Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Effects of Pedometer-Based Step-Feedback on Physical Activity of Severe COPD Patients
Authors Tsujimura Y , Akiyama A, Hiramatsu T, Mikawa K, Tabira K
Received 5 April 2023
Accepted for publication 29 September 2023
Published 17 October 2023 Volume 2023:18 Pages 2277—2287
DOI https://doi.org/10.2147/COPD.S415958
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Yasuhiko Tsujimura,1,2 Ayumu Akiyama,1 Tetsuo Hiramatsu,3 Kotaro Mikawa,4 Kazuyuki Tabira2
1Department of Rehabilitation, Hiramatsu Clinic of Internal and Respiratory Medicine, Komaki, Aichi, Japan; 2Division of Health Science, Graduate School of Health Science, Kio University, Kitakaturagi, Nara, Japan; 3Department of Respiratory Medicine, Hiramatsu Clinic of Internal and Respiratory Medicine, Komaki, Aichi, Japan; 4Department of Physical Therapy Faculty of Nursing and Rehabilitation, Chubu Gakuin University, Seki, Gifu, Japan
Correspondence: Kazuyuki Tabira, Division of Health Science, Graduate School of Health Science, Kio University, 4-2-2 Umami-naka, Koryo-cho, Kitakaturagi, Nara, Japan, Tel +81-745-54-1601, Fax +81-745-54-1600, Email [email protected]
Purpose: This study investigated whether adding step-feedback (step-FB) from a pedometer to pulmonary rehabilitation (PR) programs could increase the physical activity (PA) of low-activity patients with severe chronic obstructive pulmonary disease (COPD).
Patients and Methods: We included low-activity patients with severe COPD (step-FB group: 14 patients; control group: 17 patients) who underwent PR for the first time. The usual PR program for patients with severe COPD consisted of two 8-week sessions (PR session 1: PR1, PR session 2: PR2). The step-FB group was provided a program with step-FB added to PR2 (PR2+step-FB). Furthermore, all patients were evaluated at pre-intervention (baseline), PR1, and PR2. The primary outcome of this study was the number of daily steps (steps) and energy expenditure from activity (energy expenditure), as measured by a pedometer. The secondary outcomes were dyspnea and exercise tolerance.
Results: In PR1, dyspnea, exercise tolerance, steps, and energy expenditure were significantly improved as compared to baseline, in both groups. During PR2, dyspnea and exercise tolerance were significantly improved as compared to PR1, in both groups. Steps and energy expenditure were significantly improved in the step-FB group, but not in the control group.
Conclusion: PR improved PA by improving physical function in severe COPD patients. Adding step-FB improved PA in severe COPD patients by presenting an activity goal for improving PA. Therefore, pedometer-based step-FB is a viable addition to PR and has the potential to improve PA continuously in these patients.
Keywords: dyspnea, exercise tolerance, monitoring, pulmonary rehabilitation, step counter
Introduction
Low physical activity (PA) levels are associated with increased healthcare utilization and reduced survival in patients with chronic obstructive pulmonary disease (COPD).1–5 PA levels are associated with disease severity,6–11 with lower PA levels observed in severe COPD patients than in patients with mild to moderate COPD. Thus, improving PA levels is a key COPD treatment goal.12,13 However, improving PA in severe COPD patients is difficult, as they have marked dyspnea and decreased exercise tolerance.
Pulmonary rehabilitation (PR) is the cornerstone of COPD management, with well-documented effects on dyspnea, exercise capacity, and health-related quality of life. The primary effect of PR programs is to break the cycle of dyspnea and deconditioning through prescribed exercises, which may be ideal for promoting PA in COPD patients.14–16 Furthermore, PA levels may be enhanced by including behavioral strategies in PR programs.17,18 Such strategies can be used to raise the patient’s awareness of their actual PA level, as such awareness is a potential determinant of their willingness to increase their PA levels.
A pedometer can be used to present a target for the daily number of steps to take, ie, it can provide step-feedback (step-FB). Pedometers offer new perspectives in changing PA behavior because patients can monitor themselves and set concrete goals for increasing their own PA. Indeed, behavioral strategies using pedometers have been reported to be effective for improving PA.19–24 However, many of the participants in previous studies on this topic were relatively mild cases with stage 1–2 of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification of COPD severity,25 and had relatively good exercise tolerance (for example, a 6-minute walk distance [6MWD] ≥ 350 m). In addition, some reports have targeted participants with a relatively high number of steps at the start of the survey (for example, around 4000 steps). Nevertheless, no reports have evaluated improvements in activity in severe COPD patients with low PA. Therefore, the effect of using step-FB in low-activity patients with severe COPD is unknown.
This study investigated whether PR increased PA in low-activity patients with severe COPD. Furthermore, we investigated whether the addition of pedometer-based step-FB could increase the PA of low-activity, severe COPD patients.
Materials and Methods
Study Design
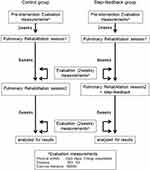
Figure 1 shows the study flowchart. The PR program for patients with severe COPD consisted of a 2-week pre-intervention investigation (baseline) and two 8-week sessions of PR (pulmonary rehabilitation sessions 1 [PR1] and 2 [PR2]). Participants who had previously completed this PR program without step-FB were retrospectively evaluated as controls (control group). Additionally, a group who underwent the same PR program, but with step-FB added to PR2, were prospectively investigated (step-FB group).
All patients were evaluated at baseline, PR1, and PR2, with or without step-FB. All outcomes were assessed by the same physical therapist. The primary outcome was the number of steps taken and activity-related energy expenditure, as measured by a pedometer. The secondary outcomes were dyspnea (ie, baseline dyspnea index [BDI] / transition dyspnea index [TDI]) and exercise tolerance (ie, 6-minute walking test [6MWT]).
Participants
Participants in both groups were patients with severe COPD with low PA, who underwent first-time PR in our outpatient clinic. We defined severe COPD as GOLD stages 3 and 4. Low PA was defined as taking fewer than 2800 steps daily, as this is below 50% of the average number of steps taken daily by a Japanese person aged > 65 years (5744 steps).26 Other inclusion criteria were as follows: no prior PR; no clinical exacerbations in the previous 3 months; absence of other pathological conditions that could impair PA in daily life; and no cognitive changes.
The control group had previously completed the PR programs and assessments, and their data were obtained from clinical records.
All participants provided written informed consent for participation. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and all study methods were approved by the ethics committee of Chubu Gakuin University (Approval # D18-0003).
Data Collection
Patient characteristics, ie, age, sex, body mass index, smoking pack years, pulmonary function (predicted forced vital capacity, predicted forced expiratory volume in 1 s, forced expiratory volume in 1 s/forced vital capacity ratio), GOLD classification of COPD severity,25 and modified British Medical Research Council Dyspnea Scale, were obtained from the clinical records.
Evaluation of Physical Activity in Daily Life
PA in daily life was evaluated as the daily number of steps and energy expenditure using a Lifecorder uniaxial accelerometer sensor pedometer (Suzuken Corporation, Nagoya, Japan). Participants were given written instructions on pedometer use. The pedometer was attached to the left anterior superior iliac spine by means of a belt. The patients were instructed to wear the pedometer throughout the day, except when sleeping or bathing. Patients in the step-FB group wore the pedometer daily during the baseline and PR2 periods. Additionally, they wore a pedometer during the PR1 evaluation period. The patients in the control group wore a pedometer only during each evaluation period. In both groups, during the baseline and PR1 evaluation periods, the pedometer screen was hidden by masking tape to avoid the displayed information influencing participants’ behavior. Additionally, for the control group, but not for the step-FB group, the screen was also masked during the PR2 evaluation period. The tape was marked to reveal tampering.
Lifecorders automatically recorded the daily number of steps and energy expenditure. The average values for these parameters over the last 14 consecutive days of each period were used for analysis. The recorded data were analyzed using dedicated software (Suzuken Corporation, Lifelyzer05 Coach, Nagoya, Japan), and an activity record summary was created to provide step-FB and to evaluate the pedometer-wearing rate.
Evaluation of Dyspnea
Dyspnea was evaluated at a single baseline state (BDI), and as a change from baseline (TDI). The BDI/TDI included three domains (functional impairment, magnitude of task, and magnitude of effort). The BDI score ranged from 0 (very severe impairment) to 4 (no impairment) for each domain. The TDI score was graded from −3 to +3, where −1 to −3 signifies deterioration, 0 signifies no change, and 1 to 3 signifies improvement (a change of 1 unit was considered clinically significant) for each domain. The TDI focal score was determined by sum of all domains and was used for analysis. BDI/TDI was evaluated at baseline and at the end of PR1 and PR2.
Evaluation of Exercise Tolerance
Exercise tolerance was evaluated using the 6MWT in accordance with the protocol proposed by the European Respiratory Society/American Thoracic Society.27 Patients performed two trials, and the best 6MWD result was used for analysis. The 6MWT was evaluated at baseline and at the end of PR1 and PR2.
PR Program for Severe COPD Patients
PR was provided once a week for 16 weeks in an outpatient supervised program, with individually prescribed exercise training and self-management education. Each time, PR consisted of 15 min of education and 1 h of exercise. All patients received identical education, including lectures on their disease, proper use of inhalation devices, and home exercises. The exercise program consisted of pursed lip breathing, quadriceps muscle training in the sitting position, sit-to-stand practice from a dining chair, and aerobic exercise using an ergometer or fast walking. The same exercises were to be performed at hospital and at home (on a daily basis). All patients were asked to maintain an activity diary to record their exercise sessions. The physical therapist checked these diaries every week.
Step-Feedback Program
In PR2, the step-FB PA enhancement program was presented to motivate the step-FB group to increase their daily PA, by being aware of the number of steps they took each day. Patients were asked to record this number in their activity diary every day. Additionally, an activity record summary was used to facilitate their awareness of the number of steps taken. We aimed to increase the number of steps by 500 steps or 10% per month. The physical therapist prescribed the target step count based on the patient’s activity diary. Moreover, the short-term goals of this training were reviewed every 2 weeks.
Compliance in Pedometer Wearing and Daily Step Recording
Data on patient compliance in pedometer use (number of days of wearing the device) were collected. Compliance data were obtained from the activity diary and pedometer recording data. Patients in the step-FB group also reported their impressions of using the pedometer via a questionnaire.
Statistical Analysis
Data analysis was performed using SPSS v23.0 statistical software (IBM SPSS Inc., Armonk, NY, USA). Data are presented as mean ± standard deviation. Patient characteristics were compared between the two groups by using the independent t-test or Mann–Whitney U-test for parametric and nonparametric data, respectively. Intervention effects were compared at baseline, PR1, and PR2. The daily number of steps and energy expenditure of the step-FB group were compared using Friedman test. Wilcoxon signed-rank tests with Bonferroni adjusted critical values were used for multiple comparisons. The daily number of steps and energy expenditure of the control group, as well as the 6MWD of both groups, were compared using repeated-measures one-way analysis of variance. The Tukey–Kramer method was used for multiple comparisons. The TDI was compared between PR1 and PR2 using the Wilcoxon signed-rank test. To assess the additional effect of step-FB, the amount of improvement in the daily number of steps and energy expenditure from baseline to PR1 and from PR1 to PR2 were compared between the two groups, using the Mann–Whitney U-test. Statistical significance was set at p < 0.05.
Results
Study Subjects and Baseline Characteristics
Patients’ characteristics are summarized in Table 1. Fourteen patients were included in the step-FB group (between September 2016 and January 2020), none of whom dropped out, while 17 patients were included in the control group (between December 2014 and April 2016). The baseline characteristics of the two groups were similar.
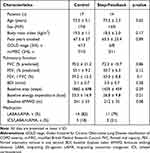 |
Table 1 Characteristics of Patients |
Compliance with Pedometer Wearing and Daily Step Recording
All patients in both groups reported pedometer use as instructed. The pedometer recording data showed a wearing rate of 100% during the requested period. In each case, the tape covering the pedometer was intact, so that blinding was considered to have been maintained. Additionally, the step-FB group’s activity diaries showed daily entries of the number of steps, implying that these patients self-managed their steps every day.
The questionnaire revealed that participants found the pedometer easy to use, simple, motivating, etc. There were no negative comments.
Outcome Measures
The daily life PA levels at each measurement point in both groups are shown in Figure 2. In the step-FB group, the number of steps and energy expenditure during PR1 (2181 ± 843 steps, 38.7 ± 17.8 kcal) and during PR2 (3050 ± 1045 steps, 52.6 ± 20.1 kcal) were significantly increased as compared to baseline (1635 ± 459 steps, 26.8 ± 9.8 kcal) (all p < 0.01). Furthermore, the number of steps and energy expenditure during PR2 were significantly higher than those during PR1 (both p < 0.01). In the control group, the number of steps and energy expenditure during PR1 (2540 ± 1012 steps, 46.4 ± 27.9 kcal) and during PR2 (2720 ± 1026 steps, 53.4 ± 25 kcal) were significantly increased as compared to baseline (1860 ± 698 steps, 33.3 ± 16.9 kcal) (all p < 0.01). However, both variables were similar for PR1 and PR2 (p = 0.076 and p = 0.052, respectively).
The mean pre-intervention BDI focal score was 3.0 ± 0.7 units in the step-FB group and 3.1 ± 0.7 units in the control group. The TDI values at each assessment in both groups are shown in Figure 3A. In the step-FB and control groups, the TDI focal score improved during PR1 (1.3 ± 0.7 units, 1.5 ± 0.7 unit, respectively) and during PR2 (2.4 ± 0.9 units, 2.1 ± 0.3 unit, respectively), with statistically significant differences between PR1 and PR2 (both p < 0.01).
The 6MWD for each assessment in both groups is shown in Figure 3B. In the step-FB and control groups, the 6MWDs at the PR1 assessment (248 ± 40 m, 293 ± 52 m, respectively) and at the PR2 assessment (285 ± 47 m, 317 ± 53 m, respectively) were significantly increased as compared to baseline (212 ± 35 m, 241 ± 53 m, respectively) (both p < 0.01). Furthermore, the 6MWD was significantly greater at the PR2 than at the PR1 assessment (both p < 0.01).
Table 2 shows the changes in the daily number of steps and energy expenditure from baseline to PR1 and to PR2 in both groups. Changes in the daily number of steps and energy expenditure from baseline to PR1 were similar in both groups. However, the change from PR1 to PR2 was significantly greater in the step-FB than in the control group.
 |
Table 2 Comparison of Changes in Steps and Energy Expenditure from Baseline to PR1 and from PR1 to PR2 |
Discussion
To identify approaches for improving PA in low-activity, severe COPD patients, we investigated whether PR increased PA in such patients, and whether adding pedometer-based step-FB to PR could increase their PA. We found that dyspnea, exercise tolerance, number of steps, and energy expenditure improved significantly with PR, and that the number of steps and energy expenditure were significantly improved with the added step-FB.
Effects of Pulmonary Rehabilitation on Dyspnea, Exercise Tolerance, and Daily Physical Activity in Severe COPD Patients
In COPD patients, the most significant causes of PA decline are dyspnea and decreased exercise tolerance, particularly in severe COPD cases. Watz et al6 and Hernandes et al28 found that PA decreased with the dyspnea degree. Barriga et al reported that the worse the dyspnea, the higher the activities of daily living restraints, and the fewer the number of daily steps.10 We previously reported that PA level was related to 6MWD at any COPD stage.29 Additionally, we suggested that, although daily routine housework and hobbies provide motivation, exercise tolerance is required for leading an active life. Thus, severe COPD patients lack the physical function necessary for PA. Troosters et al suggested that, in patients with very low exercise tolerance or significant symptom burden, enhancing PA is difficult, and interventions should first focus on enhancing exercise tolerance.30 Therefore, dyspnea and exercise tolerance should first be improved to increase PA, rather than focusing on education or behavior changes. Zwerink et al reported that, in COPD patients participating in physiotherapy exercise programs, changes in exercise capacity correlated moderately with changes in daily PA.31 They argued that increased exercise capacity in COPD patients reduced dyspnea and fatigue symptoms during and after exercise, removing a barrier to being active. Osadnik et al reported that PR increased the number of steps taken, but the higher the 6MWD improvement rate after PR, the more the number of steps increased.32 Mesquita et al reported that enhanced post-PR exercise tolerance influenced PA improvement.33 They argued that increased functional capacity is needed for PA improvement. Our PR program for severe COPD patients consisted of conditioning, strength training, and many aerobic exercises (walking, and ergometer and treadmill exercise). Additionally, we used an activity diary to promote daily exercise continuation at home. We found that PA increased, and dyspnea and exercise tolerance improved. Taken together, improved dyspnea and exercise tolerance due to PR contributed to significantly increased daily PA. Moreover, increased daily PA likely contributed to improved dyspnea and exercise tolerance.
Effect of Step-Feedback on Daily Physical Activity in Severe COPD Patients
Dyspnea and the 6MWD showed significant improvement from PR1 to PR2 in both groups. Additionally, the number of steps taken and the energy expended increased significantly in the step-FB group, but not in the control group, despite the latter’s dyspnea and exercise tolerance improvements. This suggested that the PA increase in the step-FB group was due to behavioral changes caused by step-FB. Overall, low-activity, severe COPD patients lack the physical function necessary to perform PA, have no activity purpose, and have a narrow activity range. Therefore, even if they achieve incremental gains in physical function, their PA levels will not show equal increments. Clinically, these patients are commonly encountered. Therefore, patients with severe COPD who have acquired the physical function necessary for performing PA require an activity goal as the next step. Zwerink et al suggested that, while COPD gradually worsened, the patient developed a sedentary lifestyle, necessitating more than an increase in exercise capacity to increase PA.31 They argued that behavioral techniques, such as motivational interviews and cognitive behavioral therapy, could be helpful for achieving and maintaining an improved daily PA level. We considered that patient education and exercise training alone would make it difficult to improve physical activity continuously. Osadnik et al suggested that behavioral therapy-based interventions may be appropriate for patients who are accepting of these approaches.32 Spruit et al suggested that both exercise capacity increase and adaptive behavior change are necessary to achieve significant and lasting increases in daily PA in patients.17
A recent report highlighted the importance of having an activity purpose to improve PA. Behavioral strategies using a pedometer have been reported to be effective for improving PA.19–24 Bertine et al34 and Cruz et al35 investigated the effects of a lifestyle PA counseling program using pedometer-based step-FB during PR in patients with mild to severe COPD. They reported that PA-focused behavioral intervention during PR may improve patients’ PA levels. Kawagoshi et al reported that PR with added step-FB significantly increased walking time per day.36 Armstrong et al conducted a systematic review and meta-analysis to examine the use of pedometers as tools to promote daily PA levels in COPD patients.24 They concluded that pedometer-based PA promotion, as well as PR, induced meaningful improvements in daily PA levels (steps per day). Therefore, our results likely reflect the effect of adding behavioral change, based on step-FB, to PR.
Furthermore, our study included only patients with severe COPD who had low activity levels. To date, no studies have reported improved PA in low-activity patients with severe COPD. However, our study demonstrated that using step-FB to improve PA during PR is feasible and increases the daily number of steps and energy expenditure, even in low-activity, severe COPD patients. Moreover, our participants’ compliance was satisfactory, and they reported positive experiences with the use of pedometers. Therefore, we concluded that the combination of step-FB and PR raised patient awareness and motivated them to improve their daily PA.
Limitations
This study had some limitations. First, the sample size was small. It was difficult to recruit patients that met the study inclusion criteria, given that many severe COPD patients have undertaken PR. We would require about 15 additional participants to achieve adequate statistical power.37 In future, data from additional patients should be accumulated. Second, because our sample included only male patients, the results may not apply to female patients. Although the prevalence of COPD in women is increasing in Japan, it remains much higher in men, which hampered recruitment of female COPD patients. Third, this study was not a standard randomized controlled study, implying a possibility of selection bias, performance bias, and detection bias. However, although the control group was based on historical data, basic treatments (such as pharmacotherapy and rehabilitation) did not differ, so that the impact thereof is considered to be small. Fourth, the timing of the measured PA (PR1 and PR2) coincided with the PR program, and we cannot rule out the influence of the PR program on increased PA. Pedometers should not have been worn during the PR program. Fifth, this was a short-term study. We did not investigate the long-term effects of the intervention; therefore, sustainability of the results could not be determined. Thus, further studies with more robust designs are needed to investigate the value of providing step-FB to severe COPD patients during PR.
Conclusion
This study showed that a combination of PR and step-FB was an effective method for improving PA in low-activity patients with severe COPD. We believe that step-FB may be a better addition for patients with severe COPD who do not achieve an increase in PA with PR alone.
Abbreviations
6MWD, 6-minute walking distance; 6MWT, 6-minute walking test; BDI, baseline dyspnea index; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; PA, physical activity; PR, pulmonary rehabilitation; TDI, transition dyspnea index.
Acknowledgments
We express our gratitude to the patients who participated in this study. We also thank all the staff who assisted in data collection and analysis.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Disclosure
All authors report that there are no conflicts of interest to declare for this work.
References
1. Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61(9):772–778. doi:10.1136/thx.2006.060145
2. Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331–342. doi:10.1378/chest.10-2521
3. Garcia-Rio F, Rojo B, Casitas R, et al. Prognostic value of the objective measurement of daily physical activity in patients with COPD. Chest. 2012;142(2):338–346. doi:10.1378/chest.11-2014
4. Vaes AW, Garcia-Aymerich J, Marott JL, et al. Changes in physical activity and all-cause mortality in COPD. Eur Respir J. 2014;44(5):1199–1209. doi:10.1183/09031936.00023214
5. Demeyer H, Donaire-Gonzalez D, Gimeno-Santos E, et al. Physical activity is associated with attenuated disease progression in chronic obstructive pulmonary disease. Med Sci Sports Exerc. 2019;51(5):833–840. doi:10.1249/MSS.0000000000001859
6. Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J. 2009;33(2):262–272. doi:10.1183/09031936.00024608
7. Troosters T, Sciurba F, Battaglia S, et al. Physical inactivity in patients with COPD, a controlled multi-center pilot-study. Respir Med. 2010;104(7):1005–1011. doi:10.1016/j.rmed.2010.01.012
8. Jehn M, Schmidt-Trucksäss A, Meyer A, Schindler C, Tamm M, Stolz D. Association of daily physical activity volume and intensity with COPD severity. Respir Med. 2011;105(12):1846–1852. doi:10.1016/j.rmed.2011.07.003
9. Tudorache V, Oancea C, Avram C, Fira-Mlădinescu O. Changes in physical activity in healthy people and COPD patients. Wien Klin Wochenschr. 2014;126(1–2):30–35. doi:10.1007/s00508-013-0452-x
10. Barriga S, Rodrigues F, Bárbara C. Factors that influence physical activity in the daily life of male patients with chronic obstructive pulmonary disease. Rev Port Pneumol. 2014;20(3):131–137. doi:10.1016/j.rppneu.2013.09.004
11. Minakata Y, Sugino A, Kanda M, et al. Reduced level of physical activity in Japanese patients with chronic obstructive pulmonary disease. Respir Investig. 2014;52(1):41–48. doi:10.1016/j.resinv.2013.06.002
12. Watz H, Pitta F, Rochester CL, et al. An official European Respiratory Society statement on physical activity in COPD. Eur Respir J. 2014;44(6):1521–1537. doi:10.1183/09031936.00046814
13. Qiu S, Cai X, Wang X, et al. Using step counters to promote physical activity and exercise capacity in patients with chronic obstructive pulmonary disease: a meta-analysis. Ther Adv Respir Dis. 2018;12:1753466618787386. doi:10.1177/1753466618787386
14. Sewell L, Singh SJ, Williams JEA, Collier R, Morgan MDL. Can individualized rehabilitation improve functional Independence in elderly patients with COPD? Chest. 2005;128(3):1194–1200. doi:10.1378/chest.128.3.1194
15. Pitta F, Troosters T, Probst VS, Langer D, Decramer M, Gosselink R. Are patients with COPD more active after pulmonary rehabilitation? Chest. 2008;134(2):273–280. doi:10.1378/chest.07-2655
16. Mantoani LC, Rubio N, McKinstry B, MacNee W, Rabinovich RA. Interventions to modify physical activity in patients with COPD: a systematic review. Eur Respir J. 2016;48(1):69–81. doi:10.1183/13993003.01744-2015
17. Spruit MA, Pitta F, McAuley E, ZuWallack RL, Nici L. Pulmonary rehabilitation and physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(8):924–933. doi:10.1164/rccm.201505-0929CI
18. Lahham A, McDonald CF, Holland AE. Exercise training alone or with the addition of activity counseling improves physical activity levels in COPD: a systematic review and meta-analysis of randomized controlled trials. Int J Chron Obstruct Pulmon Dis. 2016;11:3121–3136. doi:10.2147/COPD.S121263
19. Hospes G, Bossenbroek L, Ten Hacken NH, van Hengel P, de Greef MH. Enhancement of daily physical activity increases physical fitness of outclinic COPD patients: results of an exercise counseling program. Patient Educ Couns. 2009;75(2):274–278. doi:10.1016/j.pec.2008.10.005
20. Cruz J, Brooks D, Marques A. Impact of feedback on physical activity levels of individuals with chronic obstructive pulmonary disease during pulmonary rehabilitation: a feasibility study. Chron Respir Dis. 2014;11(4):191–198. doi:10.1177/1479972314552280
21. Mendoza L, Horta P, Espinoza J, et al. Pedometers to enhance physical activity in COPD: a randomised controlled trial. Eur Respir J. 2015;45(2):347–354. doi:10.1183/09031936.00084514
22. Altenburg WA, ten Hacken NH, Bossenbroek L, Kerstjens HA, de Greef MH, Wempe JB. Short- and long-term effects of a physical activity counselling programme in COPD: a randomized controlled trial. Respir Med. 2015;109(1):112–121. doi:10.1016/j.rmed.2014.10.020
23. Widyastuti K, Makhabah DN, Setijadi AR, Sutanto YS, Suradi N. Benefits and costs of home pedometer assisted physical activity in patients with COPD. A preliminary randomized controlled trial. Pulmonology. 2018;24(4):211–218. doi:10.1016/j.pulmoe.2018.01.006
24. Armstrong M, Winnard A, Chynkiamis N, Boyle S, Burtin C, Vogiatzis I. Use of pedometers as a tool to promote daily physical activity levels in patients with COPD: a systematic review and meta-analysis. Eur Respir Rev. 2019;28(154):190039. doi:10.1183/16000617.0039-2019
25. GOLD. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2023. Available from: https://goldcopd.org/2023-gold-report-2/.
26. Ministry of Health, Labour and Welfare. The National Health and Nutrition Survey in Japan. Available from: https://www.mhlw.go.jp/file/04-Houdouhappyou-10904750-Kenkoukyoku-Gantaisakukenkouzoushinka/kekkagaiyou_7.pdf.
27. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi:10.1183/09031936.00150314
28. Hernandes NANA, Teixeira DC, Probst VSVS, Brunetto AF, Ramos EM, Pitta F. Profile of the level of physical activity in the daily lives of patients with COPD in Brazil. J Bras Pneumol. 2009;35(10):949–956. doi:10.1590/s1806-37132009001000002
29. Tsujimura Y, Hiramatsu T, Kojima E, Tabira K. Factors influencing the physical activity in daily life of male patients with different levels of severity of chronic obstructive pulmonary disease. J Phys Ther Sci. 2018;30(10):1251–1256. doi:10.1589/jpts.30.1251
30. Troosters T, Blondeel A, Rodrigues FM, Janssens W, Demeyer H. Strategies to increase physical activity in chronic respiratory diseases. Clin Chest Med. 2019;40(2):397–404. doi:10.1016/j.ccm.2019.02.017
31. Zwerink M, van der Palen J, van der Valk P, Brusse-Keizer M, Effing T. Relationship between daily physical activity and exercise capacity in patients with COPD. Respir Med. 2013;107(2):242–248. doi:10.1016/j.rmed.2012.09.018
32. Osadnik CR, Loeckx M, Louvaris Z, et al. The likelihood of improving physical activity after pulmonary rehabilitation is increased in patients with COPD who have better exercise tolerance. Int J Chron Obstruct Pulmon Dis. 2018;13:3515–3527. doi:10.2147/COPD.S174827
33. Mesquita R, Meijer K, Pitta F, et al. Changes in physical activity and sedentary behaviour following pulmonary rehabilitation in patients with COPD. Respir Med. 2017;126:122–129. doi:10.1016/j.rmed.2017.03.029
34. de Blok BM, de Greef MH, ten Hacken NH, Sprenger SR, Postema K, Wempe JB. The effects of a lifestyle physical activity counseling program with feedback of a pedometer during pulmonary rehabilitation in patients with COPD: a pilot study. Patient Educ Couns. 2006;61(1):48–55. doi:10.1016/j.pec.2005.02.005
35. Cruz J, Brooks D, Marques A. Walk2Bactive: a randomised controlled trial of a physical activity-focused behavioural intervention beyond pulmonary rehabilitation in chronic obstructive pulmonary disease. Chron Respir Dis. 2016;13(1):57–66. doi:10.1177/1479972315619574
36. Kawagoshi A, Kiyokawa N, Sugawara K, et al. Effects of low-intensity exercise and home-based pulmonary rehabilitation with pedometer feedback on physical activity in elderly patients with chronic obstructive pulmonary disease. Respir Med. 2015;109(3):364–371. doi:10.1016/j.rmed.2015.01.008
37. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. doi:10.3758/BRM.41.4.1149
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.