Back to Journals » Drug Design, Development and Therapy » Volume 16
Effects of Salidroside Combined with Paclitaxel on Proliferation, Migration, and Epithelial Mesenchyme of Colorectal Cancer Cells
Authors Hao Y , Li Z, Chang M, Zhang X
Received 28 July 2022
Accepted for publication 16 November 2022
Published 28 November 2022 Volume 2022:16 Pages 4079—4089
DOI https://doi.org/10.2147/DDDT.S384151
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Yanjiao Hao, Zhiyu Li, Mingzhi Chang, Xiaoli Zhang
Department of Life Science Research Center, College of Basic Medicine, Hebei North University, Zhangjiakou, Hebei, 075000, People’s Republic of China
Correspondence: Xiaoli Zhang, Department of Life Science Research Center, College of Basic Medicine, Hebei North University, Zhangjiakou, Hebei, 075000, People’s Republic of China, Tel +8618931316301, Email [email protected]
Background: Colorectal cancer (CRC) is a multifactorial disease and one of the most common malignancies worldwide. Salidroside (Sal) is a plant with a wide range of pharmacological effects and plays an important role in the treatment of many diseases, and is considered a new hope for the treatment of tumors. The purpose of this study was to investigate the effect of the combination of Sal and paclitaxel (Pac) on colorectal cancer cells and its mechanism of action.
Methods: The effects of different mass concentrations of Sal, Pac, and the combination intervened in the cells for 48 h were examined using the CCK8 method. The inhibition rate was obtained, and the optimal concentration of the respective drug group was screened. The proliferative capacity of the respective group was obtained. Subsequently, the results of apoptosis, cloning, migration, invasion, and angiogenesis were observed through cell morphological analysis (shape observation and Hoechst staining), colony formation assay, cell scratching assay, Transwell, angiogenesis assay, and protein immunoblotting (Western blotting) to detect the expression of epithelial-mesenchymal transition (EMT)-associated proteins and PI3K pathway-associated proteins.
Results: Different concentrations of Sal, Pac, and the combined application had significant effects in inhibiting cells in a concentration-dependent manner. Compared with the control group, the Sal group, the Pac group, and the combination group significantly inhibited the clonal number, migration, invasion, and tube-forming ability of colorectal cancer cells. Besides, the combined application had a better effect than the Sal and Pac groups. The apoptosis level was up-regulated in all drug groups, and the up-regulation was more significant in the combination group. The expression of E-cad protein was up-regulated, the expression of N-cad and Vim protein was down-regulated, and the expression of PI3K and AKT phosphorylation was down-regulated in the respective group, and the difference was more significant in the combination group compared with the group of individual drugs.
Conclusion: The combined application of Sal and Pac significantly can decrease the survival rate of colorectal cancer cells, and the mechanism may be correlated with the blocking of the PI3K/AKT pathway, thus inhibiting EMT.
Keywords: salidroside, paclitaxel, colorectal cancer, apoptosis, PI3K/AKT signaling pathway, EMT
Introduction
Colorectal cancer (CRC) is the second most common cancer in women and the third most common cancer in men, with increasing incidence and mortality among cancers diagnosed and cancer-associated deaths worldwide each year.1,2 Surgery and radiotherapy continue to be the commonly employed treatments for patients with CRC, whereas the prognosis and survival rates for patients with the recurrent and metastatic diseases remain unsatisfactory. Existing research over the past few years has identified plant extracts with potential antitumor and pro-apoptotic effects on a wide variety of cancer cells. Increasing studies have suggested that some plant extracts are capable of attenuating the toxicity of chemotherapeutic drugs and increasing their antitumor effects.
Rhodiola rosea refers to a valuable plant that grows at high altitudes, and it has been extensively employed as a medicinal herb. Salidroside (Sal) is the main active ingredient of Rhodiola rosea with antioxidant, immune-enhancing, and antitumor effects.3–6 Paclitaxel (Pac) is termed one of the successful anticancer drugs and has achieved wide applications for treating ovarian cancer, breast cancer, uterine cancer, and many other cancers. However, some patients have developed drug resistance at the early stage of chemotherapy and achieved poor prognoses.7,8 Some existing research has suggested that the combination of Pac with other drugs is capable of reducing the toxic side effects of drugs while attenuating the resistance of tumors to drugs, which has promising applications compared with the combination of drugs alone.9
Tumor migration and invasion is a complex, multifactorial and dynamic process. Epithelial-mesenchymal transition (EMT), ie, a process that converts quiescent epithelial cells into active mesenchymal cells, is capable of facilitating tumor cell migration and invasion.10 The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) information transduction pathway takes on a critical significance to cell growth, transformation, migration, invasion, apoptosis, and autophagy.11 Activation of the PI3K/AKT pathway can facilitate cell cycle progression and angiogenesis, accelerate the adhesion of cancer cells and endothelial cells, and cause the migration and invasion of tumor cells. Existing research has suggested that PI3K/AKT pathway plays a direct or indirect role in the process of EMT oogenesis.12 Accordingly, this study aimed to investigate the effects of Sal combined with Pac on cell proliferation, migration, invasion, apoptosis, and EMT marker protein expression levels after drug treatment, and whether the specific pathway of drug-regulated EMT is correlated with PI3K/AKT signaling pathway lays a novel theoretical basis for clinical treatment of CRC.
Materials and Methods
Reagents
Salidroside was purchased from Solaibao (purity: ≥98%, lot number: IS0020, Beijing, China) and Paclitaxel was purchased from Huiyu Pharmaceutical Co., Ltd (Lot No. 2,412,110,042, Sichuan, China), both drugs were dissolved in culture medium and diluted to the indicated concentrations when used for cell experiments, ready to use. β-actin (ABclonal, USA), E-cadherin (Abcam, UK), N-cadherin, Vimentin, and Goat anti-mouse IgG/horseradish enzyme labeling, goat anti-rabbit IgG/horseradish enzyme labeling all purchased from Zhong Shan -Golden Bridge Biological (Beijing, China), PI3K, p-PI3K, AKT, p-AKT (CST, USA), Rapid Closure Solution (Bio-Rad, USA).
Cell Culture
HCT-8 and HCT-116 cells were purchased from the Cell Bank of the Typical Culture Collection Committee of the Chinese Academy of Sciences (Shanghai, China) and kept in the Life Science Research Center of Hebei North University, HCT-8 and HCT-116 cells were cultured in DMEM medium (CORNING, USA) with 10% fetal bovine serum (Gegrogen, USA) and 1% penicillin and streptomycin. The cells were incubated in a HEPA class 100 thermostat incubator (Thermo, USA) at 37°C with 5% CO2, and the medium was changed every 24 h. After the cells were fused to more than 70%-80%, the cells were passaged and experiments were performed with cells of the logarithmic growth phase.
Cell Viability Assay
The cells were inoculated in 96-well plates (1×104 cells/well) at the mass concentrations of 0, 20, 40, and 80µM for Sal and 0, 2.5, 5, and 10µM for Pac. The mass concentrations of the co-drugs reached Sal 20µM + Pac 2.5µM, Sal 40µM + Pac 5µM, Sal 80µM + Pac 10µM for pretreatment, and six replicate wells were set for the respective concentration. After the cells were placed in the incubator at 37°C for 48 h, 10μL CCK8 (livning, Beijing, China) was added and then incubated at 37°C for 2 h, and the absorbance (OD) at 450 nm was examined using the enzyme-linked immunoassay detector (Molecular Devices, USA). The results of the respective group were compared, and at least three independent experiments were repeated. The control group (drug concentration of 0µM), Sal group (40µM), Pac group (5µM), and combination group (Sal 40µM + Pac 5µM) were set in accordance with the measured optimal concentration of the respective drug group for subsequent experimental manipulations.
Cell Morphology Determination
The cells were inoculated in 6-well plates (5×104 cells/well) and gently shaken to make cells evenly distributed. Moreover, the cell morphology was identified and photographed after 48 h of treatment based on the above grouping. The independent experiment was repeated at least 3 times.
Plate Clone Formation Assay
The cells of the respective group were collected after drug treatment. The cells were inoculated in a 6-well plate (1×103 cells/well) and placed in a constant temperature incubator for 10–14 days. When the cells were pooled till becoming visible, they were washed with phosphate buffer solution (PBS), fixed with formaldehyde, stained with 0.1% crystalline violet, washed again with PBS, and then air-dried for photos.
Wound Healing Assay
The cells cultured to log phase were inoculated in 6-well plates (1×106 cells/well) and incubated in an incubator. When the cell density increased to 90%, a straight line was scratched at the bottom of the 6-well plate with the tip of a sterile gun. The cells were washed with PBS, and the corresponding drug concentration was added in accordance with the above grouping. Subsequently, the cells were incubated in a constant temperature oven for 48 h. Using an inverted microscope at 0 h and 48 h, photographs were taken, and cell migration images were recorded using an inverted microscope at 0 h and 48 h.
Transwell Invasion Assay
Matrigel gel (BD, USA) and DMEM medium were diluted in proportion (1:4), and 50µL of diluted Matrigel gel was added to the upper chamber, placed in an incubator, and removed after 3–4 hours. The cells were inoculated in the upper chamber of the chamber, while 600µL of the serum-containing medium was added to the lower chamber. After 48 h of incubation, the cells were fixed with formaldehyde, stained with crystal violet (0.1%), washed with PBS, and photographed under a microscope to record the number of invasive cells.
Hoechst Staining
Cells were grown in 24-well plates supplemented with sterile coverslips (2×105 cells/well), fixed with 4% formaldehyde after the action of Sal, Pac, and combination for 48 h, respectively, and then stained with Hoechst 33,258 (10μg/mL) staining solution (Beyotime, Beijing) for 20 min continuously, washed with PBS, photographed and recorded under a fluorescence microscope.
Angiogenesis Assay
Matrigel gel was spread evenly in 96-well plates with 60μL/well and placed in a 37°C thermostat for 30 min. Cells of the respective group were inoculated with 5×104 cells/well in 96-well plates supplemented with matrix gel, placed in a thermostat for 48 h, and photographed under an inverted microscope.
Western Blotting Assay
The cells of the respective group were collected and treated by adding lysis solution on ice, and the cellular proteins of the respective group were extracted, and the protein concentration was measured using a BCA protein quantification kit (Solarbio, Beijing). Electrophoresis was performed on the gel, transfer printing was performed on the PVDF membrane (MILLIPORE, USA), and the membranes were blocked in EveryBlot Blocking Buffer Fast Blocking Solution (Bio-Rad, USA) for 15 min at room temperature. The sample reacted with the primary antibody (1:1000) at 4°C overnight. After the sample was washed with TBST the next day, the corresponding secondary antibody (1:5000) was added, and slowly shaken at ambient temperature for 2 h. After TBST wash, the target protein was detected using an ultrasensitive chemiluminescence substrate kit (Bio-Rad, USA), and the images were taken using a gel imager and analyzed by ImageJ software. Independent experiments were repeated at least three times.
Statistical Analysis
The data were analyzed and plotted using Prism 8. Values are expressed as mean ± standard deviation, and the data were analyzed using SPSS 25.0 software. Statistical comparisons were evaluated through t-test and one-way or two-way ANOVA. P < 0.05 was set, and the differences achieved statistical significance.
Results
Inhibitory Effect of Sal, Pac, and Combination on Cells
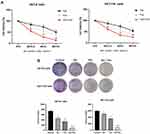
The effects of Sal, Pac, and combination on cell viability were first evaluated through CCK8 assay, and the results indicated that different concentrations of Sal, Pac, and combination inhibited cell treatment for 48 h in a concentration-dependent manner, and Sal enhanced the inhibitory effect of Pac on cell proliferation (P <0.05) (Figure 1A). The results of cell cloning experiments indicated that the number of cell colony formation was significantly down-regulated in the Sal, Pac, and combination groups, and the number of cell colonies was significantly reduced in the combination group compared with that of the individual drugs (P <0.01) (Figure 1B). The above results indicated that Sal, Pac, and combined application could inhibit the viability of the cells.
Effects of Sal and Pac Alone and in Combination on Apoptosis
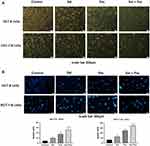
Apoptosis is a complex molecular biological mechanism of the organism, and it takes on a significance in physiology for tumor cell development. Whether Sal, Pac, and combined application can induce apoptosis and thus inhibit proliferation was investigated. The results indicated that the cells in the control group were in good condition and compact, while in the Sal and Pac groups, the number of cells was reduced and the cells were slightly rounded, and the cells in the combination group had different morphology with floating cells and cell debris in the culture medium (Figure 2A). The cells in the combined Sal and Pac groups were reduced in size, showed bright blue fluorescent signals, and had condensed chromatin in the nucleus, etc (Figure 2B). The above results suggest that Sal, Pac, and the combined drug can inhibit cell proliferation and then apoptosis.
Migration and Invasion of Cells by Sal, Pac, and Combination
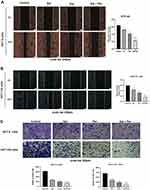
Migration and invasion are crucial in the malignant process of tumor cells, and we assessed the migration and invasion of cells by wound healing and transwell assays. Cell migration was evaluated through cell scratch assay. The results of the cell scratch assay indicated that cell motility was significantly reduced in the drug group compared with the control group (Figure 3A and B). The number of invading cells was significantly decreased in the combination group compared with the drug-alone group (Figure 3C). The above results suggest that Sal, Pac, and the combination of drugs can attenuate the motility of cells.
Effect of Sal, Pac, and Combination on Cellular Angiogenic Ability
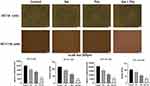
Tumor cells can secrete a variety of growth factors by themselves, which in turn induce new blood vessel formation and have an important effect on tumor development, so we investigated whether Sal, Pac, and a combination of drugs affect the angiogenic ability of cells. Compared with the control group, the number, length, and the number of branches of angiogenesis were reduced in the Sal, Pac, and combination groups. These results suggest that Sal and Pac can inhibit the ability of angiogenesis (P <0.01), and the combination of drugs is more effective than the drugs alone (Figure 4).
Effects of Sal, Pac, and Combination on Cellular EMT-Related Protein Expression
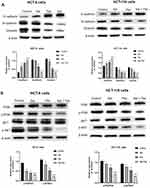
Numerous studies have suggested that EMT is correlated with an aggressive or metastatic phenotype in a complex process controlled by a wide variety of families of transcriptional regulators via different signaling pathways. Accordingly, the protein expression of EMT-associated markers was examined. As depicted in Figure 5A, the expression of E-cad protein was up-regulated, and that of N-cad and Vim protein was down-regulated in the Sal group, the Pac group, and the combination group compared with the control group (P < 0.01). Furthermore, the expression of E-cad protein was up-regulated significantly, and that of N-cad and Vim protein was down-regulated more significantly in the combination group compared with the drug group alone.
Effect of Sal, Pac, and Combination on Cellular Pathway-Related Proteins
The PI3K/AKT pathway plays a certain role in regulating a variety of tumor cell biological processes (eg, survival, proliferation, growth, metabolism, angiogenesis, and metastasis). To further investigate whether Sal, Pac, and the combination can affect cell proliferation, migration, and invasion via PI3K/AKT pathway, a Western Blot assay was performed to observe the changes in PI3K/AKT pathway proteins. As depicted in Figure 5B, the phosphorylation level of PI3K, AKT was reduced in the Sal group, the Pac group, and the combination group (P <0.01), whereas the total PI3K, AKT expression was not significantly different. The above results indicate that Sal, Pac, and the combination of drugs acted on PI3K/AKT pathway and thus inhibited cell proliferation, migration, and invasion.
Discussion
Over the past few years, the age of onset of colorectal cancer has shown a younger age, marking an increase of nearly 2 million new cases per year.13,14 The estimated result has suggested that over 1.2 million patients are diagnosed with colorectal cancer each year, and 600,000 of them die from this disease. Numerous studies have suggested that the combined application of Sal and oxaliplatin, azithromycin, and 5-fluorouracil can inhibit the proliferation of tumor cells and thus exert anti-cancer effects, and can significantly improve the therapeutic effect of chemotherapeutic drugs, and is considered one of the effective anti-tumor agents.15 In this study, Sal, Pac, and the combination drugs all inhibited cell proliferation, the inhibitory effect increased with the increase of drug concentration, and the effect of the combination group was better than that of the Sal and Pac groups. The number of cell colonies was significantly reduced in the respective drug group, further suggesting that Sal and Pac can significantly reduce the proliferation of CRC cells. Sal can attenuate the proliferation, migration, and invasion and promote apoptosis of gastric cancer cells by inhibiting the MAPK/ERK pathway.5 In this study, cells were treated with Sal, Pac, and the combination of drugs for 48 h. The results of Hoechst staining showed morphological changes (eg, cell crinkling, nuclear fragmentation, and chromatin agglutination) in the respective drug group, indicating that Sal and Pac induced apoptosis, and the effect of apoptosis in the combination of drugs was significant.
Cancer metastasis is a major cause of death in patients with colorectal cancer. EMT is a well-known biological process that has been confirmed to take on a significance to the metastasis and progression of many tumor cells.16 Abnormal activation of EMT can lead to reduced adhesion and increased wandering ability of cancer cells, which in turn promotes tumor migration and invasion. Piperine exhibits anti-inflammatory and anti-cancer properties, and existing research has suggested that piperine is capable of inhibiting the migration and invasive ability of colorectal cancer through STAT3-mediated EMT.17 The result of the cell scratch and Transwell assays indicated that the combination group significantly reduced the number of cells migrating and invading compared with the control group and the drug-alone groups, thus suggesting that Sal and Pac can synergistically inhibit the migratory and invasive ability of cells. The expression levels of some molecular markers can indicate the extent of EMT, including the down-regulation of cell adhesion protein (eg, E-cad) expression and enhanced expression of mesenchymal markers (eg, N-cad, Vim), thus confirming that down-regulation of PHLDA2 can decrease N-cad, Vim, and β-linked proteins and up-regulate E-cad levels in colorectal cancer cells.18,19 The experimental results of this study indicated that the Sal group, Pac group, and the combination group significantly up-regulated the expression of protein E-cad and down-regulated the expression of protein N-cad and Vim in cells, and the combined application of both drugs was also significantly better than the application of each drug alone, thus suggesting that Sal, Pac and the combination inhibit cell migration and invasion by mediating EMT and thus.
EMT is mediated by several different signaling pathways. Existing research has suggested that PI3K/AKT signaling pathway is a classical pathway involved in EMT, capable of affecting the status of downstream effector molecules via multiple pathways. On that basis, it can regulate a variety of effects (eg, cellular autophagy and apoptosis), and it has a close correlation with the development of various human tumors.20,21 Numerous studies have suggested that FAT4 regulates PI3K/AKT information transduction pathway, thus playing a vital role in EMT.22 Overexpression of IMPDH2 promotes cell invasion, migration, and EMT by activating the PI3K/AKT pathway.23 The anti-tumor mechanism of Sal is closely linked to the PI3K/AKT pathway. Existing research has suggested that Sal-mediated PI3K/AKT pathway inhibits the growth of gastric cancer AGS cells and induces autophagy, thus inhibiting cell viability and inducing apoptosis. However, the relationship between Sal and Pac with this pathway and EMT should be investigated in depth. To investigate more deeply the mechanism of Sal and Pac mediating the induction of EMT by the PI3K/AKT pathway, the focus was placed on the related proteins in PI3K/AKT. The results indicated that Sal, Pac, and the combination led to PI3K and AKT pathway inactivation, especially in the combination treatment group where the phosphorylation levels of PI3K and AKT proteins were significantly inhibited. The above results suggest that the combined application of Sal and Pac inhibits the EMT process, which may be regulated via the PI3K/AKT pathway. However, the inhibitory effect of the combined application of Sal and Pac on malignant tumors is very complex and requires in-depth research.
Conclusion
In brief, the results of this study suggest that the combined application of Sal and Pac can significantly inhibit cell proliferation, induce apoptosis, and regulate the cellular EMT process by participating in the PI3K/AKT pathway, which provides a novel idea for the treatment of CRC in the clinical setting.
Data Sharing Statement
The original data from the study are included in this article. For further inquiries, please contact the corresponding author.
Acknowledgments
I would like to thank my supervisor for guiding me academically, my friends for helping me in experiments and life, and Hebei North College for providing me with an experimental platform.
Author Contributions
This study was conceived and designed by Yanjiao Hao and Xiaoli Zhang. Technical support was provided by Yanjiao Hao, Zhiyu Li, and Mingzhi Chang. The original manuscript was drafted by Yanjiao Hao and revised by Xiaoli Zhang. All authors participated in data analysis, drafted or revised the article, gave final approval of the version to be published, agreed to the journal to which the article was submitted; and agreed to take responsibility for all aspects of the work.
Funding
The authors thank the Natural Science Research Program of Hebei North College for providing financial support for this study (NO. YB2018008).
Disclosure
The authors declare no competing conflict of interest in this work.
References
1. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394(10207):1467–1480. doi:10.1016/S0140-6736(19)32319-0
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249.
3. Kang DY, Sp N, Kim DH, et al. Salidroside inhibits migration, invasion and angiogenesis of MDAMB 231 TNBC cells by regulating EGFR/Jak2/STAT3 signaling via MMP2. Int J Oncol. 2018;53(2):877–885.
4. Bai XL, Deng XL, Wu GJ, Li WJ, Jin S. Rhodiola and salidroside in the treatment of metabolic disorders. Mini Rev Med Chem. 2019;19(19):1611–1626.
5. Yang L, Yu Y, Zhang Q, et al. Anti-gastric cancer effect of Salidroside through elevating miR-99a expression. Artif Cells Nanomed Biotechnol. 2019;47(1):3500–3510.
6. Ren M, Xu W, Xu T. Salidroside represses proliferation, migration and invasion of human lung cancer cells through AKT and MEK/ERK signal pathway. Artif Cells Nanomed Biotechnol. 2019;47(1):1014–1021.
7. Yang YH, Mao JW, Tan XL. Research progress on the source, production, and anti-cancer mechanisms of paclitaxel. Chin J Nat Med. 2020;18(12):890–897.
8. D’Antona L, Dattilo V, Catalogna G, et al. In preclinical model of ovarian cancer, the SGK1 inhibitor SI113 counteracts the development of paclitaxel resistance and restores drug sensitivity. Transl Oncol. 2019;12(8):1045–1055.
9. Sawatani Y, Komiyama Y, Nakashiro KI, et al. Paclitaxel potentiates the anticancer effect of cetuximab by enhancing antibody-dependent cellular cytotoxicity on oral squamous cell carcinoma cells in vitro. Int J Mol Sci. 2020;21(17):6292–6302.
10. Aiello NM, Kang Y. Context-dependent EMT programs in cancer metastasis. J Exp Med. 2019;216(5):1016–1026. doi:10.1084/jem.20181827
11. Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20(2):74–88. doi:10.1038/s41568-019-0216-7
12. Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh Migr. 2015;9(4):317–324. doi:10.1080/19336918.2015.1016686
13. La Vecchia S, Sebastian C. Metabolic pathways regulating colorectal cancer initiation and progression. Semin Cell Dev Biol. 2020;98:63–70.
14. Mauri G, Sartore-Bianchi A, Russo AG, Marsoni S, Bardelli A, Siena S. Early-onset colorectal cancer in young individuals. Mol Oncol. 2019;13(2):109–131.
15. Li H, Chen C. Inhibition of autophagy enhances synergistic effects of Salidroside and anti-tumor agents against colorectal cancer. BMC Complement Altern Med. 2017;17(1):538.
16. Menju T, Date H. Lung cancer and epithelial-mesenchymal transition. Gen Thorac Cardiovasc Surg. 2021;69(5):781–789.
17. Song L, Wang Y, Zhen Y, et al. Piperine inhibits colorectal cancer migration and invasion by regulating STAT3/Snail-mediated epithelial-mesenchymal transition. Biotechnol Lett. 2020;42(10):2049–2058.
18. Wang J, Cai H, Liu Q, et al. Cinobufacini inhibits colon cancer invasion and metastasis via suppressing wnt/beta-catenin signaling pathway and EMT. Am J Chin Med. 2020;48(3):703–718.
19. Ma Z, Lou S, Jiang Z. PHLDA2 regulates EMT and autophagy in colorectal cancer via the PI3K/AKT signaling pathway. Aging. 2020;12(9):7985–8000.
20. Kim KY, Park KI, Kim SH, et al. Inhibition of autophagy promotes salinomycin-induced apoptosis via reactive oxygen species-mediated PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human prostate cancer cells. Int J Mol Sci. 2017;18(5):1088–1100.
21. Lee YJ, Kim D, Shim JE, et al. Genomic profiling of the residual disease of advanced high-grade serous ovarian cancer after neoadjuvant chemotherapy. Int J Cancer. 2020;146(7):1851–1861.
22. Wei R, Xiao Y, Song Y, Yuan H, Luo J, Xu W. FAT4 regulates the EMT and autophagy in colorectal cancer cells in part via the PI3K-AKT signaling axis. J Exp Clin Cancer Res. 2019;38(1):112–125.
23. Duan S, Huang W, Liu X, et al. IMPDH2 promotes colorectal cancer progression through activation of the PI3K/AKT/mTOR and PI3K/AKT/FOXO1 signaling pathways. J Exp Clin Cancer Res. 2018;37(1):304–319.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.