Back to Journals » Journal of Pain Research » Volume 14
Effects of Wearable Transcutaneous Electrical Nerve Stimulation on Fibromyalgia: A Randomized Controlled Trial
Authors Jamison RN , Edwards RR, Curran S, Wan L, Ross EL, Gilligan CJ , Gozani SN
Received 28 April 2021
Accepted for publication 13 July 2021
Published 24 July 2021 Volume 2021:14 Pages 2265—2282
DOI https://doi.org/10.2147/JPR.S316371
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Krishnan Chakravarthy
Robert N Jamison,1 Robert R Edwards,1 Samantha Curran,1 Limeng Wan,1 Edgar L Ross,1 Christopher J Gilligan,1 Shai N Gozani2
1Department of Anesthesiology, Perioperative and Pain Medicine, Harvard Medical School, Brigham & Women’s Hospital, Chestnut Hill, MA, USA; 2NeuroMetrix, Inc., Woburn, MA, USA
Correspondence: Shai N Gozani
NeuroMetrix, Inc., 4b Gill St., Woburn, MA, 01801, USA
Tel +1 781 314 2789
Fax +1 781 890 1556
Email [email protected]
Purpose: Fibromyalgia is a chronic condition characterized by widespread pain and interference with daily activities. The aim of this study is to assess the benefit of transcutaneous electrical nerve stimulation (TENS) for persons diagnosed with fibromyalgia.
Patients and Methods: Adults meeting diagnostic criteria for fibromyalgia were randomized in a double-blind trial to receive either an active (n=62) or sham (n=57) wearable TENS device for 3-months. Subjects were classified as having lower or higher pain sensitivity by Quantitative Sensory Testing (QST). Patient Global Impression of Change (PGIC, primary outcome) and secondary efficacy measures including Fibromyalgia Impact Questionnaire (FIQR), Brief Pain Inventory (BPI) and painDETECT questionnaire (PDQ) were assessed at baseline, 6-weeks and 3-months. Treatment effects were determined by a mixed model for repeated measures (MMRM) analysis of the intention-to-treat (ITT) population (N=119). A pre-specified subgroup analysis of pain sensitivity was conducted using an interaction term in the model.
Results: No differences were found between active and sham treatment on PGIC scores at 3-months (0.34, 95% CI [− 0.37, 1.04], p=0.351) in the ITT population. However, in subjects with higher pain sensitivity (n=60), PGIC was significantly greater for active treatment compared to sham (1.19, 95% CI [0.24, 2.13], p=0.014). FIQR total score (− 7.47, 95% CI [− 12.46, − 2.48], p=0.003), FIQR pain item (− 0.62, 95% CI [− 1.17, − 0.06], p=0.029), BPI Interference (− 0.70, 95% CI [− 1.30, − 0.11], p=0.021) and PDQ (− 1.69, 95% CI [− 3.20, − 0.18], p=0.028) exhibited significant improvements for active treatment compared to sham in the ITT population. Analgesics use was stable and comparable in both groups.
Conclusion: This study demonstrated modest treatment effects of reduced disease impact, pain and functional impairment from wearable TENS in individuals with fibromyalgia. Subjects with higher pain sensitivity exhibited larger treatment effects than those with lower pain sensitivity. Wearable TENS may be a safe treatment option for people with fibromyalgia.
Clinicaltrials.gov Registration: NCT03714425.
Keywords: fibromyalgia, wearable, transcutaneous electrical nerve stimulation, neuromodulation, non-pharmacological treatment, clinical trial
Introduction
Fibromyalgia is a chronic condition characterized by widespread pain and tenderness. Individuals with fibromyalgia also experience sleep disturbances, fatigue, cognitive impairment, and mood disorders.1 The prevalence is estimated at 2–6% of the US adult population and is more common in women than men.2 The specific cause of fibromyalgia is unknown; however, pathological changes in the central nervous system leading to pain hypersensitivity are likely involved.3 Fibromyalgia diagnostic criteria have evolved over the past 30 years and may include characteristic symptoms, tenderness on physical exam and medical history.1,4,5 However, diagnosis remains challenging, with many patients suffering for years before identification of the disease.6 There is increasing interest in developing objective and quantitative biomarkers for the condition.7 As an example, many individuals with fibromyalgia exhibit low pressure pain thresholds throughout the body and physiological signs of central sensitization and diminished endogenous pain inhibition when assessed by Quantitative Sensory Testing (QST).3
Few treatments have been shown to be effective in managing fibromyalgia. Three drugs (pregabalin, duloxetine, and milnacipran) have been approved by the Food and Drug Administration (FDA) for management of fibromyalgia but these pharmacological agents are often associated with side effects8–10 and poor adherence.11,12 Non-pharmacological treatments such as patient education and physical exercise are recommended as first-line therapy, potentially followed by alternative approaches such as cognitive behavioral therapy and acupuncture.13 Although these non-pharmacological interventions are safe, their efficacy has not been conclusively demonstrated. In general, fibromyalgia treatments are associated with small to moderate effect sizes.14–16
Transcutaneous Electrical Nerve Stimulation (TENS) is believed to activate descending pain inhibition and reduce central excitability.17,18 Theses mechanisms align with pathological changes in pain processing that are thought to underlie fibromyalgia. TENS is usually applied to the site of pain, however its analgesic effects can be widespread,19,20 potentially making it effective for multisite pain.21,22 For these reasons, TENS has been suggested as a non-pharmacological option for patients with fibromyalgia.20,23 However, the efficacy of TENS in reducing fibromyalgia pain and symptoms is uncertain.24 As a general matter, randomized controlled trials of TENS have been criticized for small sample sizes, poor controls, the absence of compliance monitoring, and inadequate stimulation intensity.25,26 A recent trial of daily TENS use for 1-month addressed these deficiencies and demonstrated significant treatment effects in fibromyalgia patients compared to sham TENS and no TENS controls.23
Wearable TENS is an emerging category of non-invasive neuromodulation where the device is designed for placement directly on the body, typically at a fixed location such as the upper calf or arm. These devices may incorporate automated stimulation control and sensors to adaptively modulate stimulation and track objective outcomes.27 Some wearable devices are designed for extended wear, including during sleep, which addresses a key limitation of traditional TENS.28 Wearable TENS devices have been evaluated in chronic lower extremity pain,29 chronic low back pain,30 chemotherapy induced peripheral neuropathy (CIPN),31 migraine,32 and essential tremor33 with generally encouraging results. Wearable TENS has not been evaluated for management of fibromyalgia.
The present RCT compared 3-months of at-home treatment with an active or sham wearable TENS device located on the upper calf in individuals with fibromyalgia. The upper calf location enables stimulation of sensory dermatomes S2 through L4 with a circumferential electrode. These dermatomes are typically targeted when treating lower extremity and low back pain,34 which are common in fibromyalgia.4,35 The primary hypothesis was that active treatment would produce greater improvements in pain, somatic symptoms, and functional impairment compared to sham treatment. A second hypothesis was that subjects with the greatest relative baseline pain sensitivity by QST would exhibit the largest treatment effects. This hypothesis was based on the proposition that analgesic treatments that target sensitized central neurons, such as TENS,17,18 may be most effective in chronic pain characterized by hyperalgesia and central sensitization. Model-based statistical analyses were employed to maximize power to detect treatment effects in the presence of low to moderate effect sizes characteristic of fibromyalgia.
Materials and Methods
Study Design and Subject Selection
The protocol was registered at ClinicalTrials.gov (NCT03714425) prior to initiation of the study. This single-site, parallel-group trial was conducted at a tertiary academic hospital between February 2019 and June 2020. Following a screening process, eligible individuals were scheduled for a baseline visit. All subjects signed a written informed consent and were randomized to either an active or sham device. All participants were administered a brief stimulation trial with the device and if they disliked the sensation they could immediately withdraw from the study. No subject chose to withdraw. QST was performed at this initial visit. Following the baseline visit, subjects were called weekly to monitor changes in analgesic use and adverse events. This study conforms to the Consolidated Standards of Reporting Trials (CONSORT) guidelines,36 including a recently published pain-specific supplement.37 This study protocol and all amendments were approved by the Human Research Committee (Institutional Review Board) of Mass General Brigham (Massachusetts General Hospital and Brigham and Women’s Hospital, Boston, MA, USA). This study followed the principles outlined in the World Medical Association Declaration of Helsinki – Ethical Principles or Medical Research Involving Human Subjects.
The subject inclusion criteria were age 21 or older; able to speak and understand English; own a smartphone that can run the mobile app associated with the wearable TENS device; meet American College of Rheumatology 2010 diagnostic criteria for fibromyalgia;1 physician diagnosis of fibromyalgia in the medical record; and average pain intensity ≥ 4 on an 11-point numerical rating scale (NRS). Exclusion criteria included a diagnosis of cancer or other malignant disease; acute osteomyelitis or acute bone disease; present or past psychiatric diagnosis (Diagnostic and Statistical Manual of Mental Disorders: DSM-5) that was judged by the principal investigator to interfere with study participation (no subjects were excluded on this basis in the present study); pregnancy; clinically unstable systemic illness that could interfere with treatment; pain condition requiring urgent surgery; active substance use disorder that could interfere with study participation; or an implanted cardiac pacemaker, defibrillator, or other implanted electronic device. Subjects were asked to continue their pre-study analgesic medications with self-reported changes tracked through a weekly phone interview.
Randomization and Blinding
Subjects were randomized to an active or sham device with equal allocation. Active and sham devices were physically identical; only differing in whether they were loaded with standard software or modified software that implemented a sham stimulation protocol. A total of 120 devices were randomized by the manufacturer (www.graphpad.com/quickcalcs/randomize1) and provided to the trial site. The manufacturer had no interaction with the study subjects. The study coordinators and investigators could not determine whether a device was an active or sham device based on any markings or physical characteristics and did not discuss the stimulation experience with subjects. Subjects were told that two types of TENS were being evaluated, a “low intensity” device and a “high intensity” device. Blinding effectiveness for both subjects and study coordinators was assessed at the end of the study. Subjects in the sham treatment group were offered a device with standard software after completing the study.
TENS Intervention
The active treatment was a commercially available wearable TENS device (Quell, NeuroMetrix, Inc., Woburn, MA, USA) that is placed on the lower extremity of either leg, typically the upper calf.27,29 The device is comprised of a one-channel electrical stimulator, a stretchable band to secure the stimulator to the leg, an electrode array and a smartphone app. The electrode array consists of 4 hydrogel pads that provide a total stimulation surface area of 60 cm2. The stimulator generates bipolar, current-regulated pulses with a duration of 290 microseconds and alternating leading phase polarity. Stimulation frequency is random with a uniform distribution between a 60 and 100 Hz. The stimulator communicates with a smartphone application through Bluetooth®. The mobile application serves as a remote control for stimulator functions, displays device status, and tracks utilization. It is linked with a cloud database for storage of deidentified utilization data.
Prior to first use, the device calibrates to the user’s sensation threshold using an algorithm based on ascending and descending methods of limits. Subsequent stimulation is controlled automatically, although the user can also manually decrease or increase intensity. The initial stimulation level is 1.8 times the sensation threshold. This intensity is generally perceived as “strong but comfortable” by most individuals,38 which is the target sensation for effective conventional TENS.26,39 Each therapy session is 60 minutes, with sessions automatically starting every other hour as long as the device is on the body, including overnight. The active device provided 60-minutes of continuous stimulation during each therapy session. The sham device provided three 2-minute periods of stimulation during each session (at 0, 28, and 58 minutes) for a total of 6-minutes of stimulation. Sham TENS based on transient stimulation has been validated in healthy controls40 and used in earlier osteoarthritis41 and fibromyalgia RCTs.20,23 The device placement on the upper calf and usage instructions were identical for the two devices. Subjects were instructed to maintain a strong but comfortable stimulation intensity26 and to use their device for at least two 1-hour therapy sessions each day over the course of the study.
Quantitative Sensory Testing
The multimodal QST procedures included mechanical and cold stimuli.30,42 Responses to punctate mechanical stimuli were measured using a standard set of weighted probes (Touch-Test Sensory Evaluator; North Coast Medical, Inc.). Singular taps were performed on the metacarpophalangeal joint of the middle finger of the non-dominant hand.43 The lowest-force stimulator that produced a sensation of discomfort at 10 out of 100 was used to assess temporal summation of pain that occurred with rapid administration of 10 identical stimuli at 1-second intervals. Participants rated the painfulness of the first, fifth, and tenth stimulus. Mechanical temporal summation was defined as the increase in pain from the first to the tenth stimulus. A Somedic pressure algometer (Somedic SenseLab AB) was used to measure pain pressure thresholds (PPT) at the trapezius muscle and thumb joint. Each site was assessed twice on the left and right sides. Mechanical pressure was applied using a 0.5-cm2 probe covered with a 1-mm polypropylene pressure-transducing material. Pressure was increased at a steady rate of 30 kPA/s until the subject indicated that the stimulus was painful. Cuff algometry was used to assess responses to sustained mechanical pressure. A Hokanson rapid cuff inflator (D. E. Hokanson, Inc.) was used to inflate a standard blood pressure cuff around the gastrocnemius muscle of the dominant leg until the subject indicated a pain level of 40 out of 100. This pressure was maintained for 2 minutes, with the subject rating their pain at 30 second intervals.
Responses to noxious cold were evaluated using a repeated cold pressor task (CPT), which involved immersion of the right hand in a circulating water bath (Neslab RTE 17, Thermo Electron Corp.) maintained at 4 °C. Participants underwent a series of CPTs, with the first 2 consisting of serial immersions of the dominant hand for 15 seconds, with 2 minutes between immersions. Once the subject removed their hand, pain ratings were asked at 0, 15, 30, and 60 seconds. If the subject was not able to remain in the water for the full 15 seconds, they were able to remove it early with the same assessment intervals. Conditioned Pain Modulation (CPM) was measured by assessing PPT at the trapezius during the water bath immersions. The final CPT involved an immersion of the dominant hand until the participant reached maximum pain tolerance (or 3 minutes). Pain was assessed at 15 second intervals while submerged and as soon as the hand was removed from the water. The participants rated the intensity of cold pain on a 0–100 scale.
Efficacy Measures
The primary outcome measure was the Patient Global Impression of Change (PGIC), which represents the subject’s overall belief about the efficacy of treatment on a 7-point categorical verbal rating scale.44 The scale ranges from (1) “no change or condition has gotten worse” to (7) “a great deal better and a considerable improvement that has made all the difference.” There were 7 pre-specified secondary efficacy measures. Disease impact and health related quality-of-life was assessed with the Fibromyalgia Impact Questionnaire (FIQR).45 The FIQR pain intensity and sleep quality items, which are components of the FIQR total score, were also separately analyzed because of their particular importance to individuals with fibromyalgia.46–48 Pain severity and pain interference with function were evaluated with the Brief Pain Inventory (BPI).49 Neuropathic pain was assessed with the 7-item painDETECT questionnaire (PDQ).50,51 Pain-related disability was evaluated with the Pain Disability Index (PDI).52 Psychological outcomes included the Hospital Anxiety and Depression Scale (HADS)53 and the Pain Catastrophizing Scale (PCS).54 These outcome measures have been widely used in prior fibromyalgia treatment trials and their psychometric properties have been validated in US patients with chronic pain.55 The psychometric characteristics of several instruments have been specifically evaluated in patients with fibromyalgia.45,56,57 All instruments were delivered in conventional paper format consistent with their original formulation and validation. All efficacy measures except for PGIC were taken at baseline, 6-weeks, and 3-months. PGIC was assessed at 6-weeks and 3-months. The 6-week outcome assessments were mailed to the study subjects, completed at home, and returned via mail. The 3-month efficacy assessments were intended to be completed by subjects at the 3-month in-person visit. Due to COVID-19 restrictions on clinic visits beginning March 2020, 27 subjects were asked to complete the 3-month outcome assessments via mail.
Statistical Analyses
The sample size calculation was designed to confirm the hypothesis that the active device would be associated with a greater PGIC score compared to the sham device at 3-months of 0.6 points with a standard deviation (SD) of 1.0.58–60 The calculation assumed 85% power and a two-sided Type I error rate of 0.05. The estimated sample size of 100 was increased to a target recruitment of 115 to account for 15% drop-out.30 The primary analysis of treatment effects was conducted in the intention-to-treat (ITT) population, which included all 119 randomized subjects. In addition, a pre-specified subgroup analysis was carried out. The study protocol predicted that subjects with higher pain sensitivity based on QST measures would demonstrate the greatest treatment effects. Pain sensitivity was classified using the first principal component of the baseline QST data.61
The mean PGIC score at 3-months and the mean baseline to 3-month change scores for the secondary efficacy measures were compared between the active and sham treatment groups by a mixed model for repeated measures (MMRM) analysis. In the presence of missing data, a MMRM analysis typically has more power than a two-sample t-test62 or ANCOVA model.63 The ITT model included fixed effects for treatment, visit (baseline, 6-weeks, 3-months), and a treatment-by-visit interaction. Covariates included the baseline value (except for PGIC), baseline pain severity (BPI average pain item) and BMI along with the three corresponding by-visit interactions. The subgroup model included all the parameters in the ITT model and an interaction between treatment and pain sensitivity. This model was first used to test for treatment heterogeneity by a significant interaction term at a two-sided p-value less than 0.15.64,65 The model was then used to estimate the marginal effects of treatment for subjects with lower and higher pain sensitivity.
A value of 1 (“no change”) was assigned as the 3-month PGIC score if there was no 6-week or 3-month data for a subject. The outcome vector of the secondary efficacy measures included a change score of zero for the initial (baseline) visit to account for subjects with no treatment data.66,67 Missing covariates were addressed with the missing-indicator approach.68 Correlations among measurements taken on the same subject were first modeled with an unstructured covariance assumption. If the model failed to converge then a first-order autoregressive covariance structure was used. Marginal effects of treatment were determined at 3-months along with corresponding two-sided p-values. In the subgroup model, the marginal effects were further conditioned on pain sensitivity. Comparisons were deemed significant if the two-sided p-value was less than 0.05. Adjustments for multiple comparisons of secondary efficacy measures were not performed to preserve Type II error rates at the risk of an elevated family-wise Type I error rate.69 Multiplicity corrections (eg, Bonferroni) generally assume that outcomes are independent and will overcompensate for correlated measures leading to increased Type II errors. In the present study, there were moderate correlations among many of the efficacy measures.
A MMRM analysis uses all available data at each visit and implicitly imputes missing data under a missing at random (MAR) assumption. A sensitivity analysis was performed using reference-based multiple imputation (MI) with the jump-to-reference method.70,71 The MI model included the same covariates as the primary MMRM analysis. The imputed datasets were analyzed with the original MMRM model and combined using Rubin’s rule.70 The impact of missing data was assessed by comparing the resulting estimates with the primary MMRM estimates.
Responder rates were compared between treatment groups using logistic regression. Missing outcomes due to study withdrawal were treated as non-responders. For subjects that completed the study, 6-week data were used if 3-month data were not available. The dependent variable in the model was a binary variable indicating whether the subject was a responder or non-responder. The model included treatment assignment as an independent variable and baseline pain severity (BPI average pain item) and BMI as covariates. An interaction term between treatment and baseline pain sensitivity was added for subgroup analyses of responder rates.
Results
Subjects
Of 170 individuals screened for the study, 119 met the inclusion/exclusion criteria and were randomized to an active (62) or a sham (57) device for 3-months (Figure 1). Among these subjects, 16 (7 active, 9 sham) withdrew and were lost to follow-up and 3 (3 active, 0 sham) withdrew but completed the 3-month assessments. The remaining 100 subjects completed 3-months of treatment, however 4 (1 active, 3 sham) did not return the 3-month assessments. A comparison of demographic data between the two groups is presented in Table 1. The active group exhibited non-significant but numerically higher efficacy measures at baseline (Table 2). The baseline characteristics were similar to other trials of pharmacological and non-pharmacological interventions for fibromyalgia.23,58 Half of the subjects (47.1%) were taking over-the-counter analgesics, 31.9% were prescribed neuroleptics, 27.7% were prescribed an antidepressant, and 21.8% were taking an opioid, including tramadol. There was no discernable change in analgesic use over the course of the study and there was no significant difference between the treatment groups for any week (Supplemental Table 1). Most subjects were compliant with the target of 2 or more TENS sessions per day and utilized adequate stimulation intensity (Supplemental Table 2). Participants averaged 3.5 (SD 2.4) sessions per day and used their device on an average of 68.9 (SD 27.1) days and 23.7 (SD 23.4) nights during the study. No differences in TENS utilization were found between groups. Both the active and sham stimulation intensities (defined relative to sensation threshold) were comparable to values reported for successful long-term users of TENS.38
 |
Table 1 Demographic Characteristics of Study Population at Baseline |
 |
Table 2 Comparison of Efficacy Measures at Baseline |
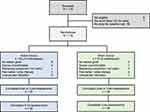 |
Figure 1 CONSORT diagram with intention-to-treat. |
Baseline Pain Sensitivity
Principal component analysis was applied to the correlation matrix of the baseline QST data (Table 3) to identify the prominent pain patterns in the study population. The loading weights for the first principal component, which accounted for 40% of the total variance, are shown in Figure 2. The component had negative loadings for mechanical pain thresholds, positive loadings for pain responses to punctate stimuli and cold stimuli, and a positive loading for temporal summation, which can be interpreted as a composite index of pain sensitivity. Subjects were classified as lower (< median) or higher (≥ median) pain sensitivity using this principal component. This yielded a lower pain sensitivity subgroup with 59 subjects and a higher pain sensitivity subgroup with 60 subjects. The proportions of subjects with higher pain sensitivity were 48.4% in the active group and 52.6% in the sham group, p=0.643. The proportions of higher pain sensitivity were similar in subjects that completed the study (50.0%) and those that withdrew early (52.6%), p=0.833.
 |
Table 3 Comparison of Quantitative Sensory Tests at Baseline |
Patient Global Impression of Change
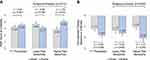
The mean PGIC score at 3-months was 3.58 (95% CI [3.09, 4.07]) in the active group and 3.24 (95% CI [2.74, 3.75]) in the sham group (Figure 3A). The mean difference of 0.34 (95% CI [−0.37, 1.04], p=0.351) was not significant and corresponded to an effect size of 0.17 (95% CI [−0.19, 0.53]). The prespecified subgroup analysis demonstrated a significant interaction between treatment and pain sensitivity (p=0.011). In the higher pain sensitivity subgroup, the mean PGIC score at 3-months was 4.05 (95% CI [3.37, 4.73]) for active treatment and 2.86 (95% CI [2.19, 3.53]) for sham treatment (Figure 3A). The mean difference of 1.19 (95% CI [0.24, 2.13], p=0.014) was significant and corresponded to an effect size of 0.63 (95% CI [0.11, 1.15]). The difference between active treatment and sham treatment in subjects with lower pain sensitivity was not significant (mean difference −0.55, 95% CI [−1.52, 0.42], p=0.268).
Secondary Efficacy Measures
After 3-months of active treatment, all secondary efficacy measures exhibited significant within-group improvement compared to their pre-treatment baseline (Table 4). Fibromyalgia disease impact (FIQR total score) decreased by 13.49 points (95% CI [−16.80, −10.18]) in the active group compared to a decrease of 6.02 points (95% CI [−9.57, −2.47]) in the sham group, which represented a significant difference of −7.47 points (95% CI [−12.46, −2.48], p=0.003) and corresponded to an effect size of 0.55 (95% CI [0.18, 0.92]). Pain intensity (FIQR pain item) decreased by 1.83 points (95% CI [−2.20, −1.46]) for active treatment compared to a decrease of 1.21 points (95% CI [−1.61, −0.82]) for sham (Figure 3B). The difference was significant (mean −0.62, 95% CI [−1.17, −0.06], p=0.029) and represented an effect size of 0.41 (95% CI [0.04, 0.77]). Differences between the active and sham treatments primarily emerged between the 6-week and 3-month assessments (Figure 4). There were also significant improvements in pain interference with function (BPI interference subscale), neuropathic symptoms (PDQ) and sleep quality (FIQR sleep item) for active treatment compared to sham (Table 4). The treatment effects estimated by the sensitivity analysis were generally smaller but consistent with those in the original MMRM analysis (Supplemental Table 3).
 |
Table 4 Mean Changes in Efficacy Measures from Baseline to 3-Months Using a MMRM Analysis |
There was a significant interaction between treatment and pain sensitivity for the baseline to 3-month change in pain intensity (p=0.042) but not for FIQR total score (p=0.245). In the higher pain sensitivity subgroup, the mean change in pain intensity was −2.05 (95% CI [−2.51, −1.60]) for active treatment and −1.05 (95% CI [−1.52, −0.57]) for sham treatment (Figure 3B). The mean difference of −1.01 (95% CI [−1.67, −0.35], p=0.003) was significant and corresponded to an effect size of 0.78 (95% CI [0.25, 1.30]). No group differences were found for lower pain sensitivity (mean −0.25, 95% CI [−0.91, 0.40], p=0.448). Treatment heterogeneity was also detected for the BPI severity subscale (p=0.106) and for PCS (p=0.111).
Responder Analysis
Responder rates after 3-months of treatment based on the PGIC score and changes in pain intensity (FIQR pain item) were examined. A PGIC responder was defined as a score ≥ 5 (“moderately better” symptoms, functional abilities and overall health). Pain intensity responders were defined at the conventional levels of ≥ 30% reduction and ≥ 50% reduction.46,72,73 The treatment-by-pain sensitivity interaction term was significant for all responder definitions. For PGIC, the active group had a numerically greater responder rate than sham in the ITT population that was not significant (42.5% vs 34.5%, difference = 8.0%, p=0.372). The treatment difference in the higher sensitivity subgroup was significant (58.0% vs 30.2%, difference = 27.8%, p=0.024). In the lower pain sensitivity subgroup, active treatment had a smaller responder rate (28.2% vs 38.9%, difference = −10.8%, p=0.386). For pain intensity, active treatment had a significantly greater responder rate compared to sham in both the ITT population and in the higher sensitivity subgroup at both the 30% (p=0.053 for ITT) and 50% levels (Figure 5). In all instances, the differences in responder rates between active and sham were larger in the subset of subjects with higher pain sensitivity than in the overall ITT population.
Blinding
Blinding was assessed by asking the coordinators and subjects whether a low intensity or high intensity device was used. The coordinator identified the correct treatment in 54.7% (95% CI [45.2, 64.2]) of the 103 subjects that completed the study (n=100) or withdrew but provided the 3-month assessment (n=3). The treatment was correctly identified 63.8% (95% CI [51.4, 76.2]) of the time for the active device and 43.8% (95% CI [29.7%, 57.8%]) of the time for the sham device. Of the 99 subjects that completed the 3-month assessment, 86 answered the blinding question. In these subjects, 50.0% (95% CI [39.4, 60.6]) named the correct treatment. Subjects in the active group correctly identified their treatment 17.4% (95% CI [6.4, 28.3]) of the time and subjects in the sham group correctly recognized their treatment 87.5% (95% CI [77.3, 97.7]) of the time. Among all subjects, 84.9% (95% CI [77.3, 92.5]) believed they received a low intensity device.
Safety
A total of 12 (5 active, 7 sham) adverse events were reported (Supplemental Table 4). They included rash at the site of the device, numbness and tingling, and muscle cramping. Six (3 active, 3 sham) were determined to be related to TENS use, 3 (1 active, 2 sham) were deemed possibly related to TENS use, and 3 (1 active, 2 sham) were judged to be unrelated to TENS use by the principal investigator. The 9 events that were definitely or possibly related to TENS use were minor and self-limited.
Discussion
This double blind, randomized, sham-controlled trial of wearable TENS did not meet its primary endpoint of a significant group difference in the 3-month PGIC score. The difference of 0.34 points was similar to milnacipran (0.47),58 duloxetine (0.35–0.87),59,60 and pregabalin (0.19–0.51),74–76 which are FDA approved drugs widely used for management of fibromyalgia. The current study was powered for an effect size of 0.6 and may have been underpowered to detect the small observed PGIC difference. The interpretation of global assessments in persons with fibromyalgia is challenging, likely reflecting the complex and heterogeneous nature of the disease.77 Another potential complication with PGIC is recency bias (ie, favoring recent events over older ones).78 As a result, therapeutic interventions should be evaluated by multiple outcomes that include measures anchored to the pre-treatment baseline.77,79 For this reason, FIQR may be a more appropriate primary endpoint for future fibromyalgia trials evaluating wearable TENS.
Although the primary endpoint was not met, comparisons between the active and sham groups suggest that wearable TENS has specific treatment effects in individuals with fibromyalgia. Overall disease impact decreased in the active group as measured by changes in the Fibromyalgia Impact Questionnaire (FIQR) total score. FIQR is a comprehensive health-related quality-of-life assessment of fibromyalgia and is a core outcome in clinical trials and practice.80 The items comprising the FIQR have high everyday relevance to people with fibromyalgia.35,81 The within-group improvement of 13.5 points for active treatment exceeded the minimal clinically important difference of 8 points, which was derived for the earlier FIQ instrument.82 Pain interference with function decreased in the active group as assessed by the BPI interference subscale. Individuals with chronic pain rank an increased ability to function and improved sleep to be important treatment objectives.83 IMMPACT recommendations include BPI Interference as a core outcome measure for clinical trials of chronic pain interventions.55 Neuropathic pain symptoms as assessed by the painDETECT questionnaire decreased. Neuropathic pain is a distressing symptom reported by many individuals with fibromyalgia.84,85 In many study participants, the specific clinical benefits of active treatment were superimposed on concurrent use of analgesic medications. It is also possible that analgesic use limited the TENS associated reduction in pain intensity, as has been reported for combination pregabalin and duloxetine therapy.86
In addition to the findings in the ITT population, the interaction between treatment and pain sensitivity was significant in an MMRM analysis of PGIC, indicating treatment heterogeneity. In subjects with higher pain sensitivity, those receiving active treatment had a 1.2 point greater PGIC score compared to subjects receiving sham treatment. This difference was significant and clinically meaningful.72,82,87 This finding is consistent with the hypothesis that TENS is most effective in sensitized pain pathways.17,18,20,41 It also follows from the observation that placebo pain relief is less effective in individuals with hyperalgesia.88 The goal of personalized medicine is to identify treatments that are most clinically beneficial and least harmful for each patient based on their individual genetic, physiological, and psychological characteristics.89 In clinical practice, it may be useful to identify fibromyalgia patients with higher pain sensitivity as optimal candidates for wearable TENS. However, traditional QST is impractical outside of specialized laboratories. As an alternative, it may be possible to predict pain sensitivity using simplified QST methods or from self-reported clinical variables.
Responder analyses demonstrated that over 40% of subjects in the active treatment group experienced clinically meaningful benefits on the individual outcome measures. Forty-three percent (43%) reported that their symptoms, functional abilities, and overall health were at least “moderately better” based on PGIC and 40% experienced a moderate reduction in pain intensity. The responder rates for active treatment exceeded sham treatment by 8 to 16% in the ITT population. The responder rate differences in the higher pain sensitivity subgroup were larger, ranging from 28 to 38%. These differences support a specific treatment effect in individuals using the active device, which may be particularly strong in those with hyperalgesia and central sensitization. The responder rate differences in the lower pain sensitivity subgroup were small. The absolute responder rates and treatment group differences in the ITT population were similar to pregabalin73,90 and milnacipran.58
Most sham-controlled trials of non-invasive electrical stimulation utilize inactive devices as controls, which are difficult to blind.91,92 In this study, the sham device provided intermittent stimulation totaling 6 minutes during each 1-hour therapy session. This translated to an average of 23 (SD 15) minutes per day for subjects using the sham device. This duration of stimulation is comparable to “active” devices in earlier RCTs that have been criticized for possibly under dosing patients.25 Given the noticeable stimulation by the sham device, it is unlikely that subjects randomized to the sham group would conclude that they were receiving an inactive placebo. When asked at the end of the study whether they had received a low or high intensity device, nearly all subjects (85%) indicated low intensity, regardless of the assigned treatment. Therefore, it is unlikely that differential efficacy expectations biased the study results. Fibromyalgia is associated with an enhanced sensitivity to all sensory stimuli.93 It is possible that many participants assumed their device was low intensity because of a belief that high intensity would cause them discomfort.
The sham response in this study was not surprising as fibromyalgia is associated with substantial placebo effects.94 Moreover, unlike drug trials that utilize inert placebos, the sham was not passive in order to protect blinding. It is possible that the 6-minutes of stimulation per session and the average of 23 minutes per day had a direct impact on pain perception by modulating pain pathways and through decreased sympathetic activity.95 It is noteworthy that in contrast to placebo, no-treatment controls in fibromyalgia studies exhibit limited or no improvement.23,94 Therefore, the within-group improvements reported for active treatment may represent clinical benefits in real-world use.
There have been few high-quality sham-controlled RCTs that examined the efficacy of TENS in fibromyalgia.24 In a recently published RCT, Dailey and colleagues examined the benefits of TENS applied simultaneously to the lower and upper back for two hours a day for 4-weeks in 301 individuals with fibromyalgia.23 Their study demonstrated reduced movement evoked and resting pain, decreased fatigue, less pain interference with function and decreased overall disease impact (FIQR total score) relative to both sham TENS and no-TENS controls. Interestingly, the Dailey study showed a significant difference in PGIC responder rates between active and sham treatments that is similar to the higher pain sensitivity subgroup in the present study. Although there were methodological differences between the Dailey RCT and the present RCT, both demonstrated that TENS reduces fibromyalgia associated pain, symptoms, and functional impairment.
The utility of wearable TENS has been examined in additional chronic pain states. The clinical benefits vary with the population characteristics, which is expected of a complex intervention such as TENS.96 Jamison and colleagues compared wearable TENS against treatment as usual in 68 individuals with chronic low back pain in a RCT.30 In that study, subjects in the active treatment arm reported significant reductions in pain intensity (BPI severity subscale), pain interference (BPI interference subscale) and pain catastrophizing (PCS) compared to the control arm after 3-months. However, significant differences were not found for pain-related disability (PDI) and psychological burden (HADS). The positive treatment effects were larger than in the present study, which might be attributable to a comparison to an unblinded control rather than a blinded sham device.23 Gewandter and colleagues evaluated wearable TENS in 26 patients with CIPN in a 6-week open-label pilot study.31 Significant improvements were reported for a composite CIPN instrument (EORTC-CIPN2097), overall pain quality (Short-Form McGill Pain Questionnaire-298), and pain intensity, tingling, numbness and cramping (latter four outcomes based on 11-point NRS daily dairy). Interestingly, an improvement in large fiber sensation (Utah Early Neuropathy99) was also reported. Several observational studies of real-world registry data in heterogenous chronic pain have also been reported.21,28,29 These studies suggested that wearable TENS was comparatively more effective at reducing pain interference with function than pain intensity.
There are several factors that support generalizability of the treatment effects found in the present study. First, the ITT analyses included all randomized subjects and thus accounted for treatment dropouts that inevitably occur in practice. Second, subjects received limited training by the study coordinators and used their device as recommended by the manufacturer for regular use. Third, utilization levels observed in this study were similar to unsupervised real-world use of the device for chronic lower extremity and low back pain.29
Study Limitations
This study has several limitations that should be considered when interpreting the results. There were only 8 male subjects. This makes it difficult to generalize the findings to men, which may account for at least 10% of fibromyalgia cases.35,100 Although fibromyalgia was diagnosed by accepted criteria and confirmed by a physician’s diagnosis in the medical record, the study did not incorporate an in-person medical history and physical examination. A physical examination is not always necessary to accurately diagnose fibromyalgia,1 however a concurrent assessment of fibromyalgia may have influenced the makeup of the study population.101 Data on analgesic use at baseline and changes during the study were collected by self-report, which is subject to recall bias and the ability and willingness of the subjects to provide accurate account. Within these constraints, there was no evidence of group differences at baseline or over the course of the study. Despite being encouraged to not change their fibromyalgia treatments during the study, some subjects may have pursued other non-pharmacological approaches (eg, physical therapy). It is difficult to know how other treatments or environmental factors such as weather102 might have affected the study. Outside treatments were found to be evenly divided between the active and sham groups, so one group did not have an advantage in receiving more pain-related treatments compared with the other. Finally, treatment effects may be slightly larger in single-center compared to multi-center RCTs.103
Conclusions
Fibromyalgia is a common chronic pain condition that reduces quality of life and is challenging to treat. Non-pharmacological interventions are recommended as first-line therapy.13 There are three FDA approved drugs, and others are used off-label including opioids. Pharmacological agents are associated with substantial side effects8,9 and poor adherence.11,12 This study demonstrated clinical benefits of at-home wearable TENS use in individuals with fibromyalgia over a 3-month period. These included a reduction in pain and somatic symptoms, a decrease in functional impairment, and less overall disease impact. These treatment benefits were obtained with few adverse events that were minor and self-limited. Wearable TENS represents a safe and effective treatment option for people with fibromyalgia.
Abbreviations
BMI, body mass index; BPI, Brief Pain Inventory; CI, confidence interval; CIPN, chemotherapy induced peripheral neuropathy; CPM, conditioned pain modulation; CPT, cold pressor test; FDA, Food and Drug Administration; FIQR, Fibromyalgia Impact Questionnaire Revised; HADS, Hospital Anxiety and Depression Scale; ITT, intention to treat; MAR, missing at random; MI, multiple imputation; MMRM, mixed model for repeated measures; NRS, numerical rating scale; PCS, Pain Catastrophizing Scale; PDI, Pain Disability Index; PDQ, painDETECT questionnaire; PGIC, Patient Global Impression of Change; PPT, pressure pain threshold; QST, Quantitative Sensory Testing; RCT, randomized controlled trial; SD, standard deviation; SE, standard error; TENS, Transcutaneous Electrical Nerve Stimulation.
Data Sharing Statement
De-identified data will be made available to qualified academic researchers by reasonable request to the corresponding author ([email protected]).
Ethics Statement
This study protocol and all amendments were approved by the Human Research Committee (Institutional Review Board) of Mass General Brigham (Massachusetts General Hospital and Brigham and Women’s Hospital, Boston, MA, USA). This study followed the principles outlined in the World Medical Association Declaration of Helsinki – Ethical Principles or Medical Research Involving Human Subjects.
Funding
NeuroMertrix, Inc. provided partial funding for this this trial.
Disclosure
S. Gozani is an employee and shareholder of NeuroMetrix, Inc. He holds multiple patents related to the Quell device. CJ Gilligan reports Sponsored Research from Mainstay Medical and Sollis, personal fees from Medtronic, personal fees from Abbott, personal fees from Saluda, personal fees from Persica, outside the submitted work. The remaining authors report no potential conflicts of interest for this work.
References
1. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American college of rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62(5):600–610. doi:10.1002/acr.20140
2. Vincent A, Lahr BD, Wolfe F, et al. Prevalence of fibromyalgia: a population-based study in olmsted county, minnesota, utilizing the rochester epidemiology project. Arthritis Care Res. 2013;65(5):786–792. doi:10.1002/acr.21896
3. Sluka KA, Clauw DJ. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience. 2016;338:114–129. doi:10.1016/j.neuroscience.2016.06.006
4. Wolfe F, Smythe HA, Yunus MB, et al. The American college of rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33(2):160–172. doi:10.1002/art.1780330203
5. Wolfe F, Clauw DJ, Fitzcharles MA, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46(3):319–329. doi:10.1016/j.semarthrit.2016.08.012
6. Choy E, Perrot S, Leon T, et al. A patient survey of the impact of fibromyalgia and the journey to diagnosis. BMC Health Serv Res. 2010;10:102. doi:10.1186/1472-6963-10-102
7. Hackshaw KV. The search for biomarkers in fibromyalgia. Diagnostics. 2021;11(2):156.
8. Straube S, Derry S, Moore RA, McQuay HJ. Pregabalin in fibromyalgia: meta-analysis of efficacy and safety from company clinical trial reports. Rheumatology. 2010;49(4):706–715. doi:10.1093/rheumatology/kep432
9. Branco JC, Zachrisson O, Perrot S, Mainguy Y; Multinational Coordinator Study G. A European multicenter randomized double-blind placebo-controlled monotherapy clinical trial of milnacipran in treatment of fibromyalgia. J Rheumatol. 2010;37(4):851–859. doi:10.3899/jrheum.090884
10. Evoy KE, Covvey JR, Peckham AM, Ochs L, Hultgren KE. Reports of gabapentin and pregabalin abuse, misuse, dependence, or overdose: an analysis of the food and drug administration adverse events reporting system (FAERS). Res Social Adm Pharm. 2019;15(8):953–958. doi:10.1016/j.sapharm.2018.06.018
11. Kim SC, Landon JE, Solomon DH. Clinical characteristics and medication uses among fibromyalgia patients newly prescribed amitriptyline, duloxetine, gabapentin, or pregabalin. Arthritis Care Res. 2013;65(11):1813–1819. doi:10.1002/acr.22071
12. Ben-Ami Shor D, Weitzman D, Dahan S, et al. Adherence and persistence with drug therapy among fibromyalgia patients: data from a large health maintenance organization. J Rheumatol. 2017;44(10):1499–1506. doi:10.3899/jrheum.170098
13. Macfarlane GJ, Kronisch C, Dean LE, et al. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. 2017;76(2):318–328. doi:10.1136/annrheumdis-2016-209724
14. Hauser W, Bernardy K, Uceyler N, Sommer C. Treatment of fibromyalgia syndrome with gabapentin and pregabalin–a meta-analysis of randomized controlled trials. Pain. 2009;145(1–2):69–81. doi:10.1016/j.pain.2009.05.014
15. Nuesch E, Hauser W, Bernardy K, Barth J, Juni P. Comparative efficacy of pharmacological and non-pharmacological interventions in fibromyalgia syndrome: network meta-analysis. Ann Rheum Dis. 2013;72(6):955–962. doi:10.1136/annrheumdis-2011-201249
16. Mascarenhas RO, Souza MB, Oliveira MX, et al. Association of therapies with reduced pain and improved quality of life in patients with fibromyalgia: a systematic review and meta-analysis. JAMA Intern Med. 2021;181(1):104–112. doi:10.1001/jamainternmed.2020.5651
17. DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr Rheumatol Rep. 2008;10(6):492–499. doi:10.1007/s11926-008-0080-z
18. Vance CG, Dailey DL, Rakel BA, Sluka KA. Using TENS for pain control: the state of the evidence. Pain Manag. 2014;4(3):197–209. doi:10.2217/pmt.14.13
19. Gozani SN. Remote analgesic effects of conventional transcutaneous electrical nerve stimulation: a scientific and clinical review with a focus on chronic pain. J Pain Res. 2019;12:3185–3201. doi:10.2147/JPR.S226600
20. Dailey DL, Rakel BA, Vance CG, et al. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain. 2013;154(11):2554–2562. doi:10.1016/j.pain.2013.07.043
21. Gozani SN, Kong KX. Real-world evidence for the widespread effects of fixed-site high- frequency transcutaneous electrical nerve stimulation in chronic pain. J Pain Relief. 2018;7(4). doi:10.4172/2167-0846.1000329
22. Rampazo da Silva EP, Silva VRD, Bernardes AS, Matuzawa F, Liebano RE. Segmental and extrasegmental hypoalgesic effects of low-frequency pulsed current and modulated kilohertz-frequency currents in healthy subjects: randomized clinical trial. Physiother Theory Pract. 2019;1–10. doi:10.1080/09593985.2019.1650857
23. Dailey DL, Vance CGT, Rakel BA, et al. Transcutaneous electrical nerve stimulation reduces movement-evoked pain and fatigue: a randomized, controlled trial. Arthritis Rheumatol. 2020;72(5):824–836. doi:10.1002/art.41170
24. Johnson MI, Claydon LS, Herbison GP, Jones G, Paley CA. Transcutaneous electrical nerve stimulation (TENS) for fibromyalgia in adults. Cochrane Database Syst Rev. 2017;10:CD012172.
25. Bennett MI, Hughes N, Johnson MI. Methodological quality in randomised controlled trials of transcutaneous electric nerve stimulation for pain: low fidelity may explain negative findings. Pain. 2011;152(6):1226–1232. doi:10.1016/j.pain.2010.12.009
26. Sluka KA, Bjordal JM, Marchand S, Rakel BA. What makes transcutaneous electrical nerve stimulation work? Making sense of the mixed results in the clinical literature. Phys Ther. 2013;93(10):1397–1402. doi:10.2522/ptj.20120281
27. Gozani SN. Fixed-site high-frequency transcutaneous electrical nerve stimulation for treatment of chronic low back and lower extremity pain. J Pain Res. 2016;9:469–479. doi:10.2147/JPR.S111035
28. Gozani SN, Ferree TC, Moynihan M, Kong X. Impact of transcutaneous electrical nerve stimulation on sleep in chronic low back pain: a real-world retrospective cohort study. J Pain Res. 2019;12:743–752. doi:10.2147/JPR.S196129
29. Kong X, Gozani SN. Effectiveness of fixed-site high-frequency transcutaneous electrical nerve stimulation in chronic pain: a large-scale, observational study. J Pain Res. 2018;11:703–714. doi:10.2147/JPR.S156610
30. Jamison RN, Wan L, Edwards RR, Mei A, Ross EL. Outcome of a high-frequency transcutaneous electrical nerve stimulator (hfTENS) device for low back pain: a randomized controlled trial. Pain Pract. 2019;19(5):466–475. doi:10.1111/papr.12764
31. Gewandter JS, Chaudari J, Ibegbu C, et al. Wireless transcutaneous electrical nerve stimulation device for chemotherapy-induced peripheral neuropathy: an open-label feasibility study. Support Care Cancer. 2019;27(5):1765–1774. doi:10.1007/s00520-018-4424-6
32. Yarnitsky D, Volokh L, Ironi A, et al. Nonpainful remote electrical stimulation alleviates episodic migraine pain. Neurology. 2017;88(13):1250–1255. doi:10.1212/WNL.0000000000003760
33. Pahwa R, Dhall R, Ostrem J, et al. An acute randomized controlled trial of noninvasive peripheral nerve stimulation in essential tremor. Neuromodulation. 2019;22(5):537–545. doi:10.1111/ner.12930
34. Johnson MI. Transcutaneous Electrical Nerve Stimulation (TENS): Research to Support Clinical Practice. Oxford University Press; 2014:261.
35. Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord. 2007;8:27. doi:10.1186/1471-2474-8-27
36. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi:10.1136/bmj.c869
37. Gewandter JS, Eisenach JC, Gross RA, et al. Checklist for the preparation and review of pain clinical trial publications: a pain-specific supplement to CONSORT. Pain Rep. 2019;4(3):e621. doi:10.1097/PR9.0000000000000621
38. Johnson MI, Ashton CH, Thompson JW. An in-depth study of long-term users of transcutaneous electrical nerve stimulation (TENS). Implications for clinical use of TENS. Pain. 1991;44(3):221–229. doi:10.1016/0304-3959(91)90089-G
39. Johnson MI, Bjordal JM. Transcutaneous electrical nerve stimulation for the management of painful conditions: focus on neuropathic pain. Expert Rev Neurother. 2011;11(5):735–753. doi:10.1586/ern.11.48
40. Rakel B, Cooper N, Adams HJ, et al. A new transient sham TENS device allows for investigator blinding while delivering a true placebo treatment. J Pain. 2010;11(3):230–238. doi:10.1016/j.jpain.2009.07.007
41. Vance CG, Rakel BA, Blodgett NP, et al. Effects of transcutaneous electrical nerve stimulation on pain, pain sensitivity, and function in people with knee osteoarthritis: a randomized controlled trial. Phys Ther. 2012;92(7):898–910. doi:10.2522/ptj.20110183
42. Edwards RR, Wasan AD, Michna E, Greenbaum S, Ross E, Jamison RN. Elevated pain sensitivity in chronic pain patients at risk for opioid misuse. J Pain. 2011;12(9):953–963. doi:10.1016/j.jpain.2011.02.357
43. Backonja MM, Walk D, Edwards RR, et al. Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain. 2009;25(7):641–647. doi:10.1097/AJP.0b013e3181a68c7e
44. Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther. 2004;27(1):26–35. doi:10.1016/j.jmpt.2003.11.003
45. Bennett RM, Friend R, Jones KD, Ward R, Han BK, Ross RL. The revised fibromyalgia impact questionnaire (FIQR): validation and psychometric properties. Arthritis Res Ther. 2009;11(4):R120. doi:10.1186/ar2783
46. Mease PJ, Spaeth M, Clauw DJ, et al. Estimation of minimum clinically important difference for pain in fibromyalgia. Arthritis Care Res. 2011;63(6):821–826. doi:10.1002/acr.20449
47. Cappelleri JC, Bushmakin AG, McDermott AM, Sadosky AB, Petrie CD, Martin S. Psychometric properties of a single-item scale to assess sleep quality among individuals with fibromyalgia. Health Qual Life Outcomes. 2009;7:54. doi:10.1186/1477-7525-7-54
48. Boomershine CS. A comprehensive evaluation of standardized assessment tools in the diagnosis of fibromyalgia and in the assessment of fibromyalgia severity. Pain Res Treat. 2012;2012:653714.
49. Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23(2):129–138.
50. Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–1920. doi:10.1185/030079906X132488
51. Cappelleri JC, Koduru V, Bienen EJ, Sadosky A. A cross-sectional study examining the psychometric properties of the painDETECT measure in neuropathic pain. J Pain Res. 2015;8:159–167.
52. Pollard CA. Preliminary validity study of the pain disability index. Percept Mot Skills. 1984;59(3):974. doi:10.2466/pms.1984.59.3.974
53. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
54. Sullivan MJL, Bishop S, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1996;7:524–532. doi:10.1037/1040-3590.7.4.524
55. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi:10.1016/j.pain.2004.09.012
56. Williams DA, Arnold LM. Measures of fibromyalgia: fibromyalgia impact questionnaire (FIQ), brief pain inventory (BPI), multidimensional fatigue inventory (MFI-20), medical outcomes study (MOS) sleep scale, and multiple ability self-report questionnaire (MASQ). Arthritis Care Res. 2011;63(Suppl 11):S86–97. doi:10.1002/acr.20531
57. Soer R, Koke AJ, Vroomen PC, et al. Extensive validation of the pain disability index in 3 groups of patients with musculoskeletal pain. Spine. 2013;38(9):E562–E568. doi:10.1097/BRS.0b013e31828af21f
58. Arnold LM, Gendreau RM, Palmer RH, Gendreau JF, Wang Y. Efficacy and safety of milnacipran 100 mg/day in patients with fibromyalgia: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62(9):2745–2756. doi:10.1002/art.27559
59. Russell IJ, Mease PJ, Smith TR, et al. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. Pain. 2008;136(3):432–444. doi:10.1016/j.pain.2008.02.024
60. Murakami M, Osada K, Mizuno H, Ochiai T, Alev L, Nishioka K. A randomized, double-blind, placebo-controlled Phase III trial of duloxetine in Japanese fibromyalgia patients. Arthritis Res Ther. 2015;17:224. doi:10.1186/s13075-015-0718-y
61. Cardoso JS, Riley JL, Glover T, et al. Experimental pain phenotyping in community-dwelling individuals with knee osteoarthritis. Pain. 2016;157(9):2104–2114. doi:10.1097/j.pain.0000000000000625
62. Ashbeck EL, Bell ML. Single time point comparisons in longitudinal randomized controlled trials: power and bias in the presence of missing data. BMC Med Res Methodol. 2016;16:43. doi:10.1186/s12874-016-0144-0
63. Siddiqui O, Hung HM, O’Neill R. MMRM vs. LOCF: a comprehensive comparison based on simulation study and 25 NDA datasets. J Biopharm Stat. 2009;19(2):227–246. doi:10.1080/10543400802609797
64. Alosh M, Fritsch K, Huque M, et al. Statistical considerations on subgroup analysis in clinical trials. Stat Biopharm Res. 2015;7(4):286–303. doi:10.1080/19466315.2015.1077726
65. Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine–reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189–2194. doi:10.1056/NEJMsr077003
66. Dinh P, Yang P. Handling baselines in repeated measures analyses with missing data at random. J Biopharm Stat. 2011;21(2):326–341. doi:10.1080/10543406.2011.550113
67. Liang KY, Zeger SL. Longitudinal data analysis of continuous and discrete responses for pre-post designs. Sankhya: Indian J Stat Series B. 2000;62(1):134–148.
68. Groenwold RH, White IR, Donders AR, Carpenter JR, Altman DG, Moons KG. Missing covariate data in clinical research: when and when not to use the missing-indicator method for analysis. CMAJ. 2012;184(11):1265–1269. doi:10.1503/cmaj.110977
69. Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. doi:10.1186/1471-2288-2-8
70. Cro S, Morris TP, Kenward MG, Carpenter JR. Sensitivity analysis for clinical trials with missing continuous outcome data using controlled multiple imputation: a practical guide. Stat Med. 2020;39(21):2815–2842. doi:10.1002/sim.8569
71. Cai X, Gewandter JS, He H, Turk DC, Dworkin RH, McDermott MP. Estimands and missing data in clinical trials of chronic pain treatments: advances in design and analysis. Pain. 2020;161(10):2308–2320. doi:10.1097/j.pain.0000000000001937
72. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi:10.1016/j.jpain.2007.09.005
73. Straube S, Derry S, Moore RA, Paine J, McQuay HJ. Pregabalin in fibromyalgia–responder analysis from individual patient data. BMC Musculoskelet Disord. 2010;11:150. doi:10.1186/1471-2474-11-150
74. Mease PJ, Russell IJ, Arnold LM, et al. A randomized, double-blind, placebo-controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgia. J Rheumatol. 2008;35(3):502–514.
75. Pauer L, Winkelmann A, Arsenault P, et al. An international, randomized, double-blind, placebo-controlled, phase III trial of pregabalin monotherapy in treatment of patients with fibromyalgia. J Rheumatol. 2011;38(12):2643–2652. doi:10.3899/jrheum.110569
76. Ohta H, Oka H, Usui C, Ohkura M, Suzuki M, Nishioka K. A randomized, double-blind, multicenter, placebo-controlled phase III trial to evaluate the efficacy and safety of pregabalin in Japanese patients with fibromyalgia. Arthritis Res Ther. 2012;14(5):R217. doi:10.1186/ar4056
77. Geisser ME, Clauw DJ, Strand V, Gendreau RM, Palmer R, Williams DA. Contributions of change in clinical status parameters to patient global impression of change (PGIC) scores among persons with fibromyalgia treated with milnacipran. Pain. 2010;149(2):373–378. doi:10.1016/j.pain.2010.02.043
78. Schmitt J, Di Fabio RP. The validity of prospective and retrospective global change criterion measures. Arch Phys Med Rehabil. 2005;86(12):2270–2276. doi:10.1016/j.apmr.2005.07.290
79. Rampakakis E, Ste-Marie PA, Sampalis JS, Karellis A, Shir Y, Fitzcharles MA. Real-life assessment of the validity of patient global impression of change in fibromyalgia. RMD Open. 2015;1(1):e000146. doi:10.1136/rmdopen-2015-000146
80. Mease P, Arnold LM, Choy EH, et al. Fibromyalgia syndrome module at OMERACT 9: domain construct. J Rheumatol. 2009;36(10):2318–2329. doi:10.3899/jrheum.090367
81. Bennett RM, Russell J, Cappelleri JC, Bushmakin AG, Zlateva G, Sadosky A. Identification of symptom and functional domains that fibromyalgia patients would like to see improved: a cluster analysis. BMC Musculoskelet Disord. 2010;11:134. doi:10.1186/1471-2474-11-134
82. Bennett RM, Bushmakin AG, Cappelleri JC, Zlateva G, Sadosky AB. Minimal clinically important difference in the fibromyalgia impact questionnaire. J Rheumatol. 2009;36(6):1304–1311. doi:10.3899/jrheum.081090
83. Casarett D, Karlawish J, Sankar P, Hirschman K, Asch DA. Designing pain research from the patient’s perspective: what trial end points are important to patients with chronic pain?. Pain Med. 2001;2(4):309–316.
84. Koroschetz J, Rehm SE, Gockel U, et al. Fibromyalgia and neuropathic pain–differences and similarities. A comparison of 3057 patients with diabetic painful neuropathy and fibromyalgia. BMC Neurol. 2011;11:55. doi:10.1186/1471-2377-11-55
85. Crofford LJ. The relationship of fibromyalgia to neuropathic pain syndromes. J Rheumatol Suppl. 2005;75:41–45.
86. Gilron I, Chaparro LE, Tu D, et al. Combination of pregabalin with duloxetine for fibromyalgia: a randomized controlled trial. Pain. 2016;157(7):1532–1540. doi:10.1097/j.pain.0000000000000558
87. Dworkin RH, Turk DC, McDermott MP, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009;146(3):238–244. doi:10.1016/j.pain.2009.08.019
88. Bouwense SA, Olesen SS, Drewes AM, van Goor H, Wilder-Smith OH. Pregabalin and placebo responders show different effects on central pain processing in chronic pancreatitis patients. J Pain Res. 2015;8:375–386. doi:10.2147/JPR.S84484
89. Bruehl S, Apkarian AV, Ballantyne JC, et al. Personalized medicine and opioid analgesic prescribing for chronic pain: opportunities and challenges. J Pain. 2013;14(2):103–113. doi:10.1016/j.jpain.2012.10.016
90. Derry S, Cording M, Wiffen PJ, Law S, Phillips T, Moore RA. Pregabalin for pain in fibromyalgia in adults. Cochrane Database Syst Rev. 2016;9:CD011790.
91. Gibson W, Wand BM, Meads C, Catley MJ, O’Connell NE. Transcutaneous electrical nerve stimulation (TENS) for chronic pain - an overview of cochrane reviews. Cochrane Database Syst Rev. 2019;4:CD011890.
92. Johnson MI, Jones G, Paley CA, Wittkopf PG. The clinical efficacy of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain: a protocol for a meta-analysis of randomised controlled trials (RCTs). BMJ Open. 2019;9(10):e029999. doi:10.1136/bmjopen-2019-029999
93. Geisser ME, Glass JM, Rajcevska LD, et al. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain. 2008;9(5):417–422. doi:10.1016/j.jpain.2007.12.006
94. Chen X, Zou K, Abdullah N, et al. The placebo effect and its determinants in fibromyalgia: meta-analysis of randomised controlled trials. Clin Rheumatol. 2017;36(7):1623–1630. doi:10.1007/s10067-017-3595-8
95. Abram SE, Asiddao CB, Reynolds AC. Increased skin temperature during transcutaneous electrical stimulation. Anesth Analg. 1980;59(1):22–25. doi:10.1213/00000539-198001000-00005
96. Gladwell PW, Badlan K, Cramp F, Palmer S. Direct and indirect benefits reported by users of transcutaneous electrical nerve stimulation for chronic musculoskeletal pain: qualitative exploration using patient interviews. Phys Ther. 2015;95(11):1518–1528. doi:10.2522/ptj.20140120
97. Postma TJ, Aaronson NK, Heimans JJ, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer. 2005;41(8):1135–1139. doi:10.1016/j.ejca.2005.02.012
98. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the short-form McGill pain questionnaire (SF-MPQ-2). Pain. 2009;144(1–2):35–42. doi:10.1016/j.pain.2009.02.007
99. Singleton JR, Bixby B, Russell JW, et al. The Utah early neuropathy scale: a sensitive clinical scale for early sensory predominant neuropathy. J Peripher Nerv Syst. 2008;13(3):218–227. doi:10.1111/j.1529-8027.2008.00180.x
100. Wolfe F, Walitt B, Perrot S, Rasker JJ, Hauser W. Fibromyalgia diagnosis and biased assessment: sex, prevalence and bias. PLoS One. 2018;13(9):e0203755. doi:10.1371/journal.pone.0203755
101. Arnold LM, Bennett RM, Crofford LJ, et al. AAPT diagnostic criteria for fibromyalgia. J Pain. 2019;20(6):611–628. doi:10.1016/j.jpain.2018.10.008
102. Fagerlund AJ, Iversen M, Ekeland A, Moen CM, Aslaksen PM. Blame it on the weather? The association between pain in fibromyalgia, relative humidity, temperature and barometric pressure. PLoS One. 2019;14(5):e0216902. doi:10.1371/journal.pone.0216902
103. Bafeta A, Dechartres A, Trinquart L, Yavchitz A, Boutron I, Ravaud P. Impact of single centre status on estimates of intervention effects in trials with continuous outcomes: meta-epidemiological study. BMJ. 2012;344:e813. doi:10.1136/bmj.e813
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.