Back to Journals » Drug Design, Development and Therapy » Volume 15
Effects of Exenatide on Coagulation and Platelet Aggregation in Patients with Type 2 Diabetes
Authors Zhang Y , Chen R , Jia Y, Chen M , Shuai Z
Received 23 March 2021
Accepted for publication 16 June 2021
Published 12 July 2021 Volume 2021:15 Pages 3027—3040
DOI https://doi.org/10.2147/DDDT.S312347
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Yaqin Zhang,1,* Ruofei Chen,1,* Yangyang Jia,2 Mingwei Chen,2 Zongwen Shuai1
1Department of Rheumatology, The First Affiliated Hospital of Anhui Medical University, Hefei, 230022, People’s Republic of China; 2Department of Endocrinology, The First Affiliated Hospital of Anhui Medical University, Hefei, 230022, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zongwen Shuai; Mingwei Chen Email [email protected]; [email protected]
Objective: To explore the effect of the glucagon-like peptide-1 receptor agonist exenatide on coagulation function and platelet aggregation in patients with type 2 diabetes mellitus (T2DM).
Methods: Thirty patients with newly diagnosed T2DM were enrolled as the case group, and 30 healthy people with matching age and sex were selected as the control group. Patients in the case group received exenatide treatment for 8 weeks. The general clinical data and biochemical indicators of all subjects were collected; and their peripheral blood platelet count, coagulation index, nitric oxide (NO), platelet membrane glycoprotein (CD62p), platelet activation complex-1 (PAC-1) and platelet aggregation induced by collagen, epinephrine (EPI), arachidonic acid (AA), and adenosine diphosphate (ADP) were detected.
Results: The fibrinogen, CD62p, PAC-1, and platelet aggregation rates of the case group (pretreatment) are higher than those in the control group (EPI 77.90± 6.31 vs 60.15± 5.37, ADP 52.89± 9.36 vs 47.90± 6.16, and AA 76.09± 3.14 vs.55.18± 3.55); and the NO level is lower in the case group than in the control group (p< 0.05, respectively). After 8 weeks of exenatide treatment in the case group, the CD62p, PAC-1, and platelet aggregation rates were lower than before the treatment (EPI: 61.96± 8.94 vs 77.90± 6.31 and AA: 50.98± 6.73 vs 76.09± 3.14); and the NO level was higher than before the treatment (p< 0.05, respectively). Pearson correlation analysis showed that the changes in platelet aggregation rates (Δ EPI and ΔAA) of the patients in the case group after 8 weeks of exenatide treatment were positively correlated with the changes in body mass index, waist circumference, weight, blood lipids, fasting plasma glucose, haemoglobin A1c, fibrinogen, CD62p, and PAC-1 and negatively correlated with the changes in high-density lipoprotein and NO (p< 0.05). Multiple linear regression analysis showed that the changes in NO, CD62p and PAC-1 were independent risk factors affecting the changes in platelet aggregation rates.
Conclusion: The GLP-1R agonist exenatide can inhibit the activation state of platelets in patients with T2DM and inhibit thrombosis, which is beneficial to reduce the risk of cardiovascular events.
Keywords: glucagon-like peptide-1, type 2 diabetes mellitus, exenatide, platelet activation, thrombosis
Background
Type 2 diabetes mellitus (T2DM) is a progressive metabolic disease characterized by chronic hyperglycaemia. High glucose toxicity, lipotoxicity and insulin resistance are components of the pathophysiology of T2DM. These components mainly affect the integrity of the blood vessel wall, leading to increased inflammation, endothelial dysfunction, enhanced platelet aggregation and coagulation factor dysfunction.1–3 In addition to causing microvascular complications (retinopathy, nephropathy, and neuropathy), this complicated pathophysiology also leads to a 2–4-fold increase in the risk of thrombosis and cardiovascular disease (CVD) in patients with T2DM.4,5 The survey shows that CVD is the main cause of death in T2DM patients, accounting for 65% of the mortality rate in T2DM patients.6 Therefore, the treatment of T2DM seeks to not only control blood sugar but also to reduce other risk factors for CVD, such as obesity, hypertension, hyperlipidaemia, and blood hypercoagulability.
Many studies have confirmed that diabetes is often accompanied by excessive activation of platelets, which can easily lead to thrombosis and induce adverse cardiovascular events.7 Therefore, antiplatelet aggregation therapy has become an indispensable part of preventing cardiovascular events in T2DM. In clinical work, the method of measuring platelet aggregation function is often used to reflect the platelet activation state.8 This method exposes platelets to different inducers (such as ADP, collagen, epinephrine, and AA) in vitro and uses light transmission aggregation measurement (LTA) to monitor platelet aggregation ability. It is often used clinically to monitor the effects of antiplatelet therapy.
Both CD62p and PAC-1 are highly sensitive and specific markers that reflect the activation state of platelets. CD62p is a platelet activation-dependent granular membrane protein, also known as GMP-140 and P-selectin, a member of the selectin family (one of the serum endothelial adhesion markers, which also includes vascular cell adhesion molecule-1 (VCAM-1) and intracellular adhesion molecule-1 (ICAM-1)). PAC-1 is the GPIIb/IIIa complex fibrinogen receptor. Several studies have confirmed that these two indicators can be used in the monitoring and early prevention of platelet activation in hypercoagulable diseases.9
As a new type of hypoglycaemic drug, glucagon-like peptide-1 (GLP-1) receptor agonists have received increasingly more attention in recent years. In addition to lowering blood sugar, these drugs also showed good effects in reducing the cardiovascular risk of T2DM.10,11 Exenatide is a commonly used clinical GLP-1 receptor (GLP-1R) agonist. A number of clinical trials that have been completed thus far have found that12–15 exenatide can not only effectively reduce the blood glucose level of patients with T2DM but also has obvious effects other than hypoglycaemic effects, such as weight loss, lower blood pressure, blood lipid regulation, and improved endothelial dysfunction caused by hyperglycaemia and hyperlipidaemia. Studies have confirmed that GLP-1R is highly expressed in mouse and human platelets.16 Cameron-Vendrig et al17 confirmed that the GLP-1R agonist exenatide can not only inhibit the aggregation of human and mouse platelets in vitro but also inhibit mouse arterial thrombosis in vivo. At present, research on the effect of GLP-1R agonists on platelet activation function is mostly limited to animal experiments and in vitro experiments,16–18 and clinical studies on the effect of platelet activation function in T2DM patients have not been reported.
To this end, we designed and conducted a small sample control study to explore the changes in platelet aggregation and its activation markers CD62p and PAC-1 in the peripheral blood of patients with T2DM after exenatide treatment, as well as the possible related factors. This study aims to provide a theoretical basis for the clinical application of GLP-1R agonists and the prevention and treatment of diabetes-related cardiovascular diseases.
Study Methods
Study Objects
A total of 30 newly diagnosed T2DM patients (case group), including 20 males and 10 females, admitted to the Department of Endocrinology of the First Affiliated Hospital of Anhui Medical University from October 2018 to November 2019 were selected.
Inclusion criteria: Male or female aged 20–75 years, BMI 24 ~ 40 kg/m2, HbA1c 7.5 ~ 10%, and glutamate decarboxylase antibody was negative. Received lifestyle intervention for at least 1 month; never received hypoglycaemic drugs (including oral; never received hypoglycaemic drugs, GLP-1R agonists, insulin, etc.); and not taking lipid-regulating drugs or antihypertensive drugs, antiplatelet or anticoagulant drugs, or any medication that affected weight in the past 6 months.
Exclusion criteria: Intolerance to GLP-1R agonists; history of blood system and chronic infectious diseases; cardiovascular and cerebrovascular events in the past 6 months; pregnant patients or recent pregnancy planners; type 2 diabetes with acute metabolic disorders; obvious abnormal heart, liver and kidney function; a history of medullary thyroid cancer; a history of gastrointestinal, pancreatic disease, and gastrointestinal surgery; received weight loss treatment in the past 6 months; secondary to other endocrine diseases and symptomatic obesity caused by other reasons; and those who cannot cooperate to complete the clinical research.
In addition, 30 patients with normal glucose tolerance (control group), including 21 males and 9 females, who received physical examinations at the Health Management Center of the First Affiliated Hospital of Anhui Medical University during the same period were selected.
Inclusion criteria: Males or females aged 20 to 75 years, BMI 24 to 40 kg/m2, normal glucose tolerance (FPG <6.1 mmol/L and HbA1c<5.7%), not taking lipid-regulating drugs and antihypertensive drugs, not taking antiplatelet and anticoagulant drugs, and not taking any medication that affects weight.
Exclusion criteria: History of blood system and chronic infectious diseases; severe heart, liver, and kidney function abnormalities; acute infectious diseases; pregnancy; taking lipid-regulating drugs or antihypertensive drugs, antiplatelet or anticoagulant drugs, or any medication that affected weight in the past 6 months.
This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Anhui Medical University, and all subjects signed an informed consent form.
Exenatide Treatment
Individuals included in the case group were supervised by professionals in the fields of diet, exercise, and blood sugar monitoring throughout the study. Additionally, they were started on exenatide treatment (5 μg, sc, bid). Four weeks later, the dose of exenatide was increased to 10 μg (sc, bid), and the treatment continued for another four weeks, with a total of 8 weeks of exenatide treatment. One telephone follow-up was conducted every week, while an outpatient follow-up was scheduled monthly. If nausea, vomiting, or other digestive symptoms occurred during the higher-dose exenatide treatment (10 μg, sc, bid), a reduced dose of exenatide (5 μg, sc, bid) was administered until the higher-dose treatment was resumed a week later. If the patient responded poorly to an increased dose of exenatide, he/she continued the lower-dose treatment until the end of the study. If serious hyperglycaemia (FPG levels at two different time points both exceeding 13.9 mmol/L) developed during the study, the patient was allowed to withdraw from the study upon completion of the relevant examinations.
Blood Samples
Ulnar vein blood was drawn after fasting for at least 8 hours. According to the relevant requirements, the collection, processing, and testing of blood samples were completed in a standardized manner.
General Biochemical Indicators
An automatic haematology analyzer (XE-2100, Sysmex, Japan) was applied to the calculation of blood platelet (PLT) counts at week 0 and at week 8. The hexokinase method was used to measure fasting blood glucose (FPG); a glycosylated haemoglobin analyzer (AYFY24319, BIO-RAD, Japan) was utilized to quantify glycosylated haemoglobin A1c (HbA1c). Total cholesterol (TCH) and triglycerides (TG) were analyzed using enzymatic colorimetric methods. High-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were detected using immunoturbidimetry (MODULE P800, Roche, Switzerland). Prothrombin time (PT), activated partial thromboplastin time (APTT), the international normalized ratio (INR), and fibrinogen (FIB) were measured by magnetic bead coagulation method; and fibrin degradation products (FDP) and dimer (DD) were detected by immunoturbidimetry (AYL-4-013, Stago, France).
LTA for Detecting Platelet Aggregation Rates
The anticoagulant blood was centrifuged at 800 r/min for 10 min at room temperature, and the upper platelet-rich plasma (PRP) was aspirated. The remaining blood was centrifuged at 3000 r/min for 10 min, and the upper plasma was taken and labelled as platelet-poor plasma (PPP). Then, 400 ul PRP and PPP were added to a cuvette, respectively, and preheated at 37°C for 3 min. Next, small silicon magnetic particles were added to the PRP; and the inducers EPI (final concentration of 200 μM), ADP (final concentration of 10.0 μM), AA (final concentration of 2.5 μg/mL), and collagen (final concentration of 5.0 µg/mL) (HYPHEN BioMed, France) were added to the PRP to make the platelets aggregate. The aggregation rates and transmittance of PRP were set to 0%, and the aggregation rates and transmittance of PPP were set to 100%. With the aggregation of platelets, the plasma turbidity decreased and the light transmittance increased, and the instrument automatically traced the platelet aggregation rate. Each subject was tested twice, and the average value was taken as the test result. The PACKS-4 platelet aggregation instrument was purchased from Helena Laboratories, USA.
Determination of the Expression Levels of CD62p and PAC-1 by Three-Colour Flow Cytometry
Each freeze-dried sample was resuspended in 4 mL of PPP. The concentration of fresh platelets was adjusted to 10×109/L with PBS. The samples were incubated with PAC-1 and CD62P for 15 min at room temperature. Each platelet suspension (100 mL) was stained with 5 mL of a fluorescent dye for 15–20 min. Subsequently, the samples were diluted in 400 mL of PBS and measured in a flow cytometer within 1 h. The control antibody of the same IgG isotype labelled with the same fluorochrome was incubated with fresh inactivated platelets under identical conditions and measured to set the dot-plot quadrants. The expression of CD62p and PAC-1 is expressed as the percentage of positive platelets for each activation marker.
Nitrogen Monoxide (NO) Test
Two mL of venous blood was collected, centrifuged and stored at −70°C for future use. After all blood samples were collected, the nitrate reductase assay (Nanjing Jiancheng Bioengineering Institute) was performed as instructed by the manufacturer. The concentration of serum NO was measured based on the optical density (OD) at 550 nm.
Each patient’s body height (m), body weight (kg), and waist circumference were measured to calculate his/her body mass index (BMI) according to the following equation: BMI = body weight (kg)/body height (m)2. Differences (Δ) in pre- and post-exenatide treatment were calculated using the values of the abovementioned clinical indicators measured at week 0 and at week 8.
Statistical Methods
The SPSS 21.0 statistical software was used to process the data, and the measurement data conforming to the normal distribution were expressed as the mean ± standard deviation ( ± s). The comparison of variables between the case group and the control group was conducted using independent sample t-test and χ2 test, and the comparison of the variables before and after treatment in the case group was conducted using the paired t-test. Pearson correlation analysis explored the correlation between the changes in platelet aggregation function before and after exenatide treatment and the changes in other variables. Multiple linear regression analysis was used to explore the independent influencing factors of platelet aggregation function before and after exenatide treatment. A significance level α=0.05 and two-sided p<0.05 indicate that the difference is statistically significant.
± s). The comparison of variables between the case group and the control group was conducted using independent sample t-test and χ2 test, and the comparison of the variables before and after treatment in the case group was conducted using the paired t-test. Pearson correlation analysis explored the correlation between the changes in platelet aggregation function before and after exenatide treatment and the changes in other variables. Multiple linear regression analysis was used to explore the independent influencing factors of platelet aggregation function before and after exenatide treatment. A significance level α=0.05 and two-sided p<0.05 indicate that the difference is statistically significant.
Results
Summary of Study Results
In this study, a total of 5 patients could not tolerate 10 μg (sc, bid) and had adverse reactions such as nausea and vomiting. After adjusting the dose of exenatide to 5 μg (sc, bid), the gastrointestinal symptoms disappeared. One week later, we tried to increase the dose of exenatide again to 10 μg (sc, bid), but the patients still could not tolerate it. Therefore, we administered 5μg exenatide (sc, bid) until the end of the study. No subjects withdrew from the study.
Comparison of the Clinical Data and Biochemical Indicators Between the Case Group and Control Group
The levels of TG, FPG, HbA1c, FIB, CD62p, and PAC-1 and the platelet aggregation rates induced by epinephrine, ADP, and AA in the case group (pretreatment) were significantly higher than those in the control group (p<0.05); the levels of HDL-C and NO were lower than those of the control group (p<0.05) and there was no significant difference in other clinical indicators between the two groups (p>0.05) as shown in Tables 1 and 2.
 |
Table 1 Comparison of Clinical Data and Biochemical Indicators Between Case Group (Pretreatment) and Control Group (%, x ± s) |
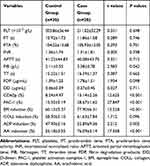 |
Table 2 Comparison of Coagulation Function and Platelet Aggregation Function Between Case Group (Pretreatment) and Control Group ( |
The Correlation of Platelet Aggregation Rates and Other Clinical Parameters in the Control Group and Case Group (Pretreatment)
Pearson correlation analysis showed that the platelet aggregation rates in the control group and case group (pretreatment) were positively correlated with BMI, weight, waist circumference, TCH, TG, LDL-C, FPG, HbA1c, CD62p and PAC-1 and negatively correlated with HDL-C and NO (p<0.05), and there was no significant correlation with SBP, DBP, PLT, PT, PTA, APTT, TT, INR, FDP, DD, and FIB (p>0.05) as shown in Figure 1A and B.
Comparison of Clinical Indicators After 0 Weeks and 8 Weeks of Exenatide Treatment in Case Group Subjects
After 8 weeks of exenatide treatment, the levels of weight, BMI, waist circumference, SBP, TCH, TG, LDL-C, FPG, HbA1c, FIB, CD62p and PAC-1 and the platelet aggregation rates induced by epinephrine and AA were significantly decreased in the case group compared with the 0-week group (p<0.05). The levels of HDL-C and NO were significantly increased (p<0.05), while DBP, PLT, PT, PTA, APTT, INR, TT, FDP, DD and platelet aggregation rates (collagen and ADP as inducers) were not significantly different before and after treatment (p>0.05) as shown in Tables 3 and 4, Figures 2 and 3.
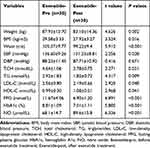 |
Table 3 Comparison of Clinical Data and Biochemical Indexes Before and After Exenatide Treatment in Case Group (x ± s) |
 |
Table 4 Comparison of Coagulation Function and Platelet Aggregation Function Before and After Exenatide Treatment in Case Group ( |
The Correlation of the Change Values of Platelet Aggregation Rates (Δ Platelet Aggregation Rates) with Epinephrine and AA as Inducers and the Change Values of Other Clinical Parameters (Δ Values) in the Case Group
Pearson correlation analysis showed that the Δ platelet aggregation rates (ΔEPI and ΔAA) before and after 8 weeks of exenatide treatment in the case group were positively correlated with ΔBMI, Δ weight, Δ waist, ΔTCH, ΔTG, ΔLDL-C, ΔFPG, ΔHbA1c, ΔFIB, ΔCD62p and ΔPAC-1 and negatively correlated with ΔHDL-C and ΔNO (P<0.05), with no significant correlation with ΔSBP (p>0.05), as shown in Figure 4.
Factors Affecting Platelet Aggregation Before and After Treatment with Exenatide
According to the results of Pearson correlation analysis, ΔBMI, Δ waist circumference, Δ weight, ΔTCH, ΔTG, ΔLDL-C, ΔHDL-C, ΔFPG, ΔHbA1c, ΔNO, ΔFIB, ΔCD62p, and ΔPAC-1 were included in the multiple linear regression analysis as independent variables, and Δ platelet aggregation rates (ΔEPI and ΔAA) was included as the dependent variable. The results show that ΔNO, ΔCD62p and ΔPAC-1 are independent risk factors affecting Δ platelet aggregation rates (ΔEPI and ΔAA) (p<0.05) as shown in Table 5.
 |
Table 5 Multiple Linear Regression Analysis of Δ Platelet Aggregation Rates and Δ Value of Other Clinical and Biochemical Indicators |
Discussion
Even though all subjects in this study were overweight or obese, in the case group, the accumulation of cardiovascular risk factors in T2DM patients was more obvious, including a significant increase in blood sugar, blood pressure, blood fat, and FIB levels, which indicated that compared with those with normal glucose tolerance in the control group, T2DM patients appeared to be at higher risk of developing CVD. Furthermore, the levels of FIB and platelet activation markers and the rates of platelet aggregation in the case group were significantly higher than those in the control group, indicating a relatively high degree of functional activation of platelets, a dramatic enhancement of aggregation function, and a prothrombotic state in T2DM patients. Pearson correlation analysis showed that the platelet aggregation rates in the control group and case group (pretreatment) were positively correlated with BMI, weight, waist circumference, TCH, TG, LDL-C, FPG, HbA1c, CD62p and PAC-1, suggesting that individuals with higher BMI (obese or overweight) or hyperlipidaemia/hyperglycaemia have higher platelet activation and a higher risk of thrombosis.
The molecular mechanism of increased platelet aggregation and adhesion in T2DM patients is not yet fully understood. Existing studies have found the following: 1) insulin resistance becomes less effective in inhibiting platelet hyperfunction;19 2) in a high-glucose environment, the number of glycosylated products increases due to nonenzymatic glycosylation of the platelet membrane, which consequently reduces the membrane fluidity, promotes platelet aggregation and adhesion and improves the sensitivity of platelets to proaggregation substances;20,21 and 3) an increase in P2Y12 on platelets in patients with diabetes mellitus (DM) can induce protein kinase A (PKA)-mediated vasodilation, leading to reduced cyclic adenosine monophosphate (cAMP)-dependent phosphorylation of vasodilator-stimulated phosphoproteins (VASP-Ps) and lower bioavailability of NO in endothelial cells.20,22 Additionally, hypertriglyceridemia and very-low-density lipoprotein (VLDL) can trigger platelet hyperfunction in DM patients through interaction between apolipoprotein E (apoE) and platelet LDL receptor.21 A study23 revealed that in T2DM, the increase in the level of FIB in the peripheral blood of patients with T2DM may be caused by the combined effect of inflammatory cytokines and insulin on the liver, resulting in increased liver synthesis.
As is well known, the potential cardiovascular protective activity of GLP-1R agonists is attributed to the antihypoglycaemic effects of these agents, which help improve insulin resistance, aid weight loss, lower blood pressure, modify lipid distribution and directly act on the myocardium and blood vessel endothelium. The study by Martinez et al24 showed an average weight loss of 3.9 kg after six months of exenatide treatment. A 30-week randomized, double-blind, controlled clinical trial found that exenatide could significantly reduce SBP in patients with newly diagnosed T2DM, while DBP was also slightly reduced.25 Sun et al26 conducted a meta-analysis of 13 studies (GLP-1R agonist treatment requires at least a 6-week duration) and showed that GLP-1R agonists could moderately reduce LDL-C, TCH, and TG levels. This study found that following the 8-week exenatide treatment, the T2DM patients lost 4.8 kg, the SBP level was reduced by 5.5 mmHg, and the DBP level was slightly decreased; however, the differences in pre- and post-treatment were not statistically significant. Likewise, blood lipids were also improved, manifested by reduced TCH, TG, and LDL-C levels and an increase in HDL-C, after 8 weeks of exenatide treatment, which was basically consistent with the aforementioned findings. Presently, it is widely accepted that GLP-1R agonist-mediated SBP reduction is probably associated with weight loss, improved endothelial function, natriuresis, and relaxation of renal vascular smooth muscles.27–29
In fact, in addition to the abovementioned beneficial effects of reducing the risk for CVD, it has become a popular interest of research to investigate the effects of GLP-1R agonists on the platelet aggregation/activation function.17,18 Haemostatic time, clotting time, platelet activating markers, and the rate of platelet aggregation are common indicators for the evaluation of platelet function.30–32 The rate of platelet aggregation is considered an important indicator of platelet aggregation function, which plays an important role in the prevention, treatment, and monitoring of thrombosis. A range of specific markers, especially CD62p and PAC-1, are released in the platelet activation process.33 CD62p is a transmembrane protein stored in α granules of platelets and Weibel–Palade bodies of endothelial cells. Under normal circumstances, CD62p has only a relatively low level of endothelial surface expression. In response to stimulation, the surface expression of platelets increases sharply to mediate the adhesion function of activated endothelial cells, mononuclear cells, and neutrophils using the lectin-like, N-terminal domain. These activated cells not only promote fibrin deposition but also play a role in the inflammatory response and thrombosis. Activated platelets treated with anti-CD62p antibodies no longer have adhesive attraction to each other, which shows that as one of the important markers of platelet activation, CD62p can mediate the adhesion between activated platelets or between endothelial cells and leukocytes.23 PAC-1, as a platelet membrane glycoprotein IIb/IIIa complex and abundant platelet surface glycoprotein, consists of binding sites specific to attachment proteins such as fibrinogen, fibronectin, and von Willebrand factor (vWF). Under normal conditions, PAC-1 exists as a monomer without ligand-binding ability. Upon platelet activation, the PAC-1 receptor reveals its ligand-binding mechanism and binds to specific attachment proteins, which promotes platelet-fibrinogen-platelet aggregation and eventually contributes to platelet thrombus formation. Furthermore, PAC-1 also plays a role in intracellular signal transduction and thus is useful for the direct detection of activated platelets.34
In terms of coagulation function, this study found that after 8 weeks of exenatide treatment, the coagulation and fibrinolysis indexes PT, APTT, INR, TT, PTA, FDP, and DD did not change significantly, but the FIB level was significantly lower than before (3.38±0.78 vs 2.89±0.63, p=0.044). The results of Pearson correlation analysis also showed that ΔFIB is positively correlated with the Δ platelet aggregation rate (ΔEPI and ΔAA). To the best of our knowledge, this finding is the first such finding to be reported. It is known that FIB is a protein with coagulation function synthesized in the liver and is the coagulation factor with the highest content in plasma. As a marker of thrombosis and inflammation, FIB is an important reaction substrate for thrombosis, participates in the key steps of thrombosis, and plays a very important role in the pathogenesis of cardiovascular diseases. In order to further explore whether the increase (or decrease) in FIB is linked to platelet aggregation/thrombosis or altered susceptibility to fibrinolysis, we used a thromboelastogram (TEG) to monitor the coagulation-fibrinolysis process of 30 patients with T2DM before and after exenatide treatment. The results showed that after 8 weeks of exenatide treatment, the K (kinetics time) value of T2DM patients was prolonged (1.3 min vs 2.3 min), the α angle was reduced (70.6 deg vs 57.1 deg), the MA (maximum amplitude) value was decreased (66.9 min vs 60.9 min), and the differences were statistically significant (p<0.05, respectively) (Figure S1). The above study results suggested that the reduction in platelet aggregation rates using exenatide may be related to its inhibition of inflammation, improvement of insulin resistance, and influence on the production of liver FIB.23
In terms of platelet activation status, this study found that after 8 weeks of exenatide treatment in T2DM patients (newly diagnosed as overweight or obese), platelet activation markers represented by CD62P and PAC-1 and platelet aggregation rates induced by epinephrine and AA were significantly reduced (EPI: 77.90±6.31 vs 61.96±8.94 and AA: 76.09±3.14 vs 50.98±6.73, p<0.05, respectively). Pearson and multiple linear regression correlation analysis showed that after exenatide treatment, the Δ platelet aggregation rates (ΔEPI and ΔAA) were significantly positively correlated with ΔCD62p and ΔPAC-1, and ΔCD62p and ΔPAC-1 were independent factors affecting Δ platelet aggregation rates, indicating that exenatide can significantly inhibit the activation of platelets in patients with T2DM, thereby decreasing the ability of peripheral blood platelets to aggregate. In addition, in order to explore the effect of exenatide dosage on the coagulation and platelet aggregation function of T2DM patients, we also compared the coagulation indicators, platelet activation markers (CD62p and PAC-1) and platelet aggregation rates of 5 T2DM patients with nausea and vomiting and 25 well-tolerated T2DM patients. The results showed that there was no statistically significant difference in the above indicators between the 25 patients who received 10 μg exenatide subcutaneously in the last 4 weeks (5 μg×4 w →10 μg×4 w) and 5 patients who could not tolerate 10 μg exenatide (5 μg×4 w→ 10 μg×1 w →5 μg×3 w) (Table S1). This reminds us that in clinical work, without considering the hypoglycaemic effect, if T2DM patients cannot tolerate 10 μg exenatide, they can also be given 5 μg exenatide, which will not affect the antiplatelet effect of exenatide. Of course, this conclusion needs to be further verified by a large sample and multicentre randomized controlled trial (RCT) study.
It is worth mentioning that in our study, no significant changes in the platelet aggregation rates with ADP and collagen as inducers were observed after 8 weeks of exenatide treatment. We speculate that this may be related to the concentration of the inducers. This result suggested that there may be differences in the detection results of platelet aggregation function with different inducers in T2DM patients, the application of a single inducer may lead to bias in the study results, and detection combined with multiple inducers can better guide clinical practice. Some studies have found that when the antiplatelet aggregation function is detected by the turbidimetric method, the decrease in the platelet aggregation rate is not obvious in the early stage, but it will decrease significantly over time.35 Therefore, the platelet aggregation rates with ADP and collagen as inducers in this study might significantly decrease with the further extension of exenatide treatment, which requires further research to confirm.
In order to further understand the other influencing factors of the Δ platelet aggregation rate after treatment with the GLP-1R agonist exenatide, we also explored the correlation between Δ platelet aggregation rates and cardiovascular risk factors in T2DM patients; and the results showed that Δ platelet aggregation rates (ΔEPI and ΔAA) were positively correlated with ΔBMI, Δ waist, weight, ΔTCH, ΔTG, ΔLDL-C, ΔFPG, ΔHbA1c and ΔFIB; negatively correlated with ΔHDL-C and ΔNO; and uncorrelated with ΔSBP. Among the factors, ΔNO is an independent risk factor that affects the platelet aggregation rates, suggesting that the effect of exenatide on improving platelet function is related not only to reducing FIB levels but also to weight loss, lipid regulation, blood sugar reduction and an increase in NO concentration.
Simeone et al36 randomly assigned 62 patients (obese subjects with prediabetes or early T2DM) to the liraglutide group and the lifestyle intervention group at a 1:1 ratio, and the two groups were monitored until the weight loss goal (−7% of their initial body weight) was achieved. The results showed that U-11-dehydro-TXB2 (a metabolite of thromboxane that reflects platelet activation) was significantly reduced after achievement of the weight loss target regardless of the intervention arm, suggesting that the inhibition of platelet activation using GLP-1R agonists is related to weight loss. Dyslipidaemia is closely related to platelet activation.37 Studies have shown that dyslipidaemia, especially hypertriglyceridaemia, is directly related to platelet function.38 Ebara et al7 believe that oxidized HDL is negatively related to blood coagulation and fibrinolysis in patients with T2DM. In addition, many studies have confirmed that hyperglycaemia can have a significant adverse effect on platelet function.20,21 In addition to decreasing the fluidity of the platelet membrane and promoting platelet aggregation, coagulation factors and PAI-1 (plasminogen activator inhibitor-1) will also increase under the condition of hyperglycaemia, breaking the balance of coagulation and fibrinolysis and promoting thrombosis.39 Besides improvements in blood sugar control and weight loss, the inhibitory effect of GLP-1R agonists on platelets has been verified in animal models17,18 and healthy volunteers.40 Therefore, GLP-1R agonists have the potential, at least in theory, to regulate platelet activation directly through the effect on platelet function and indirectly through the control of body weight and metabolism. The above arguments further support the results of this study.
NO, which can relax vascular smooth muscle and dilate blood vessels, is the most important vasodilator factor secreted by vascular endothelial cells. Liraglutide has been reported to inhibit platelet activation in animal models17 and healthy volunteers40 by increasing the concentration of NO. In vitro studies have also confirmed that liraglutide can increase the expression of endothelial nitric oxide synthase (eNOS) and reduce the expression of inducible nitric oxide synthase (iNOS) at the levels of transcription and translation by inhibiting nuclear factor kappa B (NF-p65) phosphorylation to improve endothelial cell function and prevent diabetic atherosclerosis.41 Similarly, Jia and colleagues also confirmed that GLP-1R agonists regulate platelet activity by inducing eNOS-dependent mechanisms, increase the bioavailability of NO in vascular endothelial cells, and improve vascular function.18 The above study results all suggest that GLP-1R agonists reduce platelet activation and inhibit thrombosis by enhancing the production and utilization of NO, which is basically consistent with the results of this study.
In fact, the molecular mechanism of the effect of GLP-1R agonists on platelet aggregation/activation function has not yet been elucidated. It is currently recognized that the adhesion/aggregation process of platelets is regulated by the balance between procoagulant and anti-aggregation circulatory agents (such as NO). NO stimulates soluble guanylate cyclase (sGC) in platelets, activates cyclic guanosine monophosphate (cGMP) and protein kinase G (PKG), and then inhibits platelet activation through various pathways. In addition, cGMP can indirectly increase cellular cAMP levels by inhibiting 3ʹphosphodiesterase to activate PKA42 and induce eNOS activity.43 The synergistic effect of cGMP and cAMP inhibits platelet aggregation.44,45 Second, cGMP can also inhibit the activation of phosphoinositide 3-kinase (PI3K),46 causing the activation of the GP IIb-IIIa fibrinogen receptor.47 In addition to the cGMP-dependent pathway described above, there is evidence that NO can also regulate platelet function independently of cGMP without being affected by sGC.48–50 Therefore, any change in eNOS activity or/and NO bioavailability by GLP-1R agonists is a key factor in determining platelet function.18,51
Conclusion
In short, this study found that the GLP-1R agonist exenatide can significantly reduce the platelet activation state and inhibit the platelet aggregation rate in T2DM patients. This effect may be related to weight loss, blood sugar reduction, lipid regulation, decrease in FIB and increase in NO levels; and the levels of ΔNO, ΔCD62p, and ΔPAC-1 are independent factors that affect the Δ platelet aggregation rates. Of course, there are many shortcomings in this study, such as the study being a single-centre small sample study, a lack of a placebo control, a short observation time, and all subjects being overweight/obese patients with T2DM. Whether the results can be extended to nonobese patients with T2DM still needs further study.
Abbreviations
ADP, adenosine diphosphate; AA, arachidonic acid; APTT, activated partial thromboplastin time; BMI, body mass index; COLL, collagen; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; DD, D-dimer; DBP, diastolic blood pressure; EPI, epinephrine; FPG, fasting plasma glucose; FIB, fibrinogen; FDP, fibrin degradation products; GLP-1R, glucagon-like peptide-1 receptor; HDL-C, high-density lipoprotein cholesterol; HbA1c, hemoglobin A1c; INR, international normalized ratio; LDL-C, low-density lipoprotein cholesterol; NO, nitric oxide; PLT, platelets; PT, prothrombin time; PTA, prothrombin time activity; PAC-1, platelet activation complex-1; PKG, protein kinase G; PI3K, phosphoinositide 3-kinase; PRP, platelet-rich plasma; PPP, platelet-poor plasma; RCT, randomized controlled trial; SBP, systolic blood pressure; sGC, soluble guanylate cyclase; TCH, total cholesterol; TG, triglycerides; TT, thrombin time; T2DM, type 2 diabetes mellitus; TGE, thromboelastogram.
Data Sharing Statement
The data sets used to support the findings of this study are available from the corresponding authors upon request.
Ethics Approval
According to the ethical principles of the “Measures for the Ethical Review of Biomedical Research Involving Humans”, the WMA “Declaration of Helsinki” and the CIOMS “International Ethical Guidelines for Human Biomedical Research”, the study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Anhui Medical University.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Acknowledgments
The authors thank the laboratory of Anhui Medical University and the participants of this study including statistician, doctors, nurses, and researchers from the First Affiliated Hospital of Anhui Medical University.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This work was supported by the Natural Foundation of Anhui Province [NO.1808085MH279] and Discipline Construction Project of Anhui Medical University Project number [NO. 2021lcxk004].
Disclosure
The authors declare no conflict of interest.
References
1. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287(19):2570–2581. doi:10.1001/jama.287.19.2570
2. Chilton R, Wyatt J, Nandish S, Oliveros R, Lujan M. Cardiovascular comorbidities of type 2 diabetes mellitus: defining the potential of glucagonlike peptide-1-based therapies. Am J Med. 2011;124(1 Suppl):S35–53. doi:10.1016/j.amjmed.2010.11.004
3. Vaidyula VR, Rao AK, Mozzoli M, Homko C, Cheung P, Boden G. Effects of hyperglycemia and hyperinsulinemia on circulating tissue factor procoagulant activity and platelet CD40 ligand. Diabetes. 2006;55(1):202–208. [PMID: 16380494]. doi:10.2337/diabetes.55.01.06.db05-1026
4. Mylotte D, Kavanagh GF, Peace AJ, et al. Platelet reactivity in type 2 diabetes mellitus: a comparative analysis with survivors of myocardial infarction and the role of glycaemic control. Platelets. 2012;23(6):439–446. doi:10.3109/09537104.2011.634932
5. Angiolillo DJ, Bernardo E, Sabaté M, et al. Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2007;50(16):1541–1547. doi:10.1016/j.jacc.2007.05.049
6. Strain WD, Paldánius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 2018;17(1):57. doi:10.1186/s12933-018-0703-2
7. Ebara S, Marumo M, Mukai J, Ohki M, Uchida K, Wakabayashi I. Relationships of oxidized HDL with blood coagulation and fibrinolysis in patients with type 2 diabetes mellitus. J Thromb Thrombolysis. 2018;45(2):200–205. doi:10.1007/s11239-017-1594-x
8. Osende JI, Fuster V, Lev EI, et al. Testing platelet activation with a shear-dependent platelet function test versus aggregation-based tests: relevance for monitoring long-term glycoprotein IIb/IIIa inhibition. Circulation. 2001;103(11):1488–1491. doi:10.1161/01.cir.103.11.1488
9. Zhou AM, Xiang YJ, Liu EQ, et al. Salvianolic acid a inhibits platelet activation and aggregation in patients with type 2 diabetes mellitus. BMC Cardiovasc Disord. 2020;20(1):15. doi:10.1186/s12872-019-01316-z
10. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi:10.1056/NEJMoa1603827
11. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi:10.1056/NEJMoa1607141
12. Lee YS, Jun HS. Anti-inflammatory effects of glp-1-based therapies beyond glucose control. Mediators Inflamm. 2016;2016:3094642. doi:10.1155/2016/3094642
13. Chaudhuri A, Ghanim H, Vora M, et al. Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab. 2012;97(1):198–207. doi:10.1210/jc.2011-1508
14. De Ciuceis C, Agabiti-Rosei C, Rossini C, et al. Microvascular density and circulating endothelial progenitor cells before and after treatment with incretin mimetics in diabetic patients. High Blood Press Cardiovasc Prev. 2018;25(4):369–378. doi:10.1007/s40292-018-0279-7
15. Fadini GP, Miorin M, Facco M, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45(9):1449–1457. doi:10.1016/j.jacc.2004.11.067
16. Steven S, Jurk K, Kopp M, et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br J Pharmacol. 2017;174(12):1620–1632. doi:10.1111/bph.13549
17. Cameron-Vendrig A, Reheman A, Siraj MA, et al. Glucagon-like peptide 1 receptor activation attenuates platelet aggregation and thrombosis. Diabetes. 2016;65(6):1714–1723. doi:10.2337/db15-1141
18. Jia G, Aroor AR, Sowers JR. Glucagon-like peptide 1 receptor activation and platelet function: beyond glycemic control. Diabetes. 2016;65(6):1487–1489. doi:10.2337/dbi16-0014
19. Vinik AI, Erbas T, Park TS, Nolan R, Pittenger GL. Platelet dysfunction in type 2 diabetes. Diabetes Care. 2001;24(8):1476–1485. doi:10.2337/diacare.24.8.1476
20. Carrizzo A, Izzo C, Oliveti M, et al. The main determinants of diabetes mellitus vascular complications: endothelial dysfunction and platelet hyperaggregation. Int J Mol Sci. 2018;19(10):10. doi:10.3390/ijms19102968
21. Rollini F, Franchi F, Muñiz-Lozano A, Angiolillo DJ. Platelet function profiles in patients with diabetes mellitus. J Cardiovasc Transl Res. 2013;6(3):329–345. doi:10.1007/s12265-013-9449-0
22. Suslova TE, Sitozhevskii AV, Ogurkova ON, et al. Platelet hemostasis in patients with metabolic syndrome and type 2 diabetes mellitus: cGMP- and NO-dependent mechanisms in the insulin-mediated platelet aggregation. Front Physiol. 2014;5:501. doi:10.3389/fphys.2014.00501
23. Pretorius L, Thomson GJA, Adams RCM, Nell TA, Laubscher WA, Pretorius E. Platelet activity and hypercoagulation in type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):141. doi:10.1186/s12933-018-0783-z
24. Gorgojo-Martínez JJ, Gargallo-Fernández MA, Brito-Sanfiel M, Lisbona-Catalán A. Real-world clinical outcomes and predictors of glycaemic and weight response to exenatide once weekly in patients with type 2 diabetes: the CIBELES project. Int J Clin Pract. 2018;72(3):e13055. doi:10.1111/ijcp.13055
25. Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372(9645):1240–1250. doi:10.1016/S0140-6736(08)61206-4
26. Sun F, Wu S, Wang J, et al. Effect of glucagon-like peptide-1 receptor agonists on lipid profiles among type 2 diabetes: a systematic review and network meta-analysis. Clin Ther. 2015;37(1):225–241.e228. doi:10.1016/j.clinthera.2014.11.008
27. Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016;24(1):15–30. doi:10.1016/j.cmet.2016.06.009
28. Sivertsen J, Rosenmeier J, Holst JJ, Vilsbøll T. The effect of glucagon-like peptide 1 on cardiovascular risk. Nat Rev Cardiol. 2012;9(4):209–222. doi:10.1038/nrcardio.2011.211
29. Lovshin JA, Barnie A, DeAlmeida A, Logan A, Zinman B, Drucker DJ. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care. 2015;38(1):132–139. doi:10.2337/dc14-1958
30. Gresele P. Diagnosis of inherited platelet function disorders: guidance from the SSC of the ISTH. J Thromb Haemost. 2015;13(2):314–322. doi:10.1111/jth.12792
31. Huskens D, Sang Y, Konings J, et al. Standardization and reference ranges for whole blood platelet function measurements using a flow cytometric platelet activation test. PLoS One. 2018;13(2):e0192079. doi:10.1371/journal.pone.0192079
32. Asare R, Opoku-Okrah C, Danquah KO, et al. Assessment of platelet indices and platelet activation markers in children with Plasmodium falciparum malaria. Malar J. 2020;19(1):143. doi:10.1186/s12936-020-03218-4
33. Hou M, Yu J, Zhang L, Liu C. [Changes and significance of P-selectin and PAC-1 in coronary heart disease before and after stenting]. Zhonghua Yi Xue Za Zhi. 2014;94(10):766–768. PMID: 24844962. [Chinese]
34. Caron A, Théorêt JF, Mousa SA, Merhi Y. Anti-platelet effects of GPIIb/IIIa and P-selectin antagonism, platelet activation, and binding to neutrophils. J Cardiovasc Pharmacol. 2002;40(2):296–306. doi:10.1097/00005344-200208000-00015
35. Satoh K, Ozaki Y. [Attempts for aspirin monitoring with a new assay system, Ultegra Rapid Platelet Function Assay (RPFA), based on turbidimetric platelet agglutination of whole blood samples]. Rinsho Byori. 2006;54(6):576–582. PMID: 16872006. [Japanese]
36. Simeone P, Liani R, Tripaldi R, et al. Thromboxane-Dependent Platelet Activation in Obese Subjects with Prediabetes or Early Type 2 Diabetes: effects of Liraglutide- or Lifestyle Changes-Induced Weight Loss. Nutrients. 2018;10(12):1872. doi:10.3390/nu10121872
37. Paes AMA, Gaspar RS, Fuentes E, Wehinger S, Palomo I, Trostchansky A. Lipid metabolism and signaling in platelet function. Adv Exp Med Biol. 2019;1127:97–115. doi:10.1007/978-3-030-11488-6_7
38. Boulet MM, Cheillan D, Di Filippo M, et al. Large triglyceride-rich lipoproteins from fasting patients with type 2 diabetes activate platelets. Diabetes Metab. 2020;46(1):54–60. doi:10.1016/j.diabet.2019.03.002
39. Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17(1):121. doi:10.1186/s12933-018-0763-3
40. Barale C, Buracco S, Cavalot F, Frascaroli C, Guerrasio A, Russo I. Glucagon-like peptide 1-related peptides increase nitric oxide effects to reduce platelet activation. Thromb Haemost. 2017;117(6):1115–1128. doi:10.1160/TH16-07-0586
41. Dai Y, Mehta JL, Chen M. Glucagon-like peptide-1 receptor agonist liraglutide inhibits endothelin-1 in endothelial cell by repressing nuclear factor-kappa B activation. Cardiovasc Drugs Ther. 2013;27(5):371–380. doi:10.1007/s10557-013-6463-z
42. Maurice DH, Haslam RJ. Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylate cyclase: inhibition of cyclic AMP breakdown by cyclic GMP. Mol Pharmacol. 1990;37(5):671–681. PMID: 2160060.
43. Santilli F, Simeone P, Liani R, Davì G. Platelets and diabetes mellitus. Prostaglandins Other Lipid Mediat. 2015;120:28–39. doi:10.1016/j.prostaglandins.2015.05.002
44. Bowen R, Haslam RJ. Effects of nitrovasodilators on platelet cyclic nucleotide levels in rabbit blood; role for cyclic AMP in synergistic inhibition of platelet function by SIN-1 and prostaglandin E1. J Cardiovasc Pharmacol. 1991;17(3):424–433. doi:10.1097/00005344-199103000-00011
45. Radomski MW, Palmer RM, Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987;92(3):639–646. doi:10.1111/j.1476-5381.1987.tb11367.x
46. Pigazzi A, Heydrick S, Folli F, Benoit S, Michelson A, Loscalzo J. Nitric oxide inhibits thrombin receptor-activating peptide-induced phosphoinositide 3-kinase activity in human platelets. J Biol Chem. 1999;274(20):14368–14375. doi:10.1074/jbc.274.20.14368
47. Zhang J, Zhang J, Shattil SJ, Cunningham MC, Rittenhouse SE. Phosphoinositide 3-kinase gamma and p85/phosphoinositide 3-kinase in platelets. Relative activation by thrombin receptor or beta-phorbol myristate acetate and roles in promoting the ligand-binding function of alphaIIbbeta3 integrin. J Biol Chem. 1996;271(11):6265–6272. doi:10.1074/jbc.271.11.6265
48. Tsikas D, Ikic M, Tewes KS, Raida M, Frölich JC. Inhibition of platelet aggregation by S-nitroso-cysteine via cGMP-independent mechanisms: evidence of inhibition of thromboxane A2 synthesis in human blood platelets. FEBS Lett. 1999;442(2–3):162–166. doi:10.1016/s0014-5793(98)01633-0
49. Sogo N, Magid KS, Shaw CA, Webb DJ, Megson IL. Inhibition of human platelet aggregation by nitric oxide donor drugs: relative contribution of cGMP-independent mechanisms. Biochem Biophys Res Commun. 2000;279(2):412–419. doi:10.1006/bbrc.2000.3976
50. Beghetti M, Sparling C, Cox PN, Stephens D, Adatia I. Inhaled NO inhibits platelet aggregation and elevates plasma but not intraplatelet cGMP in healthy human volunteers. Am J Physiol Heart Circ Physiol. 2003;285(2):H637–642. doi:10.1152/ajpheart.00622.2002
51. Barale C, Frascaroli C, Cavalot F, Guerrasio A, Russo I. In Type 2 diabetes mellitus the glp-1 effects on platelets are impaired. Atherosclerosis. 2016;252:e257–e258. doi:10.1016/j.atherosclerosis.2016.07.081
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.