Back to Journals » Drug Design, Development and Therapy » Volume 17
Effects of Remimazolam and Propofol on Emergence Agitation in Elderly Patients Undergoing Hip Replacement: A Clinical, Randomized, Controlled Study
Authors Duan J , Ju X, Wang X, Liu N, Xu S, Wang S
Received 18 May 2023
Accepted for publication 7 August 2023
Published 1 September 2023 Volume 2023:17 Pages 2669—2678
DOI https://doi.org/10.2147/DDDT.S419146
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Jinjuan Duan,* Xia Ju,* Xing Wang, Ning Liu, Siqi Xu, Shengbin Wang
Department of Anesthesiology, Affiliated Anqing Medical Centre of Anhui Medical University, Anqing Municipal Hospital, Anqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Siqi Xu; Shengbin Wang, Department of Anesthesiology, Affiliated Anqing Medical Centre of Anhui Medical University, Anqing Municipal Hospital, No. 87 Tianzhushan Road, Anqing, 246000, People’s Republic of China, Tel +86-13865192106 ; +86-13955665151, Email [email protected]; [email protected]
Purpose: The aim of this study was to compare the effects of remimazolam and propofol on emergence agitation (EA) in elderly patients undergoing hip replacement.
Methods: A total of 60 elderly patients undergoing hip replacement were recruited for this prospective, single-center, clinical, randomized, controlled study from February to April 2023. They were randomly assigned to two groups: the remimazolam group (group R) and the propofol group (group P). In group R, remimazolam was administered intravenously during the induction and maintenance of anesthesia, In group P, propofol was used during the induction and maintenance of anesthesia. The incidence of EA was recorded as the primary indicator. Secondary indicators included heart rate (HR) and mean arterial pressure (MAP) values at the following moments: 5 min prior to anesthetic induction (T0), 1 min following induction (T1), 5 min after the laryngeal mask was inserted (T2), the beginning of surgery (T3), the moment the laryngeal mask was removed (T4), and the overall incidence of postoperative adverse events (bleeding or splitting of the surgical incision, removal of the intravenous infusion needle, falling off the bed, hypoxemia, and hypertension).
Results: The incidence of EA was significantly lower in group R than in group P (10% vs 33%, P < 0.05). At T1, T2, and T3, the HR and MAP values of group R were higher than those of group P (P < 0.05). The overall incidence of postoperative adverse events was significantly lower in group R than in group P (P < 0.05).
Conclusion: Remimazolam further reduced the incidence of emergence agitation when compared to propofol during geriatric hip replacement. Moreover, it has a minor hemodynamic effect and lower the incidence of postoperative adverse events.
Keywords: remimazolam, propofol, emergence agitation, elderly, hip replacement
Introduction
Hip injuries and illnesses are prevalent among the elderly population, which can adversely impact their daily activities, and in extreme situations, it prevents them from walking normally.1 In such cases, hip replacement is an effective treatment option that can help patients regain their lost hip function. However, for geriatric patients, the selection of a safe and efficient anesthetic technique is crucial. They may have spinal deformities or restricted positions, which can lead to epidural anesthesia failure, hence, general anesthesia is often the preferred choice for these patients. Nevertheless, EA is an adverse post-operative complication that occurs in elderly hip replacements with an incidence of 24% to 56%,2 which constitutes a major threat to the surgical outcome and patient prognosis.3
Propofol, an intravenous anesthetic, is widely used in clinical practice, but it has several significant drawbacks, including hypotension and respiratory depression.4 Gao et al found that the incidence of EA in patients using propofol during general anesthesia can be up to 34%,5 which leads to an increase in postoperative adverse events such as incisional dehiscence, tracheal tube displacement, falling off the bed, and cardiovascular events.6 In severe cases, EA can also lead to self-harm, or violence against medical staff.7 Remimazolam is a new anesthetic sedative with an ultra-short half-life that is metabolized by non-specific plasma esterases and does not accumulate during general anesthesia,8,9 moreover, it has very slight hemodynamic impact.10 Previous studies have demonstrated that both remimazolam and propofol are effective for the induction and maintenance of general anesthesia.11,12 Yang et al reported that remimazolam did not increase the incidence of postoperative agitation.13 In the meantime, additional results demonstrated that, when compared to dexmedetomidine, remimazolam was as efficient at reducing agitation in elderly patients after orthopedic surgery.14 However, the effects of remimazalam and propofol on EA in geriatric hip replacement were rarely reported. Therefore, this study aim to mainly compare the effects of both medications on emergence agitation in elderly patients undergoing hip replacement.
Data and Methods
Design and Patients
This study was approved by the Anqing Municipal Hospital Ethics Committee (Medical Ethics Approval No: 2022027) and registered in the Chinese Clinical Trial Registry (ChiCTR2300068101) following the Helsinki Declaration. A total of 60 elderly patients undergoing hip replacement with general anaesthesia were enrolled from February 2023 to April 2023. Before surgery, all patients or their family members (because of the patient entrusted his family members) signed the informed consent form.
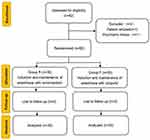
A total of 62 elderly patients undergoing hip replacement were assessed for eligibility. Out of these, 60 participants met the criteria and were randomly assigned to their respective groups. 2 patients were excluded from the study. Eventually, a total of 60 elderly patients undergoing general anesthesia were included in this study. The following inclusion criteria were applied: age between 60 and 75 years old; Body Mass Index (BMI) between 18 and 28 kg/m2; American Society of Anesthesiologists (ASA) I–III; requiring hip replacement surgery; normal coagulation function; and independent communication skills. Exclusion standards were as follows significant cardiac, pulmonary, hepatic, and renal impairment; history of psychiatric illness or delirium; use of sedatives and analgesics within the last month; history of surgery within the previous three months; allergy to propofol or benzodiazepines; SpO2 less than 95% without oxygen inhalation; sinus tachycardia or sinus bradycardia; refusal to participate in the trial. Criteria for withdrawal were as follows: more than 1000 mL of bleeding during surgery; did not use anesthetic drugs according to the anesthetic protocol of this trial.
Randomization and Masking
A total of 60 patients were randomly divided into two groups using the Excel-generated random number table method15 based on their time of admission to the hospital: the remimazolam group (group R) and the propofol group (group P), with 30 patients in each group. Because of differences in the appearance of the anesthetic agent, the anesthetists were aware of the group allocation. But they were not involved in the follow-up evaluation. Patient grouping information and anesthesia protocols were hidden in the envelopes and were not known to the assessors who performed the follow-up.
Anesthesia Method
Before surgery, patients in both groups were required to fast for eight hours. No preoperative medication was given to either group. Upon entering the operating room, venous access was established, preoxygenation was administered using a mask, and oxygen flow rate was set at 6 L/min. The patient’s HR, MAP, SpO2, temperature and partial pressure of end-tidal carbon dioxide (PETCO2) were monitored using a multifunctional Philips-MP 40 monitor (Netherlands). Anesthesia induction began with intravenous administration of either remimazolam (Jiangsu Hengrui Pharmaceutical Company, China, Lot No. 202202AK) at a dosage of 0.2–0.4 mg/kg for group R or propofol (Xi’an Lipan Pharmaceutical Company, China, Lot No. 12202191-1) at a dosage of 1.5–2 mg/kg for group P. Both groups then received intravenous sufentanil (Yichang Renfu Pharmaceutical Company, China, Lot No. 11A09051) 0.3–0.5 μg/kg and cis-atracurium (Jiangsu Hengrui Pharmaceutical Company, China, Lot No. 220301XA) 0.1–0.2mg/kg. After the patients were rendered unconscious, the laryngeal mask was inserted, and then the Fabius Plus anesthesia machine (Germany) was connected, the Volume Control Ventilation (VCV) was used, with the following settings: Tidal Volume (VT) of 6–8 mL/kg, Inspiration Time/Expiration Time (I: E) of 1:2, and Respiratory Rate (RR) of 12–16 beats per minute. Anesthesia was maintained in group R with a continuous infusion of remimazolam at a rate of 0.3–0.5mg/kg/h and in group P with propofol at a rate of 4–8 mg/kg/h. Both groups also received remifentanil (Yichang Renfu Pharmaceutical Company, China, Lot No. 20A01061) at a rate of 0.1–0.25 μg/kg/min. The infusion of remimazolam, propofol, and remifentanil was discontinued upon completion of the surgery, ondansetron 0.1 mg/kg was used intravenously as a prophylactic measure for postoperative nausea. Postoperative analgesia was provided with the same dose and concentration of lidocaine and ropivacaine used for surgical incision infiltration, combined with the patient-controlled intravenous analgesia (PCIA) with sufentanil 2 μg/kg plus granisetron 6 mg. Subsequently, the patient was transferred to the post-anesthesia care unit (PACU). Once they regained consciousness and muscle tension, the laryngeal mask was removed, and the patient was observed for 30 minutes before being transported back to their hospital room. The anesthesia induction and maintenance protocol for both groups followed standardized procedures, and vital signs were closely monitored throughout the surgery, the patient’s temperature was maintained between 36°C- 37°C and PETCO2 between 35 mmHg - 45 mmHg.
Primary Indicator
The Riker Sedation-Agitation Scale (RSAS)16 was used to evaluate the level of EA in both groups while in the PACU. The RSAS scale consisted of seven levels ranging from “unable to be aroused” to “dangerously agitated”. A score of 5 or more was considered to indicate agitation.17
Secondary Indicators
HR and MAP were monitored and recorded at several time points throughout the perioperative period, the detection time points included 5 min before anesthetic induction (T0), 1 min following induction (T1), 5 min after the laryngeal mask was inserted (T2), the beginning of surgery (T3), the moment the laryngeal mask was removed (T4). Data collected among them 5 min before the introduction of anesthesia was considered the baseline. Besides, the duration of anesthesia, duration of operation, awakening time, extubation time, intra-operative bleeding, urine output, and volume of infusion were all recorded in both groups. And the incidence of adverse events occurring in the PACU was also noted, such as bleeding or splitting of the surgical incision, removal of infusion access, falling off the bed, hypertension (defined as 20% above basal blood pressure), and hypoxemia (defined as SpO2 below 90% without oxygen inhalation).
In addition, patient demographic and clinical information such as age, sex, BMI, education level, ASA physical status, as well as preoperative comorbidities, were gathered.
Sample Size Calculation
The sample size calculation was performed using PASS 11.0 software based on preliminary pre-experimental study results. The incidence of emergence agitation, defined as RSAS scores > 4, was found to be 40% and 12% in groups P and R, respectively. To achieve a trial power of 80% at a significance level of 5%, a minimum of 27 patients in each group was needed. We ultimately enrolled 30 patients in each group to account for a potential 10% drop-out rate.
Statistical Analysis
SPSS 22.0 statistical software (IBM Corp, Armonk, NY, USA)18 was used for the statistical processing of the study data. Measures conforming to normal distribution are presented as mean ± standard deviation ( ±s). Independent samples t-test (normal distribution) was used for comparison between groups. The data of non-normal distribution was expressed as median (interquartile range [IQR]) and analyzed by Mann–Whitney U-test. Count data is expressed as a number of cases (%) and were compared using the chi-squared or Fisher’s exact test, and P<0.05 was statistically significant.
±s). Independent samples t-test (normal distribution) was used for comparison between groups. The data of non-normal distribution was expressed as median (interquartile range [IQR]) and analyzed by Mann–Whitney U-test. Count data is expressed as a number of cases (%) and were compared using the chi-squared or Fisher’s exact test, and P<0.05 was statistically significant.
Results
A total of 62 patients were initially included in the study, but one patient refused to participate, and another had a history of mental illness and was excluded. Ultimately, 60 patients (30 in each group) completed the trial (Figure 1). In terms of demographic and clinical characteristics, such as preoperative comorbidities and educational attainment, there were no statistically significant differences between the two groups (Table 1).
 |
Table 1 Comparison of the General Data of Patients Between the Two Groups |
The preoperative hip lesion types and materials for hip replacements in both groups have no statistically significant differences. The duration of anesthesia was 126.5 (117.75–133.25) min in the remimazolam group and 128.0 (122.75–137.25) min in the propofol group (P=0.325). The awakening time, extubation time, intra-operative bleeding, urine output, and volume of infusion were similar in both groups (Table 2).
 |
Table 2 Comparison of the Patient’s Situation During Surgery |
There was no statistically significant difference between the groups in terms of any score. However, the remimazolam group had only 3 cases (10%) while the propofol group had 10 cases (33%) with RSAS scores of 5 or above (P=0.028), the incidence of EA was significantly lower in group R than in group P (Table 3).
 |
Table 3 Comparison of RSAS Scores in the PACU Between the Two Groups |
At T1, T2, and T3 time points, Group R had significantly higher HR and MAP values than group P (P < 0.05) (Figure 2).
None of the patients in either group fell off the bed or voluntarily removed their urinary catheter. The venous access removal or incisional bleeding had 1 cases in group P, respectively, and had not occur in group R. The hypoxemia had 1 case (3%) in group R and had 3 cases (10%) in group P (P=0.301). The hypertension had 2 patients (7%) in the R and had 5 patients (17%) in group P (P=0.228). The overall incidence of adverse events was significantly lower in group R (10%) than in group P(33%) (P=0.028) (Table 4).
 |
Table 4 Comparison of Adverse Events of the Two Groups |
Table 5 displays the postoperative complications observed in patients. Notably, there was a single instance (3%) of deep vein thrombosis recorded in both groups R and P. Group P had 1 case (3%) of surgical site infection, while group R exhibited 1 case (3%) of hip joint dislocations. Fortunately, all of them recovered after symptomatic treatment, and neither group experienced lower limb length discrepancy, nerve injury, or fracture around the prosthesis. It is worth mentioning that there were no statistically significant differences detected between the two groups concerning postoperative complications.
 |
Table 5 Comparison of Postoperative Complications of the Two Groups |
Discussion
The results of this study showed that remimazolam reduced the incidence of EA and postoperative adverse events as well as maintained more stable hemodynamic parameters in geriatric hip replacement when compared to propofol.
The deterioration of physical reserve function and compensatory stress capacity in elderly individuals can lead to a decreased tolerance to anesthesia and surgery.19 Furthermore, postoperative adverse effects tend to increase in this population. EA is a common postoperative complication that can result in bleeding or ripping of the surgical incision, tracheal tube dislodgement, and in severe cases, aspiration, cardiovascular accidents, and asphyxia.20 This significantly jeopardizes the safety and prognosis of elderly patients during surgery. Therefore, the development of a safe and effective anesthetic plan for senior patients is of utmost importance.
The incidence of EA was found to be significantly lower in group R than in group P in this study, suggesting that remimazolam may be effective in reducing the occurrence of EA in older patients. One possible explanation for this is that propofol is a fat-soluble anesthetic agent, and older patients tend to have higher levels of body fat, which can slow down the clearance of fat-soluble drugs like propofol.21 Additionally, older patients often have impaired renal and hepatic function, further impeding drug clearance. However, elderly patients have fewer neurotransmitters and compensating receptors compared to younger individuals, leading to prolonged central nervous system depression and a diminished capacity to recover postoperatively.22 Remimazolam, on the other hand, is a novel water-soluble ultra-short-acting sedative medication that is rapidly hydrolyzed in the body by tissue esterases and it is not metabolized by the liver.23 This results in quick clearance, brief duration of action, and no accumulation of metabolites with pharmacological effects.24 As a result, remimazolam assists in restoring nerve cell activity in elderly patients and cooperates throughout the awakening period in executing commands, thereby reducing the incidence of EA. In conclusion, our study found that remimazolam was effective in reducing the incidence of EA in older patients compared to propofol. Consequently, remimazolam can be considered a viable alternative anesthetic agent for elderly patients undergoing surgery. Further studies are needed to corroborate these findings in other patient populations and surgical procedures.
Furthermore, previous studies have shown that a higher intraoperative MAP reduces the risk of EA after hip surgery, which may be associated with a corresponding increase in cerebral oxygen supply.2 Meanwhile, Qiu et al discovered that individuals receiving remimazolam had higher intraoperative blood pressure and recovered of brain function faster than those receiving propofol.25,26 Propofol has been found have a significant myocardial depressant effect which reduces cardiac output, and causes peripheral vasodilation, resulting in decreased blood pressure and heart rate after administration.27 These factors can lead to decreased in the cerebral blood flow (CBF). In contrast, remimazolam has a milder inhibition of cardiac rhythm and myocardial contractility.28 In our study, we found that patients in the remimazolam group had significantly higher HR and MAP at T1, T2, and T3 than those in the propofol group. These findings indicate that patients in the remimazolam group had more stable hemodynamic parameters, which contributed to better protection of CBF and improved regional cerebral oxygen saturation (rSO2),29 this may have contributed to the better postoperative brain function recovery observed in the remimazolam group,30 and ultimately to the reduced occurrence of EA. Therefore, the findings of this study suggest that the use of remimazolam may be a better choice for elderly patients undergoing total hip arthroplasty.
Based on the previous literature, remimazolam has a lower incidence of postoperative adverse events compared to propofol.31 Our study also observed similar findings, the overall incidence of postoperative adverse events was lower in the remimazolam group (10%) compared to the propofol group (33%). Specifically, patients in the propofol group experienced a higher rate of agitation than those in the remimazolam group, which resulted in an increased incidence of venous access removal, bleeding or splitting of incision, and hypertension in the PACU. On the other hand, propofol has a more potent respiratory depressant effect than remimazolam32 and is metabolized more slowly in elderly patients than remimazolam.21 This may explain why the propofol group experienced a higher incidence of hypoxemia compared to the remimazolam group. In conclusion, our study supports the literature findings that remimazolam has a lower incidence of postoperative adverse events compared to propofol. The higher rate of agitation observed in the propofol group, coupled with the drug’s stronger respiratory depression effect and slower metabolism in elderly patients, may account for the increased incidence of postoperative complications. Therefore, the use of remimazolam as an anesthetic agent for elderly patients undergoing surgery may be a preferred option to minimize the occurrence of postoperative adverse events.
In our experiment, we observed no statistically significant difference in postoperative complications between the two groups. This finding aligns with previous studies,33 indicating that remimazolam does not elevate the occurrence of postoperative complications among elderly hip replacement patients.
Our study has certain limitations that need to be acknowledged. Firstly, the sample size is small, and the study was conducted at a single center. As such, larger sample sizes and multi-center participation are necessary to obtain more reliable and generalizable results. Secondly, we did not monitor patients’ perioperative brain function using bispectral index (BIS), transcranial doppler (TCD), or rSO2, which could have provided insights into the patient’s brain function during the surgery. We believe that future studies with larger sample sizes and more comprehensive monitoring will provide a more comprehensive assessment of the safety and efficacy of remimazolam in geriatric patients undergoing surgery.
Conclusion
In the geriatric hip replacement, remimazolam reduced the incidence of emergence agitation compared to propofol, while maintaining more stable hemodynamic parameters and lowering the incidence of postoperative adverse events. These findings suggest that remimazolam can be considered a safe and effective alternative anesthetic agent for elderly patients undergoing hip replacement surgery.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding authors on reasonable request.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Prestmo A, Hagen G, Sletvold O, et al. Comprehensive geriatric care for patients with Hip fractures: a prospective, randomised, controlled trial. Lancet. 2015;385(9978):1623–1633. doi:10.1016/S0140-6736(14)62409-0
2. Xu XM, Hu XW, Wu Y, et al. Effects of different BP management strategies on postoperative delirium in elderly patients undergoing Hip replacement: a single center randomized controlled trial. J Clin Anesth. 2020;62:109730. doi:10.1016/j.jclinane.2020.109730
3. Kim JA, Ahn HJ, Yang M, et al. Effect of intraoperative dexmedetomidine on the postoperative agitation in thoracic surgery. J Cardiothorac Vasc Anesth. 2018;32:S12–S13. doi:10.1053/j.jvca.2018.08.044
4. Hu QX, Liu X, Wen CL, et al. Remimazolam: an updated review of a new sedative and anaesthetic. Drug Des Devel Ther. 2022;16:3957–3974. doi:10.2147/DDDT.S384155
5. Gao ZZ, Zhang JM, Nie XL, et al. Effectiveness of intravenous ibuprofen on emergence agitation in children undergoing tonsillectomy with propofol and remifentanil anesthesia: a randomized controlled trial. J Pain Res. 2022;15:1401–1410. doi:10.2147/JPR.S363110
6. Meng T, Lin X, Li X, et al. Pre-anesthetic use of butorphanol for the prevention of emergence agitation in thoracic surgery: a multicenter, randomized controlled trial. Front Med. 2022;9:1040168. doi:10.3389/fmed.2022.1040168
7. Fields A, Huang J, Schroeder D, et al. Agitation in adults in the post-anesthesia care unit after general anesthesia. Br J Anaesth. 2018;121(5):1052–1058. doi:10.1016/j.bja.2018.07.017
8. Stöhr T, Colin PJ, Ossig J, et al. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br J Anaesth. 2021;127(3):415–423. doi:10.1016/j.bja.2021.05.027
9. White Paul F. Remimazolam - can it become a cost-effective alternative to propofol for intravenous anesthesia and sedation? Clin Anesth. 2023;84:110977. doi:10.1016/j.jclinane.2022.110977
10. Sekiguchi R, Kinoshita M, Kawanishi R, et al. Comparison of hemodynamics during induction of general anesthesia with remimazolam and target-controlled propofol in middle-aged and elderly patients: a single-center, randomized, controlled trial. BMC Anesthesiol. 2023;23(1):14. doi:10.1186/s12871-023-01974-9
11. Choi JY, Lee HS, Kim JY, et al. Comparison of remimazolam-based and propofol-based total intravenous anesthesia on postoperative quality of recovery: a randomized non-inferiority trial. J Clin Anesth. 2022;82:110955. doi:10.1016/j.jclinane.2022.110955
12. Zhang JB, Cairen ZM, Shi LW, et al. Remimazolam versus propofol for procedural sedation and anesthesia: a systemic review and meta-analysis. Minerva Anestesiol. 2022;88(12):1035–1042. doi:10.23736/S0375-9393.22.16817-3
13. Yang JJ, Lei L, Qiu D, et al. Effect of remimazolam on postoperative delirium in older adult patients undergoing orthopedic surgery: a prospective randomized controlled clinical trial. Drug Des Devel Ther. 2023;17:143–153. doi:10.2147/DDDT.S392569
14. Deng Y, Qin Z, Wu Q, et al. Efficacy and safety of remimazolam besylate versus dexmedetomidine for sedation in non-intubated older patients with agitated delirium after orthopedic surgery: a randomized controlled trial. Drug Des Devel Ther. 2022;16:2439–2451. doi:10.2147/DDDT.S373772
15. Ma J, Wang F, Wang J, et al. The effect of low-dose esketamine on postoperative neurocognitive dysfunction in elderly patients undergoing general anesthesia for gastrointestinal tumors: a randomized controlled trial. Drug Des Devel Ther. 2023;17:1945–1957. doi:10.2147/DDDT.S406568
16. Riker RR, Picard JT, Fraser GL. Prospective evaluation of the sedation-agitation scale for adult critically ill patients. Crit Care Med. 1999;27(7):1325–1329. doi:10.1097/00003246-199907000-00022
17. Lee S, Sohn JY, Hwang IE, et al. Effect of a repeated verbal reminder of orientation on emergence agitation after general anesthesia for minimally invasive abdominal surgery: a randomised controlled trial. Br J Anaesth. 2023;130(4):439–445. doi:10.1016/j.bja.2022.12.009
18. Gao A, Yang H, Wang Y, et al. Radiomics for the prediction of epilepsy in patients with frontal glioma. Front Oncol. 2021;11:725926. doi:10.3389/fonc.2021.725926
19. Shin HJ, Woo NS, Kim H, et al. Postoperative delirium after dexmedetomidine versus propofol sedation in healthy older adults undergoing orthopedic lower limb surgery with spinal anesthesia: a randomized controlled trial. Anesthesiology. 2023;138(2):164–171. doi:10.1097/ALN.0000000000004438
20. Lei DX, Wu CJ, Wu ZY, et al. Efficacy of different doses of intranasal dexmedetomidine in preventing emergence agitation in children with inhalational anesthesia: a prospective randomised trial. Eur J Anaesthesiol. 2022;39(11):858–867. doi:10.1097/EJA.0000000000001743
21. Guy J, Qian Y, Zhang XJ, et al. Remimazolam tosilate compared with propofol for gastrointestinal endoscopy in elderly patients: a prospective, randomized and controlled study. BMC Anesthesiol. 2022;22(1):180. doi:10.1186/s12871-022-01713-6
22. Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi:10.1016/S0140-6736(12)62167-9
23. Dai GG, Pei LL, Duan F, et al. Safety and efficacy of remimazolam compared with propofol in induction of general anesthesia. Minerva Anestesiol. 2021;87(10):1073–1079. doi:10.23736/S0375-9393.21.15517-8
24. Chen X, Sang N, Song K, et al. Psychomotor recovery following remimazolam-induced sedation and the effectiveness of flumazenil as an antidote. Clin Ther. 2020;42(4):614–624. doi:10.1016/j.clinthera.2020.02.006
25. Qiu YW, Gu W, Zhao MY, et al. The hemodynamic stability of remimazolam compared with propofol in patients undergoing endoscopic submucosal dissection: a randomized trial. Front Med. 2022;9:938–940. doi:10.3389/fmed.2022.938940
26. Mao YY, Guo J, Yuan JJ, et al. Quality of recovery after general anesthesia with remimazolam in patients’ undergoing urologic surgery: a randomized controlled trial comparing remimazolam with propofol. Drug Des Devel Ther. 2022;16:1199–1209. doi:10.2147/DDDT.S359496
27. Zhang Y, Dai GG, Xu H, et al. Safety and efficacy of remimazolam compared with propofol in induction of general anesthesia: a reply. Minerva Anestesiol. 2022;88(3):194–195. doi:10.23736/S0375-9393.21.16169-3
28. Lu KJ, Wei SS, Ling WW, et al. Remimazolam versus propofol for deep sedation/anaesthesia in upper gastrointestinal endoscopy in elderly patients: a multicenter, randomized controlled trial. J Clin Pharm Ther. 2022;47(12):2230–2236. doi:10.1111/jcpt.13797
29. Chong SH, Ong YH, Khatib M, et al. Real-time tracking of brain oxygen gradients and blood flow during functional activation. Neurophotonics. 2022;9(4):045006. doi:10.1117/1.NPh.9.4.045006
30. Nurmi J, Laukkanen NP, Kirves H, et al. Cerebral oxygen desaturation events during and functional outcomes after prehospital anaesthesia: a prospective pilot study. Acta Anaesthesiol Scand. 2022;66(6):750–758. doi:10.1111/aas.14066
31. Wang XM, Hu XL, Bai NY, et al. Safety and efficacy of remimazolam besylate in patients undergoing colonoscopy: a multicentre, single-blind, randomized, controlled, phase III trial. Front Pharmacol. 2022;13:900723. doi:10.3389/fphar.2022.900723
32. Jiang JL, Jiao YF, Gao P, et al. Propofol differentially induces unconsciousness and respiratory depression through distinct interactions between GABAA receptor and GABAergic neuron in corresponding nuclei. Acta Biochim Biophys Sin. 2021;53(8):1076–1087. doi:10.1093/abbs/gmab084
33. Zhang JB, Wang X, Zhang Q, et al. Application effects of remimazolam and propofol on elderly patients undergoing Hip replacement. BMC Anesthesiol. 2022;22(1):118. doi:10.1186/s12871-022-01641-5
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.