Back to Journals » Cancer Management and Research » Volume 12
Efficacy and Safety of Androgen-Deprivation Therapy Combined with Docetaxel Plus Prednisone in High-Burden Metastatic Hormone-Sensitive Prostate Cancer
Authors Hu L , Zhao Q, Bai H, Xie C, Shan X, Lu D, Chen Y, Han D, Xiao Z, Tian J, Wang D, Bi X, Xing N
Received 26 December 2019
Accepted for publication 9 April 2020
Published 9 June 2020 Volume 2020:12 Pages 4369—4377
DOI https://doi.org/10.2147/CMAR.S243843
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Kenan Onel
Linjun Hu,1,* Qinxin Zhao,2,* Hongsong Bai,1 Chengming Xie,1 Xingli Shan,1 Dehu Lu,1 Yonghai Chen,1 Dongdong Han,1 Zejun Xiao,2 Jun Tian,2 Dong Wang,2 Xingang Bi,2 Nianzeng Xing2
1Department of Urology, Cancer Hospital of HuanXing Chaoyang District Beijing, Beijing, People’s Republic of China; 2Department of Urology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Nianzeng Xing
Department of Urology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 17, Panjiayuan South Li, Beijing, Chaoyang 100021, People’s Republic of China
Email [email protected]
Purpose: The aim of this study was to evaluate the efficacy and safety of hormonal and synchronous docetaxel plus prednisone (DocP) in metastatic hormone-sensitive prostate cancer (mHSPC).
Methods: One hundred fifty-one cases with high-burden mHSPC diagnosed at 1 single center from January 2014 to August 2018 were analyzed retrospectively. Among them, 85 cases received androgen-deprivation therapy (ADT) within 3 months, along with 6 cycles of docetaxel + prednisone (treatment group), whereas 66 received ADT alone (control group). The primary end point was the median overall survival (OS), while the secondary outcomes included prostate-specific antigen (PSA) progression-free survival (PFS), radiographic PFS, and the proportion of PSA falling to 0.2 ng/mL.
Results: A total of 151 patients were included and followed up for a median of 34 months in this study. The median OS time in the treatment group was unavailable, but it was remarkably longer than that of the control group (P< 0.001). In addition, the PFS of PSA in the treatment group and control group was 17.9 months and 9.2 months, respectively (P< 0.001). Meanwhile, the radiographic PFS was 43 months in the treatment group and 19.8 months in the control group, respectively (P< 0.001). The proportions of PSA falling to 0.2 ng/mL were 53.7% and 23.3%, respectively (P< 0.001). However, there was no significant difference in the incidence of ≥ 3 toxic side effects between these 2 groups (P=0. 21).
Conclusion: ADT combined with 6 cycles of docetaxel + prednisone chemotherapy benefits patients diagnosed with high-burden mHSPC in terms of the OS, PFS of PSA and radiographic, and the ratio of PSA falling to 0.2 ng/mL.
Keywords: androgen-deprivation therapy; ADT, docetaxel, high-burden, metastatic hormone-sensitive, prostate cancer;PCa
Introduction
As reported in the 2014 ASCO meeting, androgen-deprivation therapy (ADT) + synchronous chemotherapy significantly prolongs the overall survival (OS) of patients newly diagnosed with metastatic hormone-sensitive prostate cancer (mHSPC).1 At present, it is clear that ADT + docetaxel is greatly beneficial for the high-burden mHSPC.2 To evaluate the efficacy and safety of ADT + synchronous docetaxel plus prednisone (DocP), this study retrospectively analyzed 151 cases with high-burden mHSPC who were treated at the Department of Urology, Chinese Academy of Medical Sciences, Cancer Hospital of HuanXing Chaoyang District, Beijing and the Shenzhen Hospital of Cancer Hospital of Chinese Academy of Medical Sciences from January 2014 to August 2018. Among them, 66 cases were treated with ADT alone, whereas the remaining 85 cases were treated with ADT + synchronous docetaxel (at a dose of dose of 75 mg/m2 every 3 weeks for 6 cycles) plus prednisone (5mg bid).
Methods
Basic Clinical Data
A total of 151 cases diagnosed with high-burden mHSPC were included in this study.
The patients in this study were divided into 2 groups: ADT + DocP group (n=85, treatment group) and ADT group (n=66, control group). The median age of these 2 groups was 63 years (range, 41 to 81) and 68.5 years (range, 45 to 83) respectively. In the treatment group, the percentage of ECOG performance-status score of 0 was approximately 20% higher compared with control group. The proportion of Gleason score of 8 or higher also was approximately 20% higher. Besides, in terms of lymph node metastasis and bone metastasis, the percentage difference between the 2 groups was about 10%, but it was not statistically significant (P=0.09, 0.26 respectively). Furthermore, the percentage of visceral metastasis between the 2 groups is approximately equal (Table 1). In addition, we found that the number of patients who harbored both metastases in the bone and viscera in the 2 groups were 20 and 10, respectively.
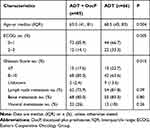 |
Table 1 Baseline Characteristics of the Patients |
Treatments
The treatment group received 6 cycles of DocP every 3 weeks; to be specific, docetaxel was administered through intravenous drip at a dose of 75 mg/m2 on day 1, while prednisone at a dose of 5 mg was administered orally for twice a day from 1 to 21 days. The decreased dose was allowed to be delayed in <2 weeks for <2 times during the course of treatment. Granulocyte colony-stimulating factors were allowed among patients with febrile granulocytopenia, antiallergic and antiemetic agents were applied appropriately during treatment, and phosphate was used in all patients with bone metastases.
All patients had signed the written informed consent prior to chemotherapy. During the course of treatment, the physician carried out necessary propaganda and education for both the patients and their families, and guided them to correctly understand and to assist in dealing with or preventing the possible treatment-related adverse events.
Evaluation of Therapeutic Effects and Adverse Events
The prostate-specific antigen (PSA) levels were monitored as planned. Meanwhile, thoracoabdominal and pelvic enhanced CT scans, together with 99 Technetium bone scans, were performed at the first visit, when the patient developed metastatic castration resistant prostate cancer (mCRPC) or when there were clinical symptoms. The criteria of radiographic progression were determined with reference to the Response Evaluation Criteria in Solid Tumors (RECIST1.0) standard.3 Besides, the complete remission of serum PSA was defined as PSA <0.2 ng/mL that lasted for more than 4 weeks. The progression of serum PSA was defined as an increase of ≥50% compared with the lowest value in first-line ADT, which continued to increase in over 1 week at an interval of more than 1 week. PSA progression survival was defined as the time when the increase of ≥50% was first recorded relative to the lowest value. If the lowest PSA value was <2 ng/mL, the absolute value of PSA rising should be over 2 ng/mL.4
OS was defined as the duration from the date of pathological diagnosis to death. Radiographic and PSA progression-free survival (PFS) were determined according to the criteria recommended by the Prostate Cancer Working Committee (PCWG2). Meanwhile, the primary end point was the median OS, whereas the secondary outcomes included PSA PFS, radiographic PFS, and the ratio of PSA falling to 0.2 ng/mL within 6 and 12 months, respectively. At the same time, all adverse drug events were also evaluated according to the common toxicity criteria (CTCAE-4).
Statistical Analysis
The SPSS 17.0 statistical software was adopted for data analyses. Descriptive analysis was carried out to test the frequency distribution, x2 test was adopted to compare between the 2 groups, the Kaplan–Meier method was employed to analyze the survival, and the Log-rank method was utilized to determine the significance of results. A difference of P<0.05 was deemed as statistically significant.
Results
Evaluation of Therapeutic Effects
The overall median follow-up period was 34 months. However, the median OS in treatment group was not attained, but it was significantly longer than that in control group (HR in treatment group, 0.41, 95% CI, 0.21–0.80, P=0.009) (Figure 1). Furthermore, stratified analysis showed that patients aged <70 years, and those with the ECOG score of 0–1, the Gleason score of 8–10, and no visceral metastasis greatly benefited from the combined treatment (Table 2).
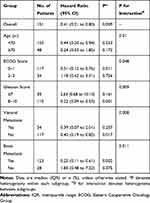 |
Table 2 Hazard Ratios for Death in Subgroups |
The PSA PFS in treatment group and control group was 17.9 months and 9.2 months, respectively (HR,0.37; 95% CI, 0.37–0.55, P<0.001) (Figure 2). Moreover, the proportions of PSA falling to 0.2 ng/mL were 53.7% and 23.3% in treatment group and control group, respectively (P<0.001). To be specific, the proportions of PSA falling to 0.2 ng/mL level within 6 months were 29.4% and 7.5% in the above 2 groups, respectively (P=0.001), and those within 12 months were 42.7% and 19.7%, separately (P<0.001). Additionally, the radiographic PFS was 43 months in the treatment group and 19.8 months in the control group (HR, 0.28; 95% CI, 0.16–0.48, P<0.001) (Figure 3). Besides, stratified analyses on both PSA and radiographic progression revealed that patients with the ECOG score of 0–1, the Gleason score of 8–10, and visceral metastasis benefited significantly from the combined treatment, regardless of age (Supplementary Table 1, 2).
Adverse Events
The rate of ≥3 toxic and side effects in treatment group was 10.9% (9/85), which was lower than that in control group (22.5%, 7/31), but there was no significant difference between these 2 groups (P=0. 21). Nine patients had grade III–IV side effects associated with chemotherapy, and no grade V side effect was reported. The primary presentations included blood toxicity, neutrophilic fever, gastrointestinal reactions, edema, fatigue, and mucositis.
Discussion
Prostate cancer (PCa) ranks first place in terms of its morbidity and second place with regard to its mortality, as suggested by the cancer statistics for 2019.5 With the development of more comprehensive screening indicators and the improvement of screening techniques, the incidence of mHSPC shows an increasing trend6 The ADT regimen has been identified as the preferred choice in the initial treatment of mHSPC.7 Subsequently, the addition of bicalutamide (the first generation antiandrogen) to the ADT regimen displays survival benefits in treating mHSPC.8 In 2004, docetaxel has been approved by the US FDA to treat metastatic castration-resistant prostate cancer (mCRPC), and it is recognized as the first drug to prolong patients' OS. Until 2015, docetaxel had proved to be the first drug that displayed significant survival benefits in treating mHSPC.9 Nowadays, the ADT + 6-cycle docetaxel chemotherapy regimen has become the standard treatment for the newly diagnosed high-burden mHSPC.10
In the CHAARTED study,2 a total of 790 patients were randomly divided into 2 groups: ADT + Doc group (n=397) and ADT group (n=393). The docetaxel regimen was docetaxel at a dose of 75 mg/m2+ prednisone at 10 mg for once a day for 3 weeks a cycle for 6 cycles. The overall median age was 63-years-old, and the follow-up period was 28.9 months. The results suggested that the median OS in the ADT + Doc group was prolonged by 13.6 months compared with the ADT group (57.6 vs. 44 months; the risk ratio [RR] was 0.61; 95% CI, 0.47–0.80; P<0.001). Meanwhile, the median time to biochemical, symptomatic, or radiographic progression in the ADT + Doc group was 20.2 months, it was 11.7 months in the ADT group (RR, 0.61; 95% CI, 0.51–0.72, P<0.001). Furthermore, subgroup analysis suggested that the median survival of high-burden ADT + Doc group was 17 months longer than that of the ADT group (49.2 vs. 32.2 months, RR, 0.60; 95% CI, 0.45–0.81, P<0.001). However, the low-burden patients did not benefit significantly from the combined treatment.
Moreover, the STAMPEDE study10 included patients with a high risk of locally advanced or metastatic PCa receiving long-term ADT. A total of 2,962 patients were randomly divided into 4 groups according to a ratio of 2:1: 1:1, ADT group (n=1184, ≥3 years ADT ± local radiotherapy); ADT + Doc group (n=592); ADT + zoledronic acid (ZA) group (n=593); ADT + Doc + ZA group (n=593). The docetaxel regimen was docetaxel at a dose of 75 mg/m2+ prednisone at 10 mg for once a day for 3 weeks a cycle for 6 cycles. The follow-up period was 43 months. The results indicated that compared with the ADT group, the OS of the ADT + Doc group was significantly prolonged by 10 months (p=0.006), and the survival improvement of the ADT + Doc + ZA group was similar to that of ADT + Doc group, which were consistent with those obtained from the CHAARTED study. However, median OS was not reached for the ADT + ZA group (HR 0·94, 95% CI 0.79–1.11; P=0.450).
The GETUG-15 study11 suggested that ADT + Doc showed no survival benefits for the uncastrated metastatic PCa. Nonetheless, the authors thought the result was partly due to the problem of methodology and early chemotherapy should be discussed with all patients. Furthermore, it was found that the ADT + Doc group had longer biochemical PFS compared with the ADT group.
The results of the above 3 clinical trials were mainly summarized as follows: first, ADT + Doc had significant survival benefits for M1 patients CHAARTED and STAMPEDE studies compared with ADT; second, ADT + Doc had longer median time to progression in CHAARTED and GETUG-15 studies. Third, although the results of these 3 trials are not exactly the same due to different characteristics of each experiment, they all show that ADT + Doc is beneficial to patients in some way (Table 3). Moreover, the meta-analysis on CHAARTED, GETUG-15 and STAMPEDE studies12 indicated that docetaxel had significant survival benefits for M1 patients, which increased the 4-year survival rate by nearly 10%. In addition, Ramamurthy et al13 performed a cost-effectiveness study to illustrate the value of abiraterone acetate (AA) vs. Doc in the first-line treatment of metastatic PCa. The results confirmed that Doc was substantially more cost-effective than AA in treating metastatic hormone-naïve PCa.
 |
Table 3 Characteristics of Clinical Trials Related to Docetaxel |
Findings in this study revealed no significant difference in other indicators between the 2 groups, except for age and the pathological Gleason score in terms of basic clinical characteristics. This might be associated with the high risk of metastasis in older patients receiving a low-dose of chemotherapy and with high pathological malignancy. The median follow-up period was 34 months. In terms of efficacy, the median OS of the treatment group was not reached, but it was significantly longer than that of 31.6 months in the control group (P<0.001). The PSA PFS in treatment group was 17.9 months, and that in control group was 9.2 months (P<0.001). Meanwhile, the radiographic PFS was 43 months in the treatment group and 19.8 months in the control group, respectively (P<0.001). The incidence of ≥3 toxic and adverse events in the treatment group was 10.9% (9/85), which was lower than that of the control group (22.5%; 7/31), but there was no significant difference between the 2 groups (P=0. 21). With regard to the proportion of PSA falling to 0.2 ng/mL within 12 months, the values of control group in our study and CHAARTED study were 19.7% and 19.6%, respectively, which were quite similar. However, the value of the treatment group was 42.7%, which was significantly higher than that of the control group (P<0.001), and that of 27.7% in the CHAARTED study. At present, no direct comparative study is available on the effect of DocP combined with ADT and ADT + Doc on the high-burden mHSPC patients. Besides, it remains unclear whether docetaxel + prednisone has an advantage in some aspects. Therefore, it is necessary to further establish a comparative study comprising the ADT + DocP (test group) and the ADT + Doc (control group) treatments for the high-burden mHSPC patients, so as to verify the differences in the proportion of PSA falling to 0.2 ng/mL, the median OS, PSA PFS and rate of ≥ 3 adverse events between the 2 groups.
As suggested by the 2017 LATITUDE study,14 Abiraterone + prednisone significantly reduced the risks of death and disease progression among the high-risk HSPC patients (with at least 2 of the following 3 criteria, Gleason score of ≥ 7, over 3 bone metastases and visceral metastasis). As a result, some clinicians had different opinions in the choice of docetaxel or Abiraterone. Wallis et al15 carried out a systematic review and network meta-analysis to evaluate the OS for men with hormone-naïve high-risk and metastatic PCa who were treated with AA plus prednisone/prednisolone combined with ADT (AA + ADT) versus docetaxel combined with ADT (Doc + ADT). They concluded that there was no significant difference in the OS between the AA + ADT and Doc + ADT regimens for men with hormone-naïve high-risk or metastatic PCa. Sydes et al16 conducted a head-to-head comparative study on the data from patients treated with standard treatment (ADT) combined with AA (ADT + AA) and those treated with standard treatment combined with docetaxel + prednisone (ADT + DocP) in the STAMPEDE trial. Altogether 566 patients were enrolled, including 189 in the ADT + DocP group and 377 in the ADT + AAP group. The median age was 66-years-old, and the median PSA level was 56 ng/mL, and the median follow-up was 4 years. These results showed that ADT + AAP group had obvious advantages in terms of FFS and PFS outcomes, but it had no significant advantage in the MFS and SRE outcomes; by contrast, the ADT + DocP group had no significant advantage in the OS outcome. Further, the maximum toxic reaction grades of the 2 groups were similar. In that study, docetaxel was administered for a total of 6 cycles (in about 4 months), while Abiraterone was administered for 24 months. Considering the cost-effectiveness and patient compliance, docetaxel, when used in combination with ADT, may be more suitable than abiraterone for patients with high burden mHSPC.17 Moreover, Sydes et al recommended docetaxel as the first-line treatment for those newly-diagnosed patients with high-burden mHSPC, and Abiraterone is recommended for patients who are intolerant to docetaxel.
In addition, the 2018 ESMO conference16 reported that, the STAMPEDE H group might further improve the prognosis for patients with low-burden PCa who received local radiotherapy combined with the standard treatment (ADT ± Doc). Results from the STAMPEDE G group suggested that, only the ADT combined with the abiraterone regimen exhibited survival benefits (HR, 0.63), which were similar to those obtained in the H group combined with radiotherapy (HR, 0.68). Therefore, an appropriate therapeutic regimen should be selected in clinical practice according to the specific patient conditions, such as health-related quality of life, volume of disease, age at treatment initiation, duration of therapy, and even patient preferences.18
There were also several limitations in this study. First, it was a non-randomized retrospective study, which may have existed in the study design account for selection bias. Second, the sample size was not large enough to make the convincing conclusion. More large-sample and high-quality RCTs are needed to confirm these preliminary findings.
Conclusion
In summary, the early ADT combined with DocP protocol benefits patients with high-burden mHSPC in many aspects (such as OS, PFS of PSA and radiographic, the ratio of PSA falling to 0.2 ng/mL), it also displays high safety. Moreover, patients with no contraindications of chemotherapy should be treated with docetaxel-based systemic chemotherapy for 6 cycles on the basis of ADT.
Abbreviations
ADT, androgen-deprivation therapy; PCa, prostate cancer; mHSPC, metastatic hormone-sensitive prostate cancer; mCRPC, metastatic castration resistant prostate cancer; OS, overall survival; PSA, prostate-specific antigen; PFS, progression-free survival; PCWG2, Prostate Cancer Working Committee; ECOG, Eastern Cooperative Oncology Group; RCT, randomized controlled trial; Doc, docetaxel; AA, abiraterone acetate; AAP, abiraterone acetate with prednisolone; DocP, docetaxel plus prednisone; HR, hazard ratio; RR, relative risk; IQR, interquartile range; ZA, zoledronic acid; LHRH, luteinizing hormone releasing hormone.
Data Sharing Statement
Data can be provided upon reasonable request.
Ethics and Consent Statement
The study was approved by Research Ethics Committee of the Chinese Academy of Medical Sciences, Cancer Hospital of HuanXing Chaoyang District, Beijing and Shenzhen Hospital of Cancer Hospital of Chinese Academy of Medical Sciences and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. All the patients in this study provided written informed consent.
Acknowledgments
This study was financially supported by the Capital Science and Technology Leading Talent Project (Project number: Z181100006318007) and the Beijing Chaoyang District Science and Technology Plan (Project number: CYSF1918).
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work
Disclosure
The authors declare that there is no conflict of interest.
References
1. Iacovelli R, Santoni M, de Braud F, Cascinu S, Procopio G. Highlights from the ASCO genitourinary symposium 2014: focus on renal and prostate cancer. Expert Rev Anticancer Ther. 2014;14(12):1407–1409. doi:10.1586/14737140.2014.953937
2. Sweeney CJ, Chen Y-H, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–746. doi:10.1056/NEJMoa1503747
3. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi:10.1093/jnci/92.3.205
4. Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol. 2008;26(7):1148–1159. doi:10.1200/JCO.2007.12.4487
5. Siegel RL, Miller KD, Ahmedin J. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
6. Kelly SP, Anderson WF, Rosenberg PS, Cook MB. Past, current, and future incidence rates and burden of metastatic prostate cancer in the United States. Eur Urol Focus. 2018;4(1):121–127. doi:10.1016/j.euf.2017.10.014
7. Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. J Urol. 2002;168(1):9–12. doi:10.1016/S0022-5347(05)64820-3
8. Prostate Cancer Trialists’ Collaborative Group. Maximum Androgen Blockade in Advanced Prostate Cancer: an Overview of the Randomised Trials. Vol. 355. London, UK: Prostate Cancer Trialists’ Collaborative Group; Lancet; 2000:1491–1498.
9. Cattrini C, Castro E, Lozano R, et al. Current treatment options for metastatic hormone-sensitive prostate cancer. Cancers (Basel). 2019;11(9):1355–1373. doi:10.3390/cancers11091355
10. James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–1177. doi:10.1016/S0140-6736(15)01037-5
11. Gravis G, Fizazi K, Joly F, et al. Androgen Deprivation Therapy (ADT) Plus Docetaxel Versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur Urol. 2016;70(2):256–262. doi:10.1016/j.eururo.2015.11.005
12. Vale CL, Burdett S, Rydzewska LHM, et al. Addition of docetaxel or bispho- sphonates to standard of care in men with localised or metastatic, hormone- sensitive prostate cancer: a systematic review and meta-analyses of aggregate data. Lancet Oncol. 2016;17(2):243–256. doi:10.1016/S1470-2045(15)00489-1
13. Ramamurthy C, Handorf EA, Correa AF, et al. Cost-effectiveness of abiraterone versus docetaxel in the treatment of metastatic hormone naïve prostate cancer[C]. Urol Oncol. 2019;37(10):688–695. doi:10.1016/j.urolonc.2019.05.017
14. Karim F, NamPhuong T, Luis F, et al. Abiraterone plus prednisone in metastatic castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352–360. doi:10.1056/NEJMoa1704174
15. Wallis Christopher JD, Klaassen Z, Bhindi B, et al. Comparison of abiraterone acetate and docetaxel with androgen deprivation therapy in high-risk and metastatic hormone-naïve prostate cancer: a systematic review and network meta-analysis. Eur Urol. 2018;73(6):834–844. doi:10.1016/j.eururo.2017.10.002
16. Sydes MR, Spears MR, Mason MD, et al. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. ESMO Ann Oncol. 2018;29(5):1235–1248. doi:10.1093/annonc/mdy072
17. Bilgin B, Şendur MA, Hızal M, et al. Docetaxel or Abiraterone in Addition to Androgen Deprivation Therapy in Metastatic Castration- Sensitive Prostate Cancer: Future Oncol; Aug 22, 2017.
18. Kretschmer A, Todenhöfer T. Are there still patients with metastatic hormone- sensitive prostate cancer who should be treated with androgen deprivation monotherapy? Eur Urol Focus. 2019;5(2):114–116. doi:10.1016/j.euf.2018.11.008
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



