Back to Journals » International Journal of General Medicine » Volume 17
Effects of Percutaneous Kyphoplasty for the Treatment of Thoracic Osteoporotic Vertebral Compression Fractures with or without Intravertebral Cleft in Elderly Patients
Authors He W , Zhou Q, Lv J, Shen Y, Liu H, Yang H , Yang P, Liu T
Received 12 November 2023
Accepted for publication 8 January 2024
Published 20 January 2024 Volume 2024:17 Pages 193—203
DOI https://doi.org/10.2147/IJGM.S447623
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Wei He,* Quan Zhou,* Jiaheng Lv,* Yujie Shen, Hao Liu, Huilin Yang, Peng Yang, Tao Liu
Department of Orthopaedics, The First Affiliated Hospital of Soochow University, Suzhou, 215006, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Peng Yang; Tao Liu, Department of Orthopaedics, The First Affiliated Hospital of Soochow University, No. 899 Pinhai Road, Suzhou, Jiangsu, 215006, People’s Republic of China, Tel +86-512-67781420, Fax +86-512-67781165, Email [email protected]; [email protected]
Background: Few studies have focused on percutaneous kyphoplasty (PKP) in the treatment of thoracic osteoporotic vertebral compression fractures (OVCFs) with intervertebral cleft (IVC). Hence, the objective of this retrospective study was to compare the clinical and radiographic outcomes of PKP in elderly patients with thoracic OVCFs, with or without IVC.
Methods: A total of 106 patients were enrolled in this study and divided into two groups: the IVC group and the NIVC group (without IVC). Radiographic measures included anterior vertebral height (AVH), thoracic kyphosis (TK), lumbar lordosis (LL), pelvic incidence (PI), pelvic tilt (PT), and sacral slope (SS). Clinical function measures included Oswestry disability index (ODI) and visual analog scale (VAS) scores.
Results: There were no significant differences in the preoperative basic data between the groups classified as IVC and NIVC. However, both groups showed significant improvements in AVH and TK throughout the follow-up periods compared to the preoperative measurements (P< 0.05). The recovery of AVH in the IVC group was found to be inferior to that in the NIVC group at 3 years after operation (P< 0.05). There were no significant differences in LL, PI, PT and SS in both groups compared with the preoperative results and no statistically significant differences between the two groups at the same follow-up time (P> 0.05). The VAS and ODI scores during all follow-up periods were significantly lower than those before operation (P< 0.05). At 3 years after operation, the VAS and ODI scores of the IVC group were higher than those of the NIVC group (P< 0.05).
Conclusion: PKP is an adoptable measure to treat thoracic OVCFs with or without IVC. Our study revealed that the NIVC group was superior to the IVC group in terms of improved vertebral height and pain recovery at long-term follow-up (3 years).
Keywords: percutaneous kyphoplasty, thoracic, osteoporotic vertebral compression fracture, intervertebral cleft, sagittal balance
Background
Aging populations worldwide have raised concerns about osteoporosis and osteoporotic vertebral compression fractures (OVCFs), and the incidence of OVCFs continues to increase as the elderly population increases.1 OVCFs are known to affect quality of life and mortality by causing persistent pain, kyphosis, and mobility limitation.2 In 1987, Galibert introduced vertebroplasty, in which medical bone cement (polymethyl methacrylate) is injected into a fractured vertebra.3 Subsequently, the utilization of this technique has become widespread in minimally invasive surgery for osteoporotic vertebral fractures due to its capacity to promptly alleviate pain and facilitate early recuperation.4 OVCFs often frequently manifest in the thoracolumbar spine; however, the thoracic vertebra exhibits distinctive characteristics in comparison to the lumbar vertebra, including steep and diminutive pedicles, a reduced angle between the pedicle and the vertebral body, and a narrower anterior margin of the vertebral body.5 In response to these characteristics of thoracic vertebrae, PKP for thoracic vertebra is more risky and more difficult to puncture. Vertebrae above T10 are often operated on with the extrapedicular approach, which is different from the transpedicular approach of lumbar vertebrae. Consequently, it is imperative to differentiate between thoracic osteoporotic compression fractures and lumbar osteoporotic compression fractures.
The occurrence of intravertebral cleft (IVC) following OVCF is not a rare phenomenon.6 These clefts, also known as intravertebral vacuum phenomena or Kümmell signs, have been extensively documented in the literature on medical imaging.7 IVC was first described by Maldague in 1978, has long been considered the result of local bone ischemia associated with nonunion vertebral collapse.8 On radiographs, they manifest as a radiolucent shadow within the vertebra, appearing as a transverse, linear, or semilunar shape. Magnetic resonance imaging reveals the presence of air or fluid accumulation within the cleft.9 IVC is considered a significant risk factor for severe vertebral collapse, progressive kyphosis, persistent back pain, and neurological impairments.
Most patients with IVC occur as a benign lesion in OVCFs, but the treatment of OVCFs with IVC was difficult.10 Although some of the OVCFs with IVC could be fixed up by conservative method, long-term clinotherapy causes various complications, such as pain and pneumonia.11 Hence, most patients chose surgical treatment for faster recovery. Many surgical approaches have been selected for the treatment of OVCFs with IVC, such as open surgery, percutaneous kyphoplasty (PKP) and percutaneous vertebroplasty (PVP).12 PKP is an effective and currently widely used method for the treatment of OVCF, the procedure was done usually under local anesthesia, and the patient was well tolerated.1 We can also find its advantages in lower bone cement leakage rate and better recovery of vertebral height in follow-up radiographic outcomes.11 At the same time, PKP has achieved good results in restoring spinal stability and relieving severe pain in patients with OVCFs during the initial follow-up.13
However, there is a scarcity of comprehensive studies examining the long-term effects of PKP on thoracic OVCFs with and without IVC. Furthermore, there is a dearth of detailed reports investigating the impact of PKP on spinopelvic sagittal balance in thoracic OVCFs. Consequently, the objective of this retrospective study was to compare the clinical and radiographic outcomes of PKP in elderly patients with thoracic OVCFs, with or without IVC.
Patients and Methods
Patients
Inclusion criteria were the following: (1) patients over 60 years of age; (2) patients suffered single-level thoracic vertebral compression fracture; (3) patients underwent bilateral PKP; (4) patients clinically diagnosed osteoporosis patients; (5) patients with back pain but no symptoms of nerve damage.
Exclusion criteria were the following: (1) patients with other spine-related diseases, such as thoracic slippage, ankylosing spondylitis, spinal tuberculosis and spinal tumors; (2) history of previous surgical interventions on thoracic vertebral; (3) patients whose data was lost during 36 months of follow-up.
Based on rigorous inclusion and exclusion criteria, a cohort of 106 elderly individuals were selected for participation in the First Affiliated Hospital of Soochow University. Pertinent patient information, such as age, gender, body mass index (BMI), bone mineral density (BMD), time of hospitalization and presence of hypertension, diabetes, hyperlipidemia and smoking status were extracted from medical records. Radiographic data pertaining to the patients was assessed by two experienced orthopedic surgeons. According to the results of radiographic images, all patients were divided into NIVC group (without IVC) and IVC group (with IVC). All data was retrieved from the electronic medical record management system of our institute.
Surgical Procedures and Management
All patients underwent surgery performed by a single group of surgeons utilizing a bilateral approach in a prone position, with a cushion placed under the waist and chest. Initially, the fractured vertebra was localized using C-arm fluoroscopy. Subsequently, guided by the C-arm, guide needles were inserted through the lateral edges of the bilateral pedicles and gradually advanced through the pedicles into the vertebral body. If thin pedicles above T10 were encountered, an extrapedicular approach was used. Following this, expansion cannulas were introduced to establish two working channels, through which balloon tamps were inserted. Simultaneously, the lateral perspective demonstrates the balloon’s ability to provide support to the fractured vertebra anteriorly and restore its height. Subsequently, the balloon was deflated and extracted, and the needle tips were advanced into the IVC of the collapsed vertebral body. A mixture of powder cement polymer and liquid monomer was prepared to form polymethylmethacrylate (PMMA) cement. With meticulous fluoroscopic monitoring, PMMA and non-ionic contrast medium were cautiously injected into the vertebrae using a bone cement injector. To mitigate the occurrence of bone cement leakage, our institution adopted incremental temperature cement delivery and graded infusion techniques.14 During the surgical procedure, intraoperative C-arm fluoroscopy was employed to meticulously observe the dispersion and potential seepage of the bone cement injection. Subsequently, on the initial postoperative day, the patient exhibited the ability to ambulate with spinal support. Throughout the subsequent monitoring period, all patients engaged in functional exercises and adhered to a prescribed regimen of anti-osteoporosis medications, as directed by medical professionals.
Radiographic and Clinical Evaluation
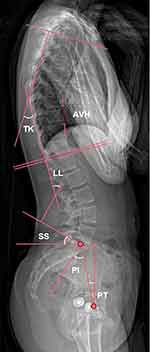
We chose sagittal balance as a measure to assess the postoperative improvement in radiographic outcomes. Sagittal balance encompasses both pelvic and spinal parameters and goes beyond mere spinal alignment. Anterior vertebral height (AVH), thoracic kyphosis (TK), lumbar lordosis (LL), pelvic incidence (PI), pelvic tilt (PT) and sacral slope (SS) were evaluated using segmental X-ray radiographs (Figure 1). Spinopelvic parameters were measured on spine radiographs in a standing position. The radiographic film was aligned parallel to both the horizontal and vertical axes, ensuring consistent measurement of all radiological parameters using the same instrument settings. All measurements were averaged from the results measured by the three co-authors, with a low margin of error. The AVH ratio was determined by comparing the anterior height of the fractured vertebrae to the average anterior height of the adjacent upper and lower vertebrae. TK was measured using Cobb’s method, which involved calculating the angle between the upper endplate of T4 and the lower endplate of T12. This approach was necessary as the visualization of T1 to T3 on lateral spine films is often hindered by the presence of shoulders and scapulae.15 LL was determined using Cobb’s method as the angle between the upper endplate of the L1 and S1 vertebrae. Pelvic incidence (PI) is defined as the angle between the perpendicular to the sacral plate and the line connecting the midpoint of the sacral plate to the centroid of femoral heads. Pelvic tilt (PT) corresponds to the angle between the line connecting the midpoint of the sacral plate to the centroid of femoral heads and the vertical line. Sacral slope (SS) corresponds to the angle between the sacral plate and the horizontal plane, serving as a positional parameter that varies based on the position of the pelvis. As said for the SS, the PT is also a positional parameter. The PI represents the algebraic sum of the SS and the PT: PI = SS + PT.
In order to assess clinical outcomes, patients were required to complete a series of questionnaires at various time points: before surgery, 1 month after surgery, 1 year after surgery and 3 years after surgery. The Oswestry disability index (ODI) scores were utilized to measure the patients’ improvement in quality of life, while the visual analogue scale (VAS) scores for back pain were employed to evaluate the patients’ subjective perception of pain (rated on a scale of 0–10, with 0 indicating no pain and 10 indicating the most severe pain; moderate-to-severe residual back pain was defined as a VAS score equal to or greater than 4).1,16 In this study, the back pain we investigated was mainly at levels T4-T12.
Statistical Analysis
Statistical analysis was performed using SPSS 26.0 software, and the results were presented as mean ± standard. Independent t‑test and One-Way ANOVA were performed to evaluate intergroup differences for continuous variables. Paired sample t‑test was used to compare preoperative and postoperative differences for continuous variables in the same group.
Results
Patients’ Data
The patients’ data of both groups can be seen in Table 1. Overall, 106 elderly patients who accepted PKP and completed final follow-up in our hospital were enrolled into this study. Among them, the average age was 66.14±13.16 years old, male patients (23.58%) were less than female patients (76.42%). The mean age of the NIVC group was slightly higher than that of the IVC group, but the difference was not statistically significant (P>0.05). There were no significant differences between the two groups in terms of gender, fractured segment, BMI and BMD (P>0.05). Many patients in both groups had different comorbidities, such as high blood pressure, diabetes, hyperlipidemia, or smoking habits. There was no significant difference between the two groups in data of comorbidities (P>0.05). The mean length of hospitalization was 5.42±0.91 days, and there was no statistically significant difference between the two groups (P>0.05), and no adverse events occurred during hospitalization. Patients’ average follow-up duration was 40.36±3.33 months, and there was no significant difference between the two groups (P>0.05).
 |
Table 1 General Characteristics of the Patients |
Radiographic Outcomes
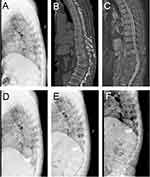
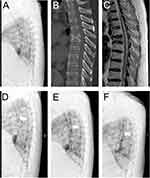
All the radiographic data are shown in Table 2. There was no significant difference between the three groups in the preoperative radiographic data, including AVH, TK, LL, PI, PT, and SS (P>0.05). Compared with the results of pre-operation, AVH and TK in both IVC and NIVC groups improved significantly at all follow-up periods (P<0.05), but the radiographic results deteriorated along with the follow-up time. The change was most pronounced at one-month follow-up, with AVH and KT recovering 28.9% and 2.8° in the IVC group and 32.0% and 3.0° in the NIVC group, respectively. The improvement decreased with the extension of follow-up time, and the AVH recovery in the IVC group was worse than that in the NIVC group at 3 years of follow-up (P<0.05). Compared with pre-operation, LL in both groups decreased at the three-year follow-up, the difference was statistically significant (P<0.05), but there was no statistically significant difference between the two groups during all follow-up periods (P>0.05). We found that there was no obvious difference between the two groups in the data of PI, PT and SS and there was no significant change compared with that pre-operation (P>0.05) (One typical case in each group is shown in Figures 2 and 3, respectively).
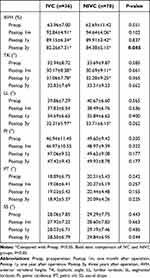 |
Table 2 Comparison of Radiographic Parameters Between the Two Groups |
Clinical Functional Outcomes
The functional outcomes are shown in Table 3. There was no significant difference in the preoperative VSA and ODI between the two groups (P>0.05). VAS and ODI of both groups were significantly lower at 1 month, 1 year and 3 years after operation than those before operation (P>0.05), but the scores of both groups deteriorated at the 3-year follow-up compared to the one-year follow-up. At the 3-year follow-up, VAS and ODI scores were significantly different between the two groups (P<0.05), and the indexes of IVC group were higher.
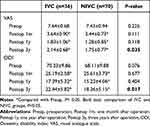 |
Table 3 Comparison of Functional Outcomes Between the Two Groups |
Discussion
OVCFs are becoming a common source of back pain and progressive spinal deformity, reducing quality of life and becoming an increasingly serious health problem around the world.17–19 The incidence of IVCs in OVCFs has varied from 13.8% to 42.4% in multiple recent studies.20–25 IVC, which is a cavity inside the vertebral body, accounted for 12.1–42.4% of OVCFs,26 and it is generally considered to be a marker of avascular osteonecrosis of the vertebral body.27,28 Most of IVCs occur in the thoracolumbar junction, there is one of the most dynamic of spinal flexion and extension.28,29 A number of studies have also shown whether the patient has IVC or PVP surgery provides satisfactory clinical and radiological results.11,23,25,30–32 In this study, we selected PKP as our surgical method to compare the clinical and radiographic outcomes of patients who suffered thoracic OVCFs, with or without intravertebral cleft.
Spinal sagittal plane parameters are very important evaluation indexes, they involve a delicate integration of sensorineural function, spinal anatomy, and biomechanics.33,34 The sagittal balance of the spine is a crucial determinant of treatment outcomes and plays a vital role in maintaining a stable standing posture,35 we can often assess the progression of lumbar disease by changes in spinal sagittal balance parameters. OVCF patients are often accompanied by severe spinal deformities, especially changes in AVH. Chang et al27 demonstrated that pyramidal height and kyphosis were significantly corrected and decreased significantly over time in both IVC and NIVC groups after PKP. In our study, we examined six variables, namely AVH, TK, LL, PI, PT and SS. The results indicated that AVH exhibited a significant increase in both groups across all follow-up periods than preoperation, and TK exhibited a significant reduction in both groups across short follow-up periods (1 month and 1 year after operation). To some extent, this indicated that surgical treatment had a better recovery effect on the vertebral height of thoracic OVCFs and could improve the thoracic kyphosis in a short follow-up period. However, the remaining radiographic indexes did not demonstrate substantial improvement. Notably, a statistically significant difference between the IVC and NIVC groups was only observed in AVH at the three-year follow-up. This finding supports the conclusion that patients in the NIVC group exhibited superior recovery in sagittal balance compared to those in the IVC group and is the most intuitive manifestation of the effect of surgery. Simultaneously, the correction of AVH and TK in all groups 1 month after operation indicated that AVH and TK can serve as effective indicators for evaluating the therapeutic outcome. Conversely, no notable differences were observed in LL, PI, PT and SS between the NIVC group and IVC group. This implies that the existence of IVC in the thoracic spine slightly influences the pelvic parameters in sagittal balance subsequent to PKP. Nevertheless, these assertions remain speculative and necessitate further investigation and extended follow-up periods for validation.
The functional outcomes of patients who underwent percutaneous kyphoplasty demonstrate significant improvement in VAS and ODI scores, which aligns with the findings of other researchers.2,27,36 It is not solely attributed to the surgical intervention itself; rather, we posit that the amelioration of sagittal balance, particularly the correction of thoracic kyphosis, contributes to a slight enhancement in pain reduction and functional improvement. The mitigation of chronic pain resulting from muscle traction in the back is facilitated by the improvement of thoracic kyphosis, and Kim et al37 also proposed that the improvement of PT and LL after surgery plays an important role in achieving good clinical outcomes. The NIVC group demonstrated superior improvement compared to the IVC group following surgery, particularly evident at the three-year follow-up. This suggests that the pain recovery efficacy of NIVC group surpasses that of the IVC group over an extended recovery period. Li et al38 also found that compared with patients with IVC, pain relief was better in patients without IVC. Consequently, it is imperative to implement enhanced rehabilitation interventions aimed at alleviating pain and enhancing the quality of life for IVC patients. These interventions commonly involve the utilization of suitable orthoses, the implementation of lumbar and back muscle strengthening exercises and the establishment of long-term follow-up procedures, among others.
Overall, it is our belief that PKP is an efficacious approach for managing OVCFs involving thoracic vertebrae with IVC. However, there have been concerns regarding the use of PKP for treating OVCFs with IVC in lower thoracic vertebrae. A common complication associated with PKP is the leakage of bone cement.21 Nevertheless, a study has demonstrated that the rate of cement leakage among patients with IVC was lower compared to those without IVC during PKP. The researchers inferred that the presence of an avascular process within the intravertebral cleft, surrounded by a fibrocartilaginous membrane, may impede cement leakage.39 In our study, we also found that AVH, scores of VAS and ODI improved better in the NIVC group during follow-up after operation than IVC group, and we believed that it was related to the special distribution of bone cement in the cleft. Vacuum space of the vertebral body might be increased in the IVC group when patients’ posture was changed from standing position to prone position, while the injected cement was blocky in the cleft rather than radially distributed as in the NIVC group. The unique distribution pattern of cement in this study may lead to reduced contact between cement and cancellous bone, resulting in increased pressure on the delicate cancellous bone surrounding the cement block. This pressure could potentially exacerbate the postoperative improvement of AVH, TK, VAS and ODI scores.
It is important to note that this study has some limitations. Firstly, the sample size was relatively small, which may limit the generalizability of the findings. Additionally, the study design was retrospective and comparative, which provides less robust evidence compared to a case–control study. Regarding radiographic outcomes, our study lacked sufficient radiological material from follow-up periods, including digital radiography of the pelvis, computed tomography (CT) and magnetic resonance imaging (MRI). Consequently, measurements of PI, PT, and SS may have been subject to some deviations. In future research, it would be beneficial to include a larger sample size or conduct a randomized controlled trial to further explore this topic. We aim to gain a more comprehensive understanding of the optimal treatment for thoracic OVCFs with IVC.
Conclusions
PKP is an effective treatment option for OVCFs affecting thoracic vertebrae, with or without IVC, resulting in satisfactory clinical and radiographic outcomes post-surgery. However, the radiographic outcomes and clinical scores, as measured by the AVH, VAS and ODI, were inferior in the IVC group compared to the NIVC group, potentially attributed to suboptimal distribution of bone cement. Therefore, future advancements in the management of OVCFs with IVC should prioritize the improvement of bone cement distribution within the vertebra, and it is advisable to implement effective rehabilitation intervention measures, including the use of suitable orthosis and the implementation of lumbar back muscle strengthening exercises, among others.
Abbreviations
OVCF, osteoporotic vertebral compression fracture; IVC, intravertebral cleft; NIVC, without intravertebral cleft; PKP, percutaneous kyphoplasty; PVP, percutaneous vertebroplasty; PMMA, Polymethylmethacrylate; AVH, anterior vertebral height; TK, thoracic kyphosis; LL, lumbar lordosis; PI, pelvic incidence; PT, pelvic tilt; SS, sacral slope; CT, computed tomography; MRI, magnetic resonance imaging.
Data Sharing Statement
Data is available via a request to both corresponding authors.
Ethics Approval and Consent to Participate
Approval was obtained from the ethics committee of First Affiliated Hospital of Soochow University [No.2023375]. Informed consent was obtained from all individual participants included in this study. Our study complied with the Declaration of Helsinki.
Acknowledgments
Thank you for all the support from the First Affiliated Hospital of Soochow University.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China [82072476].
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. doi:10.1016/0304-3959(83)90126-4
2. Baek SW, Kim C, Chang H. The relationship between the spinopelvic balance and the incidence of adjacent vertebral fractures following percutaneous vertebroplasty. Osteoporos Int. 2015;26(5):1507–1513. doi:10.1007/s00198-014-3021-x
3. Galibert P, Deramond H, Rosat P, Le Gars D. Note préliminaire sur le traitement des angiomes vertébraux par vertébroplastie acrylique percutanée [Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty]. Neurochirurgie. 1987;33(2):166–168. French. PMID: 3600949.
4. Voormolen MHJ, Mali WPTM, Lohle PNM, et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. AJNR Am J Neuroradiol. 2007;28(3):555–560.
5. Ge J, Cheng X, Li P, Yang H, Zou J. The clinical effect of kyphoplasty using the extrapedicular approach in the treatment of thoracic osteoporotic vertebral compression fracture. World Neurosurg. 2019;131:e284–e289. doi:10.1016/j.wneu.2019.07.133
6. Matzaroglou C, Georgiou CS, Wilke HJ, et al. Kümmell’s disease: is ischemic necrosis or vertebral ”microcracking” the first step in the sequence? Med Hypotheses. 2013;80(4):505. doi:10.1016/j.mehy.2012.12.003
7. Kawaguchi S, Horigome K, Yajima H, et al. Symptomatic relevance of intravertebral cleft in patients with osteoporotic vertebral fracture. J Neurosurg Spine. 2010;13(2):267–275. doi:10.3171/2010.3.SPINE09364
8. Theodorou DJ. The intravertebral vacuum cleft sign. Radiology. 2001;221(3):787–788. doi:10.1148/radiol.2213991129
9. Yu CW, Hsu CY, Shih TTF, Chen BB, Fu CJ. Vertebral osteonecrosis: MR imaging findings and related changes on adjacent levels. AJNR Am J Neuroradiol. 2007;28(1):42–47.
10. Dong L, Dong C, Zhu Y, Wei H. Intravertebral cleft in pathological vertebral fracture resulting from spinal tuberculosis: a case report and literature review. BMC Musculoskelet Disord. 2020;21(1):619. doi:10.1186/s12891-020-03642-2
11. Li Z, Liu T, Yin P, et al. The therapeutic effects of percutaneous kyphoplasty on osteoporotic vertebral compression fractures with or without intravertebral cleft. Int Orthop. 2019;43(2):359–365. doi:10.1007/s00264-018-4007-7
12. Wiggins MC, Sehizadeh M, Pilgram TK, Gilula LA. Importance of intravertebral fracture clefts in vertebroplasty outcome. AJR Am J Roentgenol. 2007;188(3):634–640. doi:10.2214/AJR.06.0542
13. Yu W, Liang D, Jiang X, Yao Z, Qiu T, Ye L. Efficacy and safety of the target puncture technique for treatment of osteoporotic vertebral compression fractures with intravertebral clefts. J Neurointerv Surg. 2017;9(11):1113–1117. doi:10.1136/neurintsurg-2016-012690
14. Yang H, Liu H, Wang S, Wu K, Meng B, Liu T. Review of percutaneous kyphoplasty in China. Spine. 2016;41(Suppl 19):B52–B58. doi:10.1097/BRS.0000000000001804
15. Woods GN, Huang M-H, Lee J-H, et al. Factors associated with kyphosis and kyphosis progression in older men: the MrOS Study. J Bone Miner Res. 2020;35(11):2193–2198. doi:10.1002/jbmr.4123
16. Kimura A, Shiraishi Y, Inoue H, Endo T, Takeshita K. Predictors of persistent axial neck pain after cervical laminoplasty. Spine. 2018;43(1):10–15. doi:10.1097/BRS.0000000000002267
17. Lau E, Ong K, Kurtz S, Schmier J, Edidin A. Mortality following the diagnosis of a vertebral compression fracture in the medicare population. J Bone Joint Surg Am. 2008;90(7):1479–1486. doi:10.2106/JBJS.G.00675
18. Lindsay R, Burge RT, Strauss DM. One year outcomes and costs following a vertebral fracture. Osteoporos Int. 2005;16(1):78–85. doi:10.1007/s00198-004-1646-x
19. Ong KL, Beall DP, Frohbergh M, Lau E, Hirsch JA. Were VCF patients at higher risk of mortality following the 2009 publication of the vertebroplasty ”sham” trials? Osteoporos Int. 2018;29(2):375–383. doi:10.1007/s00198-017-4281-z
20. Yu W, Jiang X, Liang D, et al. Intravertebral vacuum cleft and its varied locations within osteoporotic vertebral compression fractures: effect on therapeutic efficacy. Pain Physician. 2017;20(6):E979–E986.
21. Nieuwenhuijse MJ, Van Erkel AR, Dijkstra PDS. Cement leakage in percutaneous vertebroplasty for osteoporotic vertebral compression fractures: identification of risk factors. Spine J. 2011;11(9):839–848. doi:10.1016/j.spinee.2011.07.027
22. Tanigawa N, Kariya S, Komemushi A, et al. Cement leakage in percutaneous vertebroplasty for osteoporotic compression fractures with or without intravertebral clefts. AJR Am J Roentgenol. 2009;193(5):W442–W445. doi:10.2214/AJR.09.2774
23. Ding J, Zhang Q, Zhu J, et al. Risk factors for predicting cement leakage following percutaneous vertebroplasty for osteoporotic vertebral compression fractures. Eur Spine J. 2016;25(11):3411–3417. doi:10.1007/s00586-015-3923-0
24. Xie W, Jin D, Ma H, et al. Cement leakage in percutaneous vertebral augmentation for osteoporotic vertebral compression fractures: analysis of risk factors. Clin Spine Surg. 2016;29(4):E171–E176. doi:10.1097/BSD.0000000000000229
25. Zhong B-Y, He S-C, Zhu H-D, et al. Nomogram for predicting intradiscal cement leakage following percutaneous vertebroplasty in patients with osteoporotic related vertebral compression fractures. Pain Physician. 2017;20(4):E513–E520.
26. Kim YJ, Lee JW, Kim K-J, et al. Percutaneous vertebroplasty for intravertebral cleft: analysis of therapeutic effects and outcome predictors. Skeletal Radiol. 2010;39(8):757–766. doi:10.1007/s00256-009-0866-8
27. Chang J-Z, Bei M-J, Shu D-P, Sun C-J, Chen J-B, Xiao Y-P. Comparison of the clinical outcomes of percutaneous vertebroplasty vs. kyphoplasty for the treatment of osteoporotic Kümmell’s disease: a prospective cohort study. BMC Musculoskelet Disord. 2020;21(1):238. doi:10.1186/s12891-020-03271-9
28. Mirovsky Y, Anekstein Y, Shalmon E, Peer A. Vacuum clefts of the vertebral bodies. AJNR Am J Neuroradiol. 2005;26(7):1634–1640.
29. Kim D-Y, Lee S-H, Jang JS, Chung SK, Lee H-Y. Intravertebral vacuum phenomenon in osteoporotic compression fracture: report of 67 cases with quantitative evaluation of intravertebral instability. J Neurosurg. 2004;100(1 Suppl Spine):24–31. doi:10.3171/spi.2004.100.1.0024
30. Tang B, Xu S, Chen X, et al. The impact of intravertebral cleft on cement leakage in percutaneous vertebroplasty for osteoporotic vertebral compression fractures: a case-control study. BMC Musculoskelet Disord. 2021;22(1):805. doi:10.1186/s12891-021-04685-9
31. Nieuwenhuijse MJ, van Rijswijk CSP, van Erkel AR, Dijkstra SPD. The intravertebral cleft in painful long-standing osteoporotic vertebral compression fractures treated with percutaneous vertebroplasty: diagnostic assessment and clinical significance. Spine. 2012;37(11):974–981. doi:10.1097/BRS.0b013e318238bf22
32. Nakamae T, Fujimoto Y, Yamada K, Takata H, Shimbo T, Tsuchida Y. Percutaneous vertebroplasty for osteoporotic vertebral compression fracture with intravertebral cleft associated with delayed neurologic deficit. Eur Spine J. 2013;22(7):1624–1632. doi:10.1007/s00586-013-2686-8
33. Abelin-Genevois K. Sagittal balance of the spine. Orthop Traumatol Surg Res. 2021;107(1S):102769. doi:10.1016/j.otsr.2020.102769
34. Le Huec JC, Thompson W, Mohsinaly Y, Barrey C, Faundez A. Sagittal balance of the spine. Eur Spine J. 2019;28(9):1889–1905. doi:10.1007/s00586-019-06083-1
35. Endo K, Suzuki H, Tanaka H, Kang Y, Yamamoto K. Sagittal spinal alignment in patients with lumbar disc herniation. Eur Spine J. 2010;19(3):435–438. doi:10.1007/s00586-009-1240-1
36. Liu H, Zhou Q, Shao X, et al. Percutaneous kyphoplasty in patients with severe osteoporotic vertebral compression fracture with and without intravertebral cleft: a Retrospective Comparative Study. Int J Gen Med. 2022;15:6199–6209. doi:10.2147/IJGM.S369840
37. Kim MK, Lee S-H, Kim E-S, Eoh W, Chung -S-S, Lee C-S. The impact of sagittal balance on clinical results after posterior interbody fusion for patients with degenerative spondylolisthesis: a pilot study. BMC Musculoskelet Disord. 2011;12:69. doi:10.1186/1471-2474-12-69
38. Li D, Zhou Y, Cui H, et al. Analysis of the curative effect of percutaneous kyphoplasty in the treatment of osteoporotic vertebral compression fracture with intravertebral clefts. Medicine. 2021;100(22):e25996. doi:10.1097/MD.0000000000025996
39. Krauss M, Hirschfelder H, Tomandl B, Lichti G, Bär I. Kyphosis reduction and the rate of cement leaks after vertebroplasty of intravertebral clefts. Eur Radiol. 2006;16(5):1015–1021. doi:10.1007/s00330-005-0056-6
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.