patients with EOC. Collectively, ORR of the 32 patients with EOC who received anlotinib combined with PD-1 blockades was 37.5%, DCR was 81.3% and the median PFS was 6.8 months, which yielded an acceptable efficacy and promising PFS numerically. To our knowledge, as an oral multi-target TKI with inhibition of VEGFR2-3, FGFR1-4, PDGFRα-β, c-Kit and Ret,31 anlotinib was a potential agent to inhibit angiogenesis and could be safety for EOC. Interestingly, a previous retrospective study included a total of 38 patients with platinum-refractory EOC who received anlotinib-based regimen.32 And the results indicated that ORR of anlotinib monotherapy was 23.5%. On the other hand, pembrolizumab and nivolumab as monotherapy had been investigated among patients with advanced ovarian cancer. Keynote-028 trial recruited a total of 26 patients with PD-1 positive advanced metastatic ovarian cancer who received pembrolizumab of 10mg/kg administration.33 And the result found that ORR of pembrolizumab among patients with ovarian cancer was 11.5%. Study of nivolumab included 20 patients with platinum-resistant ovarian cancer who were treated with nivolumab of 1 or 3 mg/kg. And the ORR of nivolumab monotherapy was 15%.16 Obviously, PD-1 blockades monotherapy among ovarian cancer yielded an ORR of approximately <15% regardless of PD-L1 expression. Therefore, the overall response of anlotinib plus PD-1 blockades in this study suggested the potentially synergistic action for cancer therapy, which was in accordance with the conclusion that observed in patients with hepatocellular carcinoma who received atezolizumab plus bevacizumab combination therapy.19 Interestingly, a previous study initiated by YL Yang et al investigated the potential effects of anlotinib on tumor-infiltrating immune cell using a syngeneic lung cancer mouse model.18 And they found that anlotinib might augment active NK cell infiltration, accelerate APC recruitment and attenuate the percentage of M2-like tumor-associated macrophages in the tumor immune microenvironment, thus exhibiting a bona fide innate immune-modulating effect. These results highlighted a role for anlotinib in the innate immune cell of the tumor microenvironment and a potentially synergistic action combination with PD-1 blockades. Similarly, a recent study initiated by JF Liu et al performed a phase II clinical trial to identify the efficacy and safety of nivolumab plus bevacizumab among patients with relapsed ovarian cancer.24 A total of 38 patients with relapsed EOC who had disease recurrence within 12 months of the lase platinum-based treatment and received the combination of bevacizumab (10mg/kg) plus nivolumab (240mg). And this regimen yielded an ORR of 28.9% for patients with previously treated relapsed ovarian cancer. Additionally, the DCR in this study was.55.3% and median PFS was 9.4 months. Obviously, the ORR and DCR in JF Liu’s study were inferior to those in our study, which might be explained by the fact that antiangiogenic targeted drug anlotinib was different from bevacizumab. Anlotinib was a multi-targets TKI that had dual effects for both anti-angiogenesis and tumor cell inhibition.34 However, it should be noted that the median PFS in our study was shorter than that in JF Liu’s trial (median PFS: 6.8 months vs 9.4 months). We speculated one reason might be attribute to the baseline characteristics of the patients. Majority patients in JF Liu’s study only received first-line previous systemic chemotherapy, whereas patients included in our study were those who failed after the previous standard treatment. Therefore, patients in our study were heavily treated with relatively worse prognosis. Another explanation could be attributed to the retrospective design of our study. It had been proved that the adherence of the patients in retrospective study was inferior to that in clinical trial. Besides, the discrepancy of ECOG performance status proportion should be taken into consideration, previous study indicated that poor PS status was associated with worse prognosis.35 Patients with ECOG 2 score in our study accounted for 43.8%, which was higher than that in JF Liu’s study (0.0%). Furthermore, multivariate Cox analysis performed in our study suggested that ECOG PS status was an independent factor for PFS. And the result was consistent with that of the previous study.36 Interestingly, the multivariate Cox regression analysis for PFS in our study also found that FIGO stage and first-line platinum response might be independent factors to predict the PFS, which was in concert with the results in a previous retrospective study regarding apatinib in EOC.37
Additionally, given that the follow-up duration in our study was long enough (median follow-up duration: 17.5 months), OS was analyzed in our study. It should be noted that the median OS in our study was slightly longer than that in the Keynote-028 trial (median OS: 18.5 vs 13.8 months) and the median OS in the retrospective study regarding anlotinib in EOC (median OS: 18.5 vs 16.5 months).32,33 We speculated the possible explanation could be the license of another immunotherapy and PARP inhibitors (Olaparib and Niraparib) since 2018. To our knowledge, PARP inhibitors also demonstrated potential clinical benefit for patients with treatment-refractory EOC as the subsequent line administration.38 As a result, another PD-L1 blockades and PARP inhibitors were still available for the patients when they progressed after anlotinib combined with PD-1 blockades administration, thus providing the patients with survival benefits consecutively. Interestingly, it should be noted that patients with platinum-refractory or platinum-resistant conferred a worse PFS than those with platinum sensitive, even the difference was not statistically significant in multivariate Cox regression analysis (P=0.069). Therefore, this result demonstrated that patients with platinum-refractory or resistant might benefit from anlotinib plus PD-1 blockades regimen similarly. However, the conclusion in our study should be validated in prospective clinical trials subsequently.
The overall adverse reactions of the combination regimen were tolerable and manageable, which was consistent with the safety profile of a recent report regarding the combination therapy of anlotinib plus PD-1 blockades among patients with advanced refractory solid tumors.39 Incidence of grade 3–4 TRARs in our study was 37.5%, which was higher than that among patients with relapsed ovarian cancer who received the treatment of nivolumab and bevacizumab (grade ≥3 TRARs was 23.7%). Even though, it seemed that safety profile of anlotinib plus PD-1 blockades regimen was acceptable for the patients with EOC. Additionally, the most common TRARs of the combination regimen were hypertension, hand and foot syndrome, proteinuria and bleeding, which could be attributed to the treatment of anlotinib and were consistent with the safety profile of the previous study regarding anlotinib in patients with EOC.32 Another immune-related adverse reactions such as rash, reactive cutaneous capillary endothelial proliferation (RCCEP) and pneumonia were observed with relatively low incidence, which might be resulted from the treatment of PD-1 blockades.40 Interestingly, RCCEP was deemed as the specific adverse reaction of camrelizumab that was administered among 18 patients in our study. Therefore, the actual incidence of RCCEP for camrelizumab administration was 22.2%, which might be slightly lower than that of the camrelizumab monotherapy for the other cancer (approximately 60%).41 This discrepancy of RCCEP incidence could be attributed to the therapy of anlotinib that might play a key role to attenuate the occurrence of RCCEP during camrelizumab administration.42 Collectively, the overall adverse reactions of anlotinib plus PD-1 blockades were controllable and manageable.
Certainly, limitations existed in our study inevitably. Firstly, the sample size was comparatively small as a real-world study, only 32 subjects were enrolled. More patients were needed to be included to confirm the efficacy and safety of anlotinib combined with PD-1 blockades in the future. Secondly, this study was designed as a retrospective analysis and some potential biases could not be avoided. Toxicity data was not collected and documented completely in retrospective study. Furthermore, not all the target lesions were scanned at two cycle intervals, which might be a large source for the potential bias toward calculating PFS data. Thirdly, PD-L1 expression test failed to performed in our study currently. Available tumor tissue specimens should be collected subsequently to explore the relevance of PD-L1 expression to the efficacy of the combination regimen. Still and all, we thought our study was of potentially clinical significance to provide the real-world evidence regarding anlotinib combined with PD-1 blockades for patients with previously treated EOC.
Conclusion
Anlotinib combined with PD-1 blockades demonstrated satisfactory efficacy and tolerable safety profile for patients with previously treated advanced EOC preliminarily. The conclusion should be confirmed in more patients with advanced EOC subsequently.
Acknowledgments
The authors would like to express sincere gratitude to the patients and their relatives for participating in this study. We would thank all the staff who took part in this study.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296. doi:10.3322/caac.21456
2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Guo H, Wei J, Li X, et al. Do socioeconomic factors modify the effects of PM1 and SO2 on lung cancer incidence in China? Sci Total Environ. 2021;756:143998. doi:10.1016/j.scitotenv.2020.143998
4. Li X, Zhang Y, Chai X, et al. Overexpression of MEF2D contributes to oncogenic malignancy and chemotherapeutic resistance in ovarian carcinoma. Am J Cancer Res. 2019;9(5):887–905.
5. Nash Z, Menon U. Ovarian cancer screening: current status and future directions. Best Pract Res Clin Obstet Gynaecol. 2020;65:32–45. doi:10.1016/j.bpobgyn.2020.02.010
6. Rong Y, Li L. Early clearance of serum HE4 and CA125 in predicting platinum sensitivity and prognosis in epithelial ovarian cancer. J Ovarian Res. 2021;14(1):2. doi:10.1186/s13048-020-00759-9
7. Kurnit KC, Fleming GF, Lengyel E. Updates and new options in advanced epithelial ovarian cancer treatment. Obstet Gynecol. 2021;137(1):108–121. doi:10.1097/aog.0000000000004173
8. Champer M, Huang Y, Hou JY, et al. Adherence to treatment recommendations and outcomes for women with ovarian cancer at first recurrence. Gynecol Oncol. 2018;148(1):19–27. doi:10.1016/j.ygyno.2017.11.008
9. Oronsky B, Ray CM, Spira AI, et al. A brief review of the management of platinum-resistant-platinum-refractory ovarian cancer. Med Oncol. 2017;34(6):103. doi:10.1007/s12032-017-0960-z
10. Lim D, Do Y, Kwon BS, et al. Angiogenesis and vasculogenic mimicry as therapeutic targets in ovarian cancer. BMB Rep. 2020;53(6):291–298. doi:10.5483/BMBRep.2020.53.6.060
11. Singh N, Badrun D, Ghatage P. State of the art and up-and-coming angiogenesis inhibitors for ovarian cancer. Expert Opin Pharmacother. 2020;21(13):1579–1590. doi:10.1080/14656566.2020.1775813
12. Cable J, Greenbaum B, Pe’er D, et al. Frontiers in cancer immunotherapy-A symposium report. Ann N Y Acad Sci. 2021;1489(1):30–47. doi:10.1111/nyas.14526
13. Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi:10.1056/NEJMoa020177
14. Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124(2):192–198. doi:10.1016/j.ygyno.2011.09.039
15. Matulonis UA, Shapira-Frommer R, Santin AD, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the Phase II KEYNOTE-100 study. Ann Oncol. 2019;30(7):1080–1087. doi:10.1093/annonc/mdz135
16. Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of Anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015–4022. doi:10.1200/jco.2015.62.3397
17. Song Y, Fu Y, Xie Q, et al. Anti-angiogenic agents in combination with immune checkpoint inhibitors: a promising strategy for cancer treatment. Front Immunol. 2020;11:1956. doi:10.3389/fimmu.2020.01956
18. Yang Y, Li L, Jiang Z, Wang B, Pan Z. Anlotinib optimizes anti-tumor innate immunity to potentiate the therapeutic effect of PD-1 blockade in lung cancer. Cancer Immunol Immunother. 2020;69(12):2523–2532. doi:10.1007/s00262-020-02641-5
19. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
20. Chu T, Zhong R, Zhong H, et al. Phase 1b study of sintilimab plus anlotinib as first-line therapy in patients with advanced NSCLC. J Thorac Oncol. 2021;16(4):643–652. doi:10.1016/j.jtho.2020.11.026
21. Hao YY, Qiao YP, Cheng JD. Clinical activity and safety of anlotinib combined with PD-1 blockades for patients with previously treated small cell lung cancer. Int J Gen Med. 2021;14:10483–10493. doi:10.2147/ijgm.s337316
22. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
23. Miller TP, Fisher BT, Getz KD, et al. Unintended consequences of evolution of the common terminology criteria for adverse events. Pediatr Blood Cancer. 2019;66(7):e27747. doi:10.1002/pbc.27747
24. Liu JF, Herold C, Gray KP, et al. Assessment of combined nivolumab and bevacizumab in relapsed ovarian cancer: a Phase 2 clinical trial. JAMA Oncol. 2019;5(12):1731–1738. doi:10.1001/jamaoncol.2019.3343
25. Lheureux S, Cristea MC, Bruce JP, et al. Adavosertib plus gemcitabine for platinum-resistant or platinum-refractory recurrent ovarian cancer: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2021;397(10271):281–292. doi:10.1016/s0140-6736(20)32554-x
26. Lee JM, Annunziata CM, Hays JL, et al. Phase II trial of bevacizumab and sorafenib in recurrent ovarian cancer patients with or without prior-bevacizumab treatment. Gynecol Oncol. 2020;159(1):88–94. doi:10.1016/j.ygyno.2020.07.031
27. Mansi L, Demarchi M, Bazan F, et al. Impact of chemotherapy beyond the third line in patients with recurrent epithelial ovarian cancer. Int J Gynecol Cancer. 2016;26(2):261–267. doi:10.1097/igc.0000000000000592
28. Harries M, Gore M. Part I: chemotherapy for epithelial ovarian cancer-treatment at first diagnosis. Lancet Oncol. 2002;3(9):529–536. doi:10.1016/s1470-2045(02)00846-x
29. Morgan RD, Banerjee S, Hall M, et al. Pazopanib and Fosbretabulin in recurrent ovarian cancer (PAZOFOS): a multi-centre, phase 1b and open-label, randomised phase 2 trial. Gynecol Oncol. 2020;156(3):545–551. doi:10.1016/j.ygyno.2020.01.005
30. Miao M, Deng G, Luo S, et al. A phase II study of apatinib in patients with recurrent epithelial ovarian cancer. Gynecol Oncol. 2018;148(2):286–290. doi:10.1016/j.ygyno.2017.12.013
31. Cheng Y, Wang Q, Li K, et al. Anlotinib vs placebo as third- or further-line treatment for patients with small cell lung cancer: a randomised, double-blind, placebo-controlled Phase 2 study. Br J Cancer. 2021;125(3):366–371. doi:10.1038/s41416-021-01356-3
32. Cui Q, Hu Y, Ma D, Liu H. A retrospective observational study of anlotinib in patients with platinum-resistant or platinum-refractory epithelial ovarian cancer. Drug Des Devel Ther. 2021;15:339–347. doi:10.2147/dddt.s286529
33. Varga A, Piha-Paul S, Ott PA, et al. Pembrolizumab in patients with programmed death ligand 1-positive advanced ovarian cancer: analysis of KEYNOTE-028. Gynecol Oncol. 2019;152(2):243–250. doi:10.1016/j.ygyno.2018.11.017
34. Xie C, Wan X, Quan H, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018;109(4):1207–1219. doi:10.1111/cas.13536
35. Foster NR, Mandrekar SJ, Schild SE, et al. Prognostic factors differ by tumor stage for small cell lung cancer: a pooled analysis of North Central Cancer Treatment Group trials. Cancer. 2009;115(12):2721–2731. doi:10.1002/cncr.24314
36. Rades D, Motisi L, Veninga T, et al. Predictors of outcomes and a scoring system for estimating survival in patients treated with radiotherapy for metastatic spinal cord compression from small-cell lung cancer. Clin Lung Cancer. 2019;20(4):322–329. doi:10.1016/j.cllc.2019.04.005
37. Yan Z, Gu YY, Hu XD, et al. Clinical outcomes and safety of apatinib monotherapy in the treatment of patients with advanced epithelial ovarian carcinoma who progressed after standard regimens and the analysis of the VEGFR2 polymorphism. Oncol Lett. 2020;20(3):3035–3045. doi:10.3892/ol.2020.11857
38. Domchek SM, Aghajanian C, Shapira-Frommer R, et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol. 2016;140(2):199–203. doi:10.1016/j.ygyno.2015.12.020
39. Yuan M, Zhu Z, Mao W, et al. Anlotinib combined with Anti-PD-1 Antibodies therapy in patients with advanced refractory solid tumors: a Single-Center, Observational, Prospective Study. Front Oncol. 2021;11:683502. doi:10.3389/fonc.2021.683502
40. Zamarin D, Burger RA, Sill MW, et al. Randomized Phase II trial of nivolumab versus nivolumab and ipilimumab for recurrent or persistent ovarian cancer: an NRG Oncology Study. J Clin Oncol. 2020;38(16):1814–1823. doi:10.1200/jco.19.02059
41. Wang F, Qin S, Sun X, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol. 2020;13(1):47. doi:10.1186/s13045-020-00886-2
42. Jiang FE, Zhang HJ, Yu CY, Liu AN. Efficacy and safety of regorafenib or fruquintinib plus camrelizumab in patients with microsatellite stable and/or proficient mismatch repair metastatic colorectal cancer: an observational pilot study. Neoplasma. 2021;68(4):861–866. doi:10.4149/neo_2021_201228N1415
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.