Back to Journals » Drug Design, Development and Therapy » Volume 16
Effects of Ethanolic Extract of Schisandra sphenanthera on the Pharmacokinetics of Rosuvastatin in Rats
Received 26 February 2022
Accepted for publication 10 May 2022
Published 17 May 2022 Volume 2022:16 Pages 1473—1481
DOI https://doi.org/10.2147/DDDT.S364234
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Qing Sun,1 Li Li,1 Quan Zhou2
1Department of Pharmacy, Zhejiang Hospital, Hangzhou, People’s Republic of China; 2Department of Pharmacy, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, People’s Republic of China
Correspondence: Quan Zhou, Department of Pharmacy, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Jiefang Road No. 88, Shangcheng District, Hangzhou, 310009, Zhejiang Province, People’s Republic of China, Tel +86 571 89713473, Email [email protected] Li Li, Department of Pharmacy, Zhejiang Hospital, No. 12 Lingyin Road, Hangzhou, 310013, Zhejiang Province, People’s Republic of China, Email [email protected]
Purpose: Wuzhi capsule (WZ) is a proprietary Chinese medicine prepared from the ethanolic extract of Schisandra sphenanthera that is commonly used to treat liver injury. Statins are widely used in patients with hyperlipidemia, coronary heart disease, metabolic syndrome, type 2 diabetes mellitus, and nonalcoholic fatty liver disease. Co-administration of statins with WZ is possible in clinical practice. WZ has obvious inhibitory effects on the bioavailability of atorvastatin and simvastatin; however, the drug–herb interactions between WZ and rosuvastatin have not been addressed. We explored the effects of WZ on the pharmacokinetics of rosuvastatin in Sprague-Dawley rats to promote a rational use of statins.
Methods: Eighteen male rats were randomly and evenly divided into three groups: control group (gavage feeding of rosuvastatin 10 mg·kg− 1), single dose group (gavage feeding of a single dose of WZ 150 mg·kg− 1 followed by rosuvastatin 10 mg·kg− 1) and multiple doses group (gavage feeding of WZ 150 mg·kg− 1 for 7 days followed by rosuvastatin 10 mg·kg− 1 on the seventh day). Plasma samples were collected at different times before and after rosuvastatin administration. The other 18 female rats were tested the same way as the male rats. All samples were analyzed by a validated LC-MS/MS method, and the pharmacokinetic parameters were calculated using a non-compartmental model.
Results: In both male and female rats, there were no statistically significant differences in rosuvastatin pharmacokinetic parameters between the control group, the single dose group, and the multi-dose group.
Conclusion: Acute or long-term intake of WZ had no obvious effect on the pharmacokinetics of rosuvastatin, and therefore rosuvastatin could be used as an alternative to atorvastatin and simvastatin when WZ is clinically required in conjunction with statins. An appropriate pharmacodynamic study is needed to encourage the safe use of this combination.
Keywords: herb-drug interaction, hyperlipidemia, metabolic syndrome, pharmacokinetics, rosuvastatin, Wuzhi capsule
Introduction
Wuzhi capsule (WZ) is a preparation derived from the ethanol extract of Schisandra sphenanthera and is widely used in clinical practice in China to protect liver function in patients with chronic hepatitis or liver dysfunction. WZ attenuates liver steatosis and inflammation during the development of nonalcoholic fatty liver disease.1
Statins are widely used in the secondary and primary prevention of atherosclerotic cardiovascular disease. Furthermore, a meta-analysis showed beneficial effects of statin use in reducing inflammatory markers in patients with metabolic syndrome and related disorders.2 An exploratory analysis of data from 5 trials showed that rosuvastatin 10 mg was highly effective in comprehensively modifying the lipid profile of patients with high low-density lipoprotein cholesterol and metabolic syndrome.3 Statins can significantly increase the estimated rate of glomerular filtration, reduce serum creatinine, decrease the level of high-sensitivity C-reactive proteins and decrease the level of blood lipids in the treatment of diabetic nephropathy, thus reducing the inflammatory response and protecting the kidney.4 Stain therapy has favorable effects in hypercholesterolemic patients with regard to the atherogenic dyslipidemia associated with metabolic syndrome. Rosuvastatin had the most favorable effect on the overall atherogenic lipid profile of metabolic syndrome.5 Risk reducing statin therapy is recommended for almost all patients with type 2 diabetes mellitus (DM2) at 40 years of age or older regardless of cholesterol level.6 Clinicians should consider intensifying statin regimens to improve dyslipidemia control in statin-treated patients with DM2.7
Hepatic adverse effects are the most known adverse effects induced by statins.8 In approximately 1% of patients, statins cause asymptomatic and dose-related elevations in serum transaminases greater than 3 times the upper limit of normal. Avoiding statin use is unnecessary in patients with nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, compensated cirrhosis, and compensated chronic liver disease if there is a clear indication.9 Statins can delay the progression of hepatic fibrosis, prevent hepatic decompensation in cirrhosis, and reduce all-cause mortality in patients with chronic liver disease.10
In clinical practice, WZ and statins can be prescribed together to prevent statin-induced liver injury, to continue statin use in mild liver insufficiency, or to treat chronic liver diseases such as nonalcoholic fatty liver disease. Schisandrin B, one of the main active ingredients of WZ, interferes with the in vitro uptake of rosuvastatin mediated by the organic anion transporting polypeptide 1B1 (OATP1B1) and significantly inhibits the transport function of the transgenic breast cancer resistance protein (BCRP) cell line.11,12 Rosuvastatin is a typical substrate of both OATP1B1 and BCRP.13,14 Therefore, there is a possibility of herb-drug interaction between WZ and rosuvastatin in vivo. However, literature on this issue is not currently available. In this study, we explored the effects of WZ on the pharmacokinetics of rosuvastatin in Sprague-Dawley (SD) rats and tried to promote rational use of statins.
Methods
Reagents, Materials, and Chemicals
The reference standards for calcium rosuvastatin (purity ≥98%, lot A29O7E23856) were supplied by Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China.). The reference standards of lovastatin (purity>98%, lot L1507002) were used as the internal standard (IS) for chromatographic analysis and were purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. Ultrapure water was obtained from a Milli-Q Plus water purification system (Millipore, Bedford, MA, USA). Methanol, acetonitrile, and formic acid were of HPLC grade and were purchased from Merck, Germany.
Instrumentation and LC -MS-MS Conditions
A liquid chromatography, electrospray ionization, tandem mass spectrometry (LC-ESI-MS/MS) system consists of an Agilent 1260 HPLC system (Agilent Technologies Inc., USA), AB SCIEX API 4000+TM triple-quadruple mass spectrometer (Applied Biosystems, USA).
Separation was achieved on a 3.5 μm (2.1 mm ×100 mm, i.d.) Zorbax Eclipse Plus C18 column (Agilent Technologies Inc., USA). The mobile phase was a mixture of water (containing 0.1% formic acid)- acetonitrile (containing 0.1% formic acid) (10:90, v/v) pumped at a flow rate of 0.3 mL/min. Column oven was kept at 20°C. The autosampler was set at 4°C. Total run time was 6.5 min for each injection. Mass spectrometric analysis was performed in the positive-ion (ESI) and multiple reaction monitoring (MRM) acquisition mode. The mass spectrometer was set to monitor the transitions of precursors to product ions as follows: m/z 482.0→258.2 for rosuvastatin and m/z 427.4→325.3 for IS. Ion source gas 1 (GS1): 482.7kPa; curtain gas: 206.9kPa; collision gas (CAD): 34.5kPa; ion source gas 2 (GS2): 482.7kPa. The spray voltage was set at 5.5 kV and source temperature was 350°C. Declustering potential (DP): 96 eV for rosuvastatin and lovastatin; entrance potential (EP): 10 eV for rosuvastatin and IS. The optimized collision energy was 45 eV for rosuvastatin and 31 eV for IS. Collision cell exit potential (CXP):14 eV for rosuvastatin and 8 eV for IS.
Method Validation
The method was validated for specificity, linearity, sensitivity, accuracy, precision, absolute recovery, matrix effect, and stability according to the FDA guidelines for validation of bioanalytical methods.15
Specificity
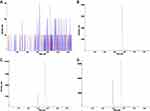
The specificity was investigated by preparing and analyzing six individual blank plasma samples from rats, a standard plasma sample of rosuvastatin and a real plasma sample from rats after rosuvastatin administration.
Linearity
Linearity was assessed by preparing and analyzing a blank sample and standard rosuvastatin samples in the range ~2–2000μg·L−1. Calibration curves were constructed from the peak area ratios of rosuvastatin to IS vs plasma concentrations using a weighted linear least-squares regression model. The correlation coefficient should not exceed 0.99, and the relative error and precision of all calibration standards must be within 15%, except at the lower limit of quantification (LLOQ), where it should not deviate by more than ±20% and rosuvastatin could be detected with a signal-to-noise ratio of 5.
Accuracy and Precision
Accuracy and precision were assessed by evaluating quality control (QC) samples at four concentration levels (six samples for each concentration) on six different validation days. Precision was determined as the relative standard deviation (RSD), and accuracy was expressed as a percentage of the measured concentration over the nominal (theoretical) concentration. The RSD did not exceed 15% and the accuracy was within 15% of the actual value.
Recovery
The absolute recovery (extraction efficiency) of analytes from rat plasma was determined by comparing the peak areas of five processed low, medium and high QC samples with those of the non-extracted pure standard that represent 100% recovery at the same theoretical concentrations of rosuvastatin.
Matrix Effects
Matrix effects were quantitatively assessed using five lots of blank matrix at low, medium, and high QC concentrations (4, 160, 1600 μg·L−1). Three different sets of solutions were prepared: a pure standard solution of the analyte and IS in quintuplicate in the mobile phase injected directly into the column (A), five different blank plasma spiked with the analyte and IS after extraction (B), and five different blank plasma spiked with the analyte and IS before extraction (C). The matrix factor (MF) was calculated for each lot of matrix, by calculating the ratio of the peak area in the presence of matrix (B) to the peak area in the absence of matrix (A). The IS normalized MF was calculated by dividing the MF of the analyte by the MF of the IS. The coefficient of variation (CV) of the IS-normalized MF calculated from the five lots of matrix should not be greater than 15%.
Stability
The stability of rosuvastatin in QC samples (4, 160, 1600 μg·L−1) was validated by four studies: short-term stability for 12 h in plasma, 30 days’ stability at –20°C, three freeze-thaw cycles and autosampler stability of 24 h of QC samples. The samples were concluded to be stable if the bias of the stability samples was not more than ±15% of the nominal concentration.
Assay of Plasma Concentrations of Rosuvastatin
A volume of 10 μL of the working solution of lovastatin (1mg·L−1 in methanol) was added to each Eppendorf tube (1.5 mL) containing 100μL of rat plasma, vortexed for 1 min; then 500 μL of methyl tert-butyl ether (containing 0.1% formic acid) was added to the samples. Samples were shaken for 5 min and centrifuged for 8 min at 10,000 r·min−1 (5415R centrifuge; Eppendorf, Germany). Then 450 μL of the supernatant was then transferred to another tube where it was evaporated to complete dryness under a gentle stream of nitrogen gas by decompression at room temperature. The samples were reconstituted with 100 μL of mobile phase, vortexed for 5 min, and centrifuged for 8 min at 10,000 r·min−1. A volume of 10 μL of the supernatant was injected directly into the LC/MS/MS system for analysis.
In vivo Study
Subjects
Healthy and clean female and male SD rats, weighing ~220–250g, were purchased from the Zhejiang Academy of Medical Sciences. The study was approved by the Animal Ethics Committee of the Zhejiang Academy of Medical Sciences (approval number: 2018–100).
Drug Treatments
The calcium tablet of rosuvastatin (lot 1902A44) was supplied by AstraZeneca Pharmaceutical Co., Ltd. WZ (lot180301), which contains 11.25 mg of Schisantherin A per capsule, was purchased from Sichuan Hezheng Pharmaceutical Co., Ltd. (China). Rosuvastatin calcium gavage solution was prepared by dissolving the rosuvastatin calcium tablet powder in suspension with 0.5% sodium carboxymethylcellulose solution (25 mg/25mL) by ultrasound. The WZ gavage solution was prepared by dissolving the WZ contents after removing the capsule shell into suspension with 0.5% sodium carboxymethylcellulose solution (375.0 mg/25 mL) by ultrasound.
Study Design
Eighteen SD male rats were randomly divided into three groups. Adaptive feeding was performed for 1 week before the experiment. The rats fasted for 12 h and were given free access to water. The control group received rosuvastatin 10 mg·kg−1 by gavage feeding. The single-dose group received a single dose of WZ contents 150 mg·kg−1 by gavage feeding, then took rosuvastatin 10 mg·kg−1 by gavage feeding 15 minutes later. The multiple dose group received consecutive daily doses of WZ contents 150 mg·kg−1 by gavage feeding for 7 days, then took rosuvastatin 10 mg·kg−1 by gavage feeding 15 minutes later the seventh day. Furthermore, 18 SD female rats were randomly divided into three groups and treated the same way as SD male rats.
Sample Collection
Approximately 0.5 mL of blood was collected from the orbital venous plexus of rats before the intake of rosuvastatin and at 0.5, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 8.0, 10.0, 12.0 and 24.0 h after the dosing of rosuvastatin. Blood samples were placed in heparinized tubes and centrifuged at 3000 ×g for 10 min, and plasma was separated and stored at –20°C until assays were performed.
Statistics
Pharmacokinetic parameters were calculated using PhoenixTM WinNonlin® software (Version 6.1, Pharsight, Mountain View, California, USA) with the non-compartmental method. The maximum plasma concentrations (Cmax) and the times at which they occurred (Tmax) were determined by inspection of the plasma concentration-time profile. The terminal elimination rate constant (λz) was determined by linear regression of the terminal portion of the log concentration-time profile. The elimination half-life (T1/2) was calculated as 0.693/λz. The area under the plasma concentration-time curve (AUC) was determined by trapezoidal rule and extrapolated to infinity by calculation of Ct/λz. The pharmacokinetic parameters between groups were statistically analyzed using SPSS 21.0 software using a paired t test (two-tailed). Data were reported as mean ± standard deviation (SD). A P-value < 0.05 was considered statistically significant.
Results
Validation of the Bioanalytical Method
The assay method was specific. Endogenous chemicals did not interfere with the determination of rosuvastatin and IS (Figure 1). The calibration curves were linear in the range of ~2–2000 μg·L−1 (r2=0.9986, n=6), with a LLOQ of 2 μg·L−1 (RSD=7.75%, n=6). The within-and between-day CV of the QC samples at low, medium, and high concentrations were less than 10%. The average recovery of rosuvastatin was 100.1%, whereas the average absolute recoveries of rosuvastatin and IS were 91.9% and 92.5%, respectively. The CV of the IS-normalized MF did not exceed 9.36% for rosuvastatin. No significant matrix effects were observed. The stability of rosuvastatin in plasma was confirmed by four studies: short-term stability for 12 h in plasma, 30-day stability at –20°C, three freeze-thaw cycles and autosampler stability for 24 h of QC samples. All bias values between the measured value and the nominal value were in the range of −13.14% to 6.99%. Specificity, linearity, sensitivity, accuracy, precision, recovery, stability, and matrix effects met the requirements for a pharmacokinetic study.
Concentration-Time Curves and Pharmacokinetics of Rosuvastatin
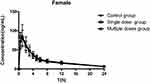
The mean plasma rosuvastatin concentration time profiles in male and female rats are shown in Figures 2 and 3, respectively. The main pharmacokinetic parameters in male rats and female rats are given in Tables 1 and 2, respectively. For both male rats and female rats, there were no statistically significant differences in pharmacokinetic parameters between the control group, the single-dose group and the multidose group. Furthermore, there was no statistically significant difference in rosuvastatin pharmacokinetic parameters between the male control group and the female control group.
 |
Table 1 Pharmacokinetic Parameters of Rosuvastatin in Male Rats |
 |
Table 2 Pharmacokinetic Parameters of Rosuvastatin in Female Rats |
Discussion
Rosuvastatin is minimally metabolized by cytochrome P450 isozymes (CYP). It is eliminated by biliary excretion.16 The hepatic uptake of rosuvastatin is mediated by OATP1B1 whereas the biliary excretion mechanism is related with BCRP.13,14 There are inconsistent findings on whether P-glycoprotein (P-gp) contributes to rosuvastatin pharmacokinetics.17–19
The main active ingredients of WZ include Schisandrin A, Schisandrin B, Schisantherin A, Schisandrin C, Schisandrol A, and Schisandrol B.20 Each capsule of WZ contains 11.25 mg Schisantherin A and the content of Schisandrin A was used as a quality control indicator. Studies have found that lignans in Schisandra sphenanthera have an inhibitory effect on CYP3A4 and have an obvious effect on tacrolimus and cyclosporine pharmacokinetics.21,22 Furthermore, Schisandrin A could inhibit P-gp-mediated drug transport at gene and protein levels.23 Schisandrin A and Schisandrin B could induce OATP1B1 expression and increase its transporter activity in the human hepatocellular liver carcinoma cell line.24 Schisandrin B could promote the in vitro uptake of rosuvastatin mediated by OATP1B1.11 Schisandrin A is a substrate of BCRP and can be transported by BCRP into Lilly Laboratories cell porcine kidney 1 (LLC-PK1) /BCRP cells whereas Schisandrol B could exhibit a marked inhibitory effect on BCRP transport function.12 Therefore, WZ ingestion may interfere OATP1B1 and/or BCRP-mediated drug transportation of rosuvastatin in vivo.
To our knowledge, this is the first study to investigate the pharmacokinetic interaction between WZ and rosuvastatin in vivo. WZ had no effect on the main pharmacokinetic parameters of rosuvastatin, which could be explained by the combined effect of WZ on efflux transporters (ie, BCRP, P-gp) and the active uptake transporter OATP1B1 in rats. Pharmacokinetic studies showed that WZ had obvious inhibitory effects on the bioavailability of atorvastatin in female rats but not in male rats,25 and a single dose of WZ could affect the pharmacokinetic parameters of simvastatin and simvastatin acid in rats.26 Atorvastatin and simvastatin are extensively metabolized by CYP3A4, therefore their fate in the body is prone to lignans in Schisandra sphenanthera. It indicates that rosuvastatin could be an alternative to atorvastatin and simvastatin in the case of adverse drug reactions caused by significant pharmacokinetic interactions when the combination of WZ and statin is clinically needed.
There was no statistically significant sex-based difference in the pharmacokinetic parameters of rosuvastatin after rosuvastatin alone administration. The results of this study are consistent with the findings that rosuvastatin does not produce clinically significant pharmacokinetic differences in Chinese patients of different sex.19,27
The limitations of this study are the following. First, the dose effects of rosuvastatin on the pharmacokinetic interaction between herb and drug were not investigated. We tested the DDI potential at the dose of rosuvastatin 10 mg/kg according to the study by Liu et al28 but did not investigate the effects at different doses of rosuvastatin (eg, 0.83 mg kg−1, 5 mg kg−1, or 100 mg kg−1).29–31 Secondly, we selected rosuvastatin powder rather than rosuvastatin tablet formulation, which could overcome any bias in the influence of excipients in pharmaceutical preparations of rosuvastatin. However, the administration of rosuvastatin in the control group, single-dose group, and multigroup was consistent; therefore, the relevant bias was negligible. Third, the interaction between cell-based metabolism and in vitro transport tests has not been studied, and information about whether the drug transport mediated by specific transporters is influenced by WZ has not been defined, but it is expected to be explored in future studies. Fourth, there has been no study of rosuvastatin on the pharmacokinetics of active herbal ingredients of WZ. The pharmacokinetic study of herbs could also be considered of great importance, and understanding the interaction between herbal medicine and chemical drugs should be further deepened. Finally, although our study provides some implications for rational drug use in clinical practice, it focused only on the pharmacokinetic interaction between WZ and rosuvastatin. It is necessary to perform an appropriate pharmacodynamic study to confirm whether there are changes in pharmacodynamic activity and thus provide additional support for our findings encouraging the safe use of this combination. Additionally, this DDI study was conducted in healthy rats, and the circumstances of moderate hepatic impairment need to be further investigated. Extension of the results of pharmacokinetic interactions in rats to clinical patients must be confirmed by randomized clinical trials or real-world data in drug combination therapy.
Conclusions
In this study, WZ, a proprietary Chinese medicine prepared from the ethanolic extract of S. sphenanthera, had no obvious negative effect on the pharmacokinetics of rosuvastatin, and thus rosuvastatin can be used as an alternative to atorvastatin when WZ is clinically indicated together with statins. A suitable pharmacodynamic study is needed to encourage the safe use of this combination.
Data Sharing Statement
Data will be available from the corresponding author upon request.
Ethics Approval and Consent to Participate
The study protocol for animal experiments was approved by the Experimental Animal Welfare Ethics Committee of the Zhejiang Academy of Medical Sciences (approval number: 2018-100) and conformed to the guidelines of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Every effort was made to minimize the pain, suffering, and death of animals.
Acknowledgments
We thank Hui-chao Chang and Pei-fang Huang for their kind help.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no potential conflicts of interest in this work.
References
1. Chen Z, Liu F, Zheng N, et al. Wuzhi capsule (Schisandra Sphenanthera extract) attenuates liver steatosis and inflammation during non-alcoholic fatty liver disease development. Biomed Pharmacother. 2019;110:285–293. doi:10.1016/j.biopha.2018.11.069
2. Tabrizi R, Tamtaji OR, Mirhosseini N, et al. The effects of statin use on inflammatory markers among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2019;141:85–103. doi:10.1016/j.phrs.2018.12.010
3. Ballantyne CM, Stein EA, Paoletti R, Southworth H, Blasetto JW. Efficacy of rosuvastatin 10 mg in patients with the metabolic syndrome. Am J Cardiol. 2003;91(5A):25C–27C. doi:10.1016/s0002-9149(03)00006-7
4. Lv J, Ren C, Hu Q. Effect of statins on the treatment of early diabetic nephropathy: a systematic review and meta-analysis of nine randomized controlled trials. Ann Palliat Med. 2021;10:11548–11557. doi:10.21037/apm-21-2673
5. Deedwania PC, Hunninghake DB, Bays HE, et al. Effects of rosuvastatin, atorvastatin, simvastatin, and pravastatin on atherogenic dyslipidemia in patients with characteristics of the metabolic syndrome. Am J Cardiol. 2005;95:360–366. doi:10.1016/j.amjcard.2004.09.034
6. Habte ML, Melka DS, Degef M, Menon MKC, Yifter H, Feyisa TO. Comparison of lipid profile, liver enzymes, creatine kinase and lactate dehydrogenase among type II diabetes mellitus patients on statin therapy. Diabetes Metab Syndr Obes. 2020;13:763–773. doi:10.2147/DMSO.S234382
7. Feher M, Greener M, Munro N. Persistent hypertriglyceridemia in statin-treated patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2013;6:11–15. doi:10.2147/DMSO.S35053
8. Jose J. Statins and its hepatic effects: newer data, implications, and changing recommendations. J Pharm Bioallied Sci. 2016;8(1):23–28. doi:10.4103/0975-7406.171699
9. Kamal S, Khan MA, Seth A, et al. Beneficial effects of statins on the rates of hepatic fibrosis, hepatic decompensation, and mortality in chronic liver disease: a systematic review and meta-analysis. Am J Gastroenterol. 2017;112:1495–1505. doi:10.1038/ajg.2017.170
10. Kostapanos MS, Milionis HJ, Elisaf MS. Rosuvastatin-associated adverse effects and drug-drug interactions in the clinical setting of dyslipidemia. Am J Cardiovasc Drugs. 2010;10:11–28. doi:10.2165/13168600-000000000-00000
11. Lu Y, Hu Q, Chen L, et al. Interaction of deoxyschizandrin and schizandrin B with liver uptake transporters OATP1B1 and OATP1B3. Xenobiotica. 2019;49:239–246. doi:10.1080/00498254.2018.1437647
12. Wu L, Liu J, Hou J, et al. Interactions of the major effective components in Shengmai formula with breast cancer resistance protein at the cellular and vesicular levels. Biomed Pharmacother. 2021;133:110939. doi:10.1016/j.biopha.2020.110939
13. Kitamura S, Maeda K, Wang Y, Sugiyama Y. Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab Dispos. 2008;36:2014–2023. doi:10.1124/dmd.108.021410
14. Hua WJ, Fang HJ, Hua WX. Transepithelial transport of rosuvastatin and effect of ursolic acid on its transport in Caco-2 monolayers. Eur J Drug Metab Pharmacokinet. 2012;37:225–231. doi:10.1007/s13318-012-0094-9
15. US Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research; 2018.
16. Cooper KJ, Martin PD, Dane AL, Warwick MJ, Raza A, Schneck DW. Lack of effect of ketoconazole on the pharmacokinetics of rosuvastatin in healthy subjects. Br J Clin Pharmacol. 2003;55:94–99. doi:10.1046/j.1365-2125.2003.01720.x
17. Hua WJ, Hua WX, Fang HJ. The role of OATP1B1 and BCRP in pharmacokinetics and DDI of novel statins. Cardiovasc Ther. 2012;30:e234–e241. doi:10.1111/j.1755-5922.2011.00290.x
18. Li J, Volpe DA, Wang Y, et al. Use of transporter knockdown Caco-2 cells to investigate the in vitro efflux of statin drugs. Drug Metab Dispos. 2011;39:1196–1202. doi:10.1124/dmd.111.038075
19. Zhou Q, Ruan ZR, Yuan H, Xu DH, Zeng S. ABCB1 gene polymorphisms, ABCB1 haplotypes and ABCG2 c.421c > A are determinants of inter-subject variability in rosuvastatin pharmacokinetics. Pharmazie. 2013;68:129–134.
20. Qin XL, Chen X, Zhong GP, et al. Effect of Tacrolimus on the pharmacokinetics of bioactive lignans of Wuzhi tablet (Schisandra sphenanthera extract) and the potential roles of CYP3A and P-gp. Phytomedicine. 2014;21:766–772. doi:10.1016/j.phymed.2013.12.006
21. Wei H, Tao X, Di P, et al. Effects of traditional Chinese medicine Wuzhi capsule on pharmacokinetics of tacrolimus in rats. Drug Metab Dispos. 2013;41:1398–1403. doi:10.1124/dmd.112.050302
22. Xue XP, Qin XL, Xu C, et al. Effect of Wuzhi tablet (Schisandra sphenanthera extract) on the pharmacokinetics of cyclosporin A in rats. Phytother Res. 2013;27:1255–1259. doi:10.1002/ptr.4849
23. Zhang ZL, Jiang QC, Wang SR. Schisandrin A reverses doxorubicin-resistant human breast cancer cell line by the inhibition of P65 and Stat3 phosphorylation. Breast Cancer. 2018;25:233–242. doi:10.1007/s12282-017-0821-9
24. Guo CX, Deng S, Yin JY, Liu ZQ, Zhang W, Zhou HH. Schisandrin A and B induce organic anion transporting polypeptide 1B1 transporter activity. Pharmazie. 2015;70:29–32.
25. Shi QL. Effects of Wuzhi capsule on pharmacokinetics of atorvastatin. Fudan University; 2014.
26. Sun Q, Sun JF, Li L, Chang HC, Zhou Q. Effects of Wuzhi capsule on the pharmacokinetics of simvastatin and its metabolite simvastatin acid in rats. Chin J Clin Pharmacol Ther. 2020;25:1242–1249.
27. Chen J, Lou H, Jiang B, et al. Effects of food and gender on pharmacokinetics of rosuvastatin in a Chinese population based on 4 bioequivalence studies. Clin Pharmacol Drug Dev. 2020;9(2):235–245. doi:10.1002/cpdd.706
28. Liu JM, Ye XY, Liu ZJ, Wen JH, Wang F. Effect of scutellarin on the pharmacokinetics of rosuvastatin in rats. Chin J Clin Pharmacol. 2017;33:1003–1006.
29. Yang J, Hasegawa J, Endo Y, Iitsuka K, Yamamoto M, Matsuda A. Pharmacokinetic drug interaction between rosuvastatin and Tanjin in healthy volunteers and rats. Yonago Acta Med. 2019;62:77–84. doi:10.33160/yam.2019.03.011
30. Basu S, Jana S, Patel VB, Patel H. Effects of piperine, cinnamic acid and gallic acid on rosuvastatin pharmacokinetics in rats. Phytother Res. 2013;27:1548–1556. doi:10.1002/ptr.4894
31. Wen JH, Xiong YQ. The effect of herbal medicine danshensu and ursolic acid on pharmacokinetics of rosuvastatin in rats. Eur J Drug Metab Pharmacokinet. 2011;36:205–211. doi:10.1007/s13318-011-0048-7
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.