Back to Journals » Journal of Pain Research » Volume 15
Effects of Esketamine Combined with Ultrasound-Guided Pectoral Nerve Block Type II on the Quality of Early Postoperative Recovery in Patients Undergoing a Modified Radical Mastectomy for Breast Cancer: A Randomized Controlled Trial
Authors Yu L, Zhou Q, Li W, Zhang Q, Cui X, Chang Y, Wang Q
Received 28 June 2022
Accepted for publication 29 September 2022
Published 11 October 2022 Volume 2022:15 Pages 3157—3169
DOI https://doi.org/10.2147/JPR.S380354
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jinlei Li
Lili Yu,1,2 Qi Zhou,1 Wei Li,1 Qin Zhang,3 Xiuling Cui,2 Yulin Chang,2 Qiujun Wang1
1Department of Anesthesiology, The Third Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China; 2Department of Anesthesiology, Cangzhou Central Hospital, Cangzhou, People’s Republic of China; 3Surgical Department of Thyroid and Mammary Tumors, Cangzhou Central Hospital, Cangzhou, People’s Republic of China
Correspondence: Qiujun Wang, Department of Anesthesiology, The Third Hospital of Hebei Medical University, No. 139, Ziqiang Road, Shijiazhuang City, Hebei, People’s Republic of China, Tel/Fax +86-311-8860-2072, Email [email protected]
Purpose: To evaluate the effect of esketamine combined with ultrasound-guided pectoral nerve block type II (Pecs II block) on the quality of early postoperative recovery in patients undergoing a modified radical mastectomy (MRM) for breast cancer.
Patients and Methods: A total of 136 female patients undergoing an elective MRM for unilateral breast cancer (UBC) for the first time were randomly divided into the control group (group C, n=68) and the experimental group (PE group, n=68). In group C, sufentanil was used for anesthesia induction and patient-controlled intravenous analgesia (PCIA). Esketamine was used for anesthesia induction and PCIA in the PE group. Ultrasound-guided Pecs II block was performed after anesthesia induction in the two groups. All other anesthetics were administered in the same way. The primary outcome was the 40-item Quality of Recovery (QoR-40) score at discharge. The secondary outcomes were postoperative Observer’s Assessment of Alertness/Sedation Scale (OAA/S) scores, time of anesthesia recovery, Numeric Rating Scale (NRS) scores, serum inflammatory cytokines interleukin-10 (IL-10), interleukin-6 (IL-6), and interleukin-1ß (IL-1ß), Hospital Anxiety and Depression Scale (HADS) scores, length of postoperative Postanesthesia Care Unit (PACU) stay, length of postoperative hospital stay and patient satisfaction score.
Results: Compared with group C, the PE group had higher QoR-40 scores at discharge (P< 0.05), decreased IL-6 levels at 24 h after surgery (P< 0.05), lower anxiety and depression scores (P< 0.05) and higher patient satisfaction scores at discharge (P< 0.05). No significant difference was found in the NRS score postoperatively between the two groups (P> 0.05). There was no significant difference in the postoperative OAA/S score, time of anesthesia recovery, length of postoperative PACU and hospital stays between the two groups (P> 0.05).
Conclusion: Esketamine combined with Pecs II block can be used for anesthesia in MRM for breast cancer, thus, improving patient quality of early postoperative recovery.
Keywords: esketamine, ultrasound-guided nerve block, thoracic nerves, mastectomy, modified radical, anesthesia, recovery
Introduction
Breast cancer is one of the most common malignancies in women,1 which is often accompanied by negative emotions such as anxiety and depression.2 Surgery is the primary treatment modality for breast cancer.3 However, pain after a radical mastectomy for breast cancer4 that is accompanied by breast loss can aggravate the negative emotions of patients, which is detrimental to physical and mental health, and affects the early postoperative recovery of patients.5–8
The popularization of the concept of multimodal analgesia and the continuous updating of anesthesia methods and drugs as well as low- or zero-dose opioids during general anesthesia have been used in clinical practice.9,10 Esketamine, the dextro-isomer of ketamine, produces sedative and analgesic effects by non-competitively inhibiting N-methyl-D-aspartate (NMDA) receptors, also having a certain affinity for u receptors.11 In addition, esketamine can relieve depressive symptoms, reduce anxiety symptoms, and improve the psychological distress of patients.12,13
Ultrasound-guided Pecs II block has been widely used in women during breast cancer surgery. Combined with general anesthesia, it can reduce the dosage of opioids, relieve perioperative pain,14 and enhance the body’s immune function,15 which is conducive to the recovery of the patients.
We conducted this study to evaluate the effect of esketamine combined with Pecs II block on the early recovery of patients after MRM for breast cancer, thereby providing a reference for clinical anesthesia.
Methods
Ethics and Participant Enrollment
This research was ethically approved by the Medical Science Research Ethics Committees of Cangzhou Central Hospital (ethical register number: 2020–236-01) and is in compliance with the Helsinki Declaration. All participants received written informed consent prior to inclusion in this study. The trial was registered at the Chinese Clinical Trial Registry (register number: ChiCTR2200056391, date of registration: February 4th, 2022). Between February 7th and June 19, 2022, 146 patients with breast cancer preparing to receive MRM at Cangzhou Central Hospital were recruited into the study.
Inclusion Criteria
(1) Female patients undergoing elective MRM for unilateral breast cancer for the first time; (2) patients aged 18–64 years; (3) ASA grade I–II; (4) BMI 18–25 kg/m2.
Exclusion Criteria
(1) Hypertension grade III; (2) acute coronary syndrome; (3) pulmonary hypertension; (4) hyperthyroidism; (5) intracranial hypertension; (6) glaucoma; (7) monoamine oxidase inhibitor use; (8) infection at the puncture site; (9) history of chronic pain; (10) pain medication taken 24 h prior to surgery; (11) history of mental or psychological diseases; (12) history of adverse reactions to esketamine treatment; (13) history of allergy to local anesthetic drugs; (14) history of lung disease; (15) obvious abnormality in preoperative chest CT; (16) language and communication disorders and barriers.
Randomization and Blinding
Participants were randomly divided into groups C and PE with a 1:1 ratio using the random number table method. The patients code number and anesthesia regimens of the two groups were placed in separate sealed envelopes by one research investigator who was not part of the investigation and delivered it to the anesthesiologist. The patient data collectors and statisticians were all blinded to the group assignments.
Anesthesia Management
Routine preparation before surgery. After entering the operating room, blood pressure (BP), electrocardiogram (ECG), oxygen saturation (SpO2), and bispectral index (BIS) were monitored. Oxygen was inhaled through a mask, with a flow rate of 2 L/min. Peripheral venous access was established, and a Ringer’s sodium acetate was infused intravenously. Radial artery puncture and catheterization were performed under local anesthesia and invasive arterial pressure measurements were performed.
Anesthesia Induction
Induction of anesthesia in group C involved intravenous injection of 0.03 mg/kg midazolam, 0.3~0.4 μg/kg sufentanil, 2~2.5 mg/kg propofol, and 0.1~0.2 mg/kg cisatracurium. Induction of anesthesia in group PE involved intravenous injection of 0.03 mg/kg midazolam, 2~2.5 mg/kg propofol, 0.5 mg/kg esketamine (Lot: 210207BL, Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, China), and 0.1~0.2 mg/kg cisatracurium.
A laryngeal mask was placed in the two groups and then connected to an anesthesia machine for mechanical ventilation. The ventilation frequency was 12–14 times/min, the tidal volume was 6–8 mL/kg, and the PETCO2 was maintained at 35–45 mmHg (1 mmHg=0.033 kPa).
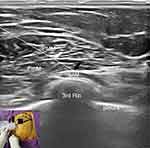
Pecs II Block
Pecs II block was performed according to a previous study.16 The skin at the puncture site was sterilized with iodophor, with the operator wearing sterile gloves and a sterile towel spread. The ultrasound probe was wrapped with a sterile endoscopic cover. A 5~13 MHz high-frequency ultrasound probe (MyLab Alpha, Esaote, Italy) was moved counterclockwise to the level of the third rib to locate the pectoralis major, pectoralis minor, and serratus anterior muscles. A 22 G puncture needle was slowly advanced along the plane using the in-plane puncture technique, paying close attention to the position of the needle tip (Figure 1). Once the needle tip reached the interfascial plane between the pectoralis minor and the anterior serratus muscles, no blood was withdrawn. Subsequently, 20 mL of 0.375% ropivacaine (LBVK, AstraZeneca AB, Sweden) was injected, followed by an injection of 10 mL of 0.375% ropivacaine into the interfascial plane between the pectoralis major and the pectoralis minor muscles.
Anesthesia Maintenance
Anesthesia maintenance propofol (5–7 mg· kg−1 h−1), remifentanil (5~10μg · kg−1·h−1) were infused intravenously in both groups, and an intermittent intravenous injection of cisatracurium 0.05~0.1 mg/kg was performed. During the surgery, the doses of propofol and remifentanil were adjusted to maintain a BIS value of 40–60. Sufentanil 0.25 μg/kg (group C) or esketamine 0.25 mg/kg (group PE) was supplemented as appropriate to maintain the changes in MAP without exceeding 20% of the base value, and in HR without exceeding 30% of the base value. Vasoactive drugs were given in case of poor response. When the skin was sutured, both groups received an intravenous infusion of 30 mg ketorolac tromethamine and 10 mg antiemetic azasetron. All drugs were withdrawn 10 min before the end of surgery.
Postoperative Analgesia Management
Both groups were given PCIA after surgery. Group C was given 2 ug/kg sufentanil + 1.5 mg/kg ketorolac tromethamine injection + 1 mg/kg dexmedetomidine + 10 mg azasetron + normal saline to 100 mL, and the PE group was given 1 mg/kg esketamine + 1.5 mg/kg ketorolac tromethamine injection + 1 mg/kg dexmedetomidine + 10 mg azasetron + normal saline to 100 mL. A PCIA pump (ZZB-150, Jiangsu Apon Medical Technology Co., Ltd., Nantong, China) was set with a loading volume of 2 mL, a background infusion rate of 2 mL/h, a PCIA dose of 2 mL, and a locking time of 15 min. When the postoperative NRS score was higher than 4 points, they were given an intramuscular injection of 50 mg tramadol for rescue analgesia.
Follow-Up
The Quality of Postoperative Recovery Evaluation
40-item Quality of Recovery (QoR-40)17 scores were recorded at discharge. The QoR-40 questionnaire is composed of two parts, and each part is evaluated on four aspects (including comfort, emotional, social, pain or independent behavior), with 1–5 points for each question and a total score of 200 points. The higher the score, the better the quality of recovery.
Quality of Anesthesia Recovery Assessment
Observer’s Assessment of Alertness/Sedation Scale (OAA/S) score18 was recorded at the end of surgery (T4), and at 30 min (T5) after surgery: (0–5 points, 0 point: no response to noxious stimuli; 1 point: response to noxious stimuli; 2 points: response to shaking shoulders or head; 3 points: response to loud or repeated calls and opening eyes; 4 points: slow response to normal voice; 5 points: rapid response to normal voice).
Pain Assessment
The Numeric Rating Scale (NRS)19 pain score was recorded at 30 min (T5), 6 h (T6), 12 h (T7), and 24 h (T8) after surgery: (0–10 points, 0 point: no pain, 10 points: unbearable severe pain). Times of postoperative PCIA pressing and postoperative rescue analgesia was recorded within 24 h after surgery.
Cytokine Measurement
Venous blood samples were collected at 0 min before anesthesia induction (T0) and 24 h (T8) after surgery. Serum inflammatory cytokines were detected according to the instructions of the 12-item cytokine ELISA detection kit (Lot: 210408, Shandong Luqing Medical Technology Co., Ltd., China); IL-10, IL-6, and IL-1ß levels were recorded.
Anxiety and Depression Assessment
The Hospital Anxiety and Depression Scale (HADS)20 was used to evaluate the patient’s anxiety and depression scores 1 d before surgery and at discharge. HADS comprises two sub-scales: hospital anxiety and depression scale-anxiety subscale (HADS-A). The hospital anxiety and the depression scale-depression subscale (HADS-d), have a total of 21 points for each sub-scale. The higher the score, the more severe the patients anxiety or depression symptoms are.
Patient Satisfaction
The patient satisfaction score was recorded (0–10 points, 0 point: not satisfied at all; 10 points: highly satisfactory) at discharge.
Other Measured Parameters
Mean arterial pressure (MAP) and heart rate (HR) were recorded 0 min before anesthesia (T0), 10 min after induction of anesthesia (T1), during skin incision (T2), 30 min after surgery (T3), at the end of surgery (T4), 30 min after surgery (T5), and 6 h (T6), 12 h (T7), and 24 h (T8) after surgery.
Nerve block complications (local anesthetic poisoning, pneumothorax, hematoma, nerve injury, puncture site infection), incidence of adverse drug reactions, and related complications (hypertension/hypotension, tachycardia/bradycardia, nausea, vomiting, dizziness, headache, blurred vision, and restlessness during recovery: Richmond agitation-sedation scale21 ≥ 5 points) were recorded.
Statistical Analysis
The sample size was calculated based on the pre-experiment, which included 20 patients with ten patients in each group. The primary outcome was a postoperative QoR-40 score at discharge. The average postoperative QoR-40 score (point) was (160.2 ± 14.5 in the control group versus 168.4± 14.5 in the experimental group). The power is set to 0.90, α to 0.05, with an equal 1:1 distribution. Finally, the calculation result suggested a minimum of 66 cases in each group. Regarding patient drop-out, we increased the number of patients by 10%-20%; therefore, 145–158 patients were required to ensure that a total of 122 patients completed the trial.
All data were analyzed using SPSS (version 22.0 for Windows, SPSS Inc., Chicago, IL, USA). Descriptive analyses were based on numbers and percentages for categorical variables and mean ± standard deviation for continuous variables. Continuous variables were tested using a Student’s t-test for normal distribution or a Mann–Whitney U-test for skewed distribution while categorical variables were analyzed through a Chi-squared test or a Fisher’s exact test. Repeated measurements were compared using repeated measure’ ANOVA. P value of less than 0.05 was considered statistically significant for all tests.
Results
A total of 146 participants were assessed for eligibility based on the inclusion and exclusion criteria. Ten participants were excluded, including 2 patients who declined to participate, 4 with grade III hypertension, 2 with a history of mental and psychological disease, and 2 due to a change in the surgical approach. Eventually, the remaining 136 patients were included and randomly divided into two groups: the control group (group C, n=68) and the experimental group (PE group, n=68) (Figure 2).
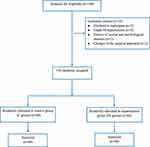 |
Figure 2 Participant flow diagram. Abbreviations: n, number; C, control; PE, pectoral nerve block type II combined with esketamine. |
Clinical Baseline Characteristics and Perioperative Data
No statistically significant differences were observed in patient baseline characteristics (age, ASA grade, BMI, comorbidities, and education) and perioperative data (duration of operation, drain output, transfusion, length of PACU stay, and length of postoperative hospital stay) (All P > 0.05, Table 1).
 |
Table 1 Clinical Baseline Characteristics and Perioperative Data in Two Groups (n=68) |
The total QoR-40 score (P < 0.05, Table 1, Figure 3) and the satisfaction score of patients were increased at discharge in group PE compared to group C (P < 0.05, Table 1).
Anesthesia Management and Consumption of Anesthetic Drugs
There were no statistically significant differences between the two groups regarding perioperative anesthesia management (anesthesia duration, the OAA/S score at each time point, time of anesthesia recovery, nerve block duration, and the times of PCIA pressing) (All P > 0.05, Table 2).
 |
Table 2 Anesthesia Management and Consumption of Anesthetic Drugs (n=68) |
The total consumption of propofol, midazolam, cisatracurium, and remifentanil as well as the times of additional analgesics (sufentanil in group C and esketamine in group PE) during operation showed no statistically significant differences between the two groups (All P > 0.05, Table 2).
No rescue analgesia after surgery was administered in either group.
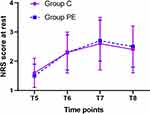
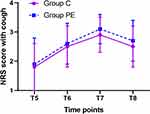
The NRS Score at Rest and During Cough
No significant differences in the NRS score at rest and during cough at all four time points during the post-operative period were observed between the two groups (Both P > 0.05, Figures 4 and 5).
IL-6, IL-10 and IL-1ß Levels
Compared to the baseline value at T0, only IL-6 serum concentration was increased at T8 in both groups (Both P > 0.05, Table 3), and IL-1ß and IL-10 levels of both groups showed no significant differences at T8 (All P > 0.05, Table 3).
 |
Table 3 Serum IL-6, IL-10 and IL-1ß Levels in Two Groups (n=68) |
In addition, the two groups showed no significant differences in serum concentrations of IL-6, IL-1ß, and IL-10 at T0 (All P > 0.05, Table 3). IL-6 serum levels were lower in the PE group compared to group C at T8 (P < 0.05, Table 3), while there was no significant difference between the two groups regarding the serum levels of IL-1ß and IL-10 at T8 (All P > 0.05, Table 3).
HADS Scores at Each Time Point
No significant differences were found in the HADS-A and HADS-d scores between the two groups 1 d before surgery (P > 0.05, Table 4), while the HADS-A and HADS-d scores were decreased in the PE group compared to group C at hospital discharge (P < 0.05, Table 4).
 |
Table 4 HADS Scores in Two Groups (n=68) |
Furthermore, the total HADS-d scores were increased at hospital discharge compared to 1 d before surgery in group C (P < 0.05, Table 4), there was no significant difference in the HADS-A and HADS-d scores 1 d before surgery and during hospital discharge in the PE group (P > 0.05, Table 4).
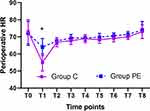
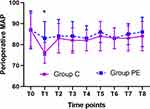
The MAP and HR at Different Time Points
The MAP and HR levels were higher 10 min after induction of anesthesia (T1) in the PE group compared to group C (P < 0.05, Figures 6 and 7), while no significant difference was found between the two groups at any other time point (P > 0.05, Figures 6 and 7). Compared to T0, the MAP and HR levels were lower at T1 in group C (P < 0.05, Figures 6 and 7). Furthermore, no statistically significance differences were found at T2-8 in group C and at T1-8 in the PE group (P > 0.05, Figures 6 and 7).
Adverse Events and Block-Related Complications
The incidence of postoperative nausea and vomiting was decreased in the PE group compared to group C (P < 0.05, Table 5). No significant differences were found in the incidence of hypertension/hypotension, tachycardia/bradycardia, and dizziness between the two groups after surgery (All P > 0.05, Table 5). Moreover, no patients in either group developed headache, blurred vision, restlessness during recovery, or block-related complications.
 |
Table 5 Adverse Events and Block-Related Complications in Two Groups (n=68) |
Discussion
In this study, we found that shortly after surgery the total QoR-40 score was higher in the PE group compared to group C. This may indicate that esketamine combined with Pecs II block improves the quality of early postoperative recovery after MRM.
The application of Pecs II block effectively reduces pain during and after surgery for breast cancer.22 Intravenous infusion of low-dose esketamine is an adjuvant therapy to perioperative management of acute and chronic pain.23 The results of our study showed no significant difference in the postoperative NRS score between the two groups, and neither group was given rescue analgesics after surgery. This suggests that the effect of perioperative analgesia was similar in the two groups, and both analgesic agents were effective for postoperative pain in patients undergoing MRM for breast cancer.
This study used low-dose esketamine combined with nonsteroidal analgesics ketorolac tromethamine and dexmedetomidine for postoperative PCIA. Dexmedetomidine has anti-anxiety, sedative, analgesic, and salivary-suppressive effects, and when used in combination with ketamine, can inhibit the increase in ketamine-induced tachycardia, BP and other side effects.24 Preclinical experiments indicated that intravenous infusion of dexmedetomidine combined with esketamine reduces the excessive excitation of brain neurons, and the mental side effects caused by esketamine.25 This explains why the PE group had stable BP and HR, low incidence of nausea and vomiting, and no obvious adverse reactions after surgery, including mental symptoms and head discomfort.
Studies have demonstrated that 20–45% of breast cancer patients experience anxiety and depression symptoms after surgery.26 Esketamine not only has sedative and analgesic effects, but also anti-anxiety and antidepressive effects. Intravenous injection rapidly ameliorates anxiety and depression symptoms in patients.27,28 This effect began a few hours after administration, peaked at 24 h, and lasted approximately one week.29 The positive psychological effects of esketamine may be attributed to an induction of neuroplasticity, which reverses the negative effect of stress and depression on neuronal cells and synapses.30 Esketamine may be the primary reason for the postoperative reductions in anxiety and depression scores observed in the PE group.
Studies have confirmed that postoperative serum inflammatory cytokines IL-10, IL-6, and IL-1ß levels are closely related to the prognosis of breast cancer.31–33 Compared with group C, the serum IL-6 levels in the PE group decreased at 24 h after surgery, and might be related to a decrease in the use of opioid analgesics during the perioperative period in the PE group. Furthermore, opioid analgesics could increase the levels of the proinflammatory factor IL-6.34,35 Studies by Welters, IDet al have shown that, compared with sufentanil, the administration of esketamine during surgery under general anesthesia increases the levels of the anti-inflammatory factor IL-10.36 In contrast, no continuous pump infusion of esketamine was given for the maintenance of anesthesia in this study, and the dose was low, which might be the reason why no significant difference was found in IL-10 levels between the two groups in this study. Several studies confirmed that compared with intravenous pump infusion of propofol, inhaled sevoflurane used for maintenance anesthesia during surgery increased the levels of pro-inflammatory factor IL-1ß.37 In this study, both groups were given intravenous pump infusion of propofol for anesthesia maintenance, which also explained why there was no significant change in IL-1ß levels between the two groups after surgery.
Our study had several limitations. First, this study only evaluated the pain score and changes in hospitalized patients; however, failed to follow up on the long-term chronic pain and quality of life after surgery, which needs further evaluation. In addition, this study has not elucidated the specific anti-inflammatory mechanism of esketamine; therefore, further research is required. A comparison between sufentanil combined with Pecs II block and esketamine combined with Pecs II block revealed significant strengths. Esketamine can prevent reductions in BP and HR during the later stages of anesthesia induction, lower the incidence of postoperative nausea and vomiting, reduce postoperative inflammatory responses, and relieve the patient’s anxiety and depression after surgery.
Conclusion
Perioperative substitution of Esketamine for sufentanil in modified radical mastectomy was associated with improved quality of postoperative recovery.
Abbreviations
Pecs II block, pectoral nerve block type II; MRM, modified radical mastectomy; UBC, unilateral breast cancer; ASA, American Society of Anesthesiologists; PCIA, patient controlled intravenous analgesia; MAP, Mean arterial pressure; HR, heart rate; OAA/S, Observer’s Assessment of Alertness/Sedation Scale; NRS, numeric rating scale; IL-10, serum inflammatory cytokines interleukin-10; IL-6, serum inflammatory cytokines interleukin-6; IL-1ß, serum inflammatory cytokines interleukin-1ß; HADS, the Hospital Anxiety and Depression Scale; PACU, Postanesthesia Care Unit; QoR-40, 40-item quality of recovery; BP, blood pressure; ECG, electrocardiogram; SpO2, oxygen saturation; BIS, bispectral index.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available upon reasonable request from Scientific Research Department of Cangzhou Central Hospital. Email: [email protected]; Telephone number: +86 0317 2072271.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No.81771134); Hebei Province Technology Innovation Guide Project Science and Technology Winter Olympics Special Project (No.19977790D); The Specialty Capability Construction and Specialty Leader Training Program Funded by Hebei Provincial Government (No.361005); Cangzhou Guidance Project of Key R&D Program (No.204106120).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Miller KD and Jemal A: cancer statistics. CA Cancer J Clin. 2016;66:7–30. doi:10.3322/caac.21332
2. So WKW, Marsh G, Ling WM, et al. Anxiety, depression and quality of life among Chinese breast cancer patients during adjuvant therapy. Eur J Oncol Nurs. 2010;14(1):17–22. doi:10.1016/j.ejon.2009.07.005
3. Li T, Mello-Thoms C, Brennan PC. Descriptive epidemiology of breast cancer in China: incidence, mortality, survival and prevalence. Breast Cancer Res Treat. 2016;159(3):395–406. doi:10.1007/s10549-016-3947-0
4. Andersen KG, Aasvang EK, Kroman N, et al. Intercostobrachial nerve handling and pain after axillary lymph node dissection for breast cancer. Acta Anaesthesiol Scand. 2014;58(10):1240–1248. doi:10.1111/aas.12393
5. Bužgová R, Jarošová D, Hajnová E. Assessing anxiety and depression with respect to the quality of life in cancer inpatients receiving palliative care. Eur J Oncol Nurs. 2015;19(6):667–672. doi:10.1016/j.ejon.2015.04.006
6. Breitbart W, Rosenfeld B, Passin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with Cancer. JAMA. 2000;284(22):2907–2911. doi:10.1001/jama.284.22.2907
7. Massie MJ, Gagnon P, Holland JC. Depression and suicide in patients with cancer. J Pain Symptom Manag. 1994;9(5):325–340. doi:10.1016/0885-3924(94)90192-9
8. Chan CM, Wan Ahmad WA, Yusof M, Ho GF, Krupat E. Effects of depression and anxiety on mortality in a mixed cancer group: a longitudinal approach using standardised diagnostic interviews. Psycho-Oncology. 2014;24(6):718–725. doi:10.1002/pon.3714
9. Chia PA, Cannesson M, Bui CCM. Opioid free anesthesia: feasible? Curr Opin Anaesthesiol. 2020;33(4):512–517. doi:10.1097/ACO.0000000000000878
10. Fancellu A, Perra T, Ninniri C, et al. The emerging role of pectoral nerve block (PECS block) in breast surgery: a case-matched analysis. Breast. 2020;26(9):1784–1787. doi:10.1111/tbj.13939
11. Hamp T, Baron-Stefaniak J, Krammel M, et al. Effect of intravenous S-ketamine on the MAC of sevoflurane: a randomised, placebo-controlled, double-blinded clinical trial. Br J Anaesth. 2018;121:1242–1248. doi:10.1016/j.bja.2018.08.023
12. Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Focus. 2019;17(1):55–56. doi:10.1176/appi.focus.17105
13. Falk E, Schlieper D, van Caster P, et al. A rapid positive influence of S-ketamine on the anxiety of patients in palliative care: a retrospective pilot study. BMC Palliat Care. 2020;19(1):1. doi:10.1186/s12904-019-0499-1
14. Schwemmer U. Breast surgery and peripheral blocks. Is It Worth It?. Curr Opin Anaesthesiol. 2020;33(3):311–315.
15. Cui X, Zhu C, Chen P, et al. Effect of pectoral nerve block type II under general anesthesia on the immune function of patients with breast cancer. Am J Surg. 2020;220(4):938–944. doi:10.1016/j.amjsurg.2020.03.008
16. Blanco R, Fajardo M, Parras Maldonado T. Ultrasound description of Pecs II (modified Pecs I): a novel approach to breast surgery. Rev Esp Anestesiol Reanim. 2012;59(9):470–475.
17. Myles PS, Weitkamp B, Jones K, et al. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth. 2000;84(1):11–15. doi:10.1093/oxfordjournals.bja.a013366
18. Bagchi D, Mandal MC, Das S, et al. Bispectral index score and observer’s assessment of awareness/sedation score may manifest divergence during onset of sedation: study with midazolam and propofol. Indian J Anaesth. 2013;57(4):351–357. doi:10.4103/0019-5049.118557
19. Thong ISK, Jensen MP, Miró J, et al. The validity of pain intensity measures: what do the NRS, VAS, VRS, and FPS-R measure? Scand J Pain. 2018;V18N1:99–107. doi:10.1515/sjpain-2018-0012
20. Cassiani-Miranda CA, Scoppetta O, Cabanzo-Arenas DF. Validity of the hospital anxiety and depression scale (Hads) in primary care patients in Colombia. Gen Hosp Psychiatry. 2022;V74N:102–109. doi:10.1016/j.genhosppsych.2021.01.014
21. Turkmen A, Altan A, Turgut N, et al. The correlation between the Richmond agitation-sedation scale and bispectral index during dexmedetomidine sedation. Eur J Anaesthesiol. 2006;V23N4:300–304. doi:10.1017/S0265021506000081
22. Senapathi TGA, Widnyana IMG, Aribawa IGNM, et al. Combined ultrasound- guided Pecs II block and general anesthesia are effective for reducing pain from modified radical mastectomy. J Pain Res. 2019;12:1353–1358. doi:10.2147/JPR.S197669
23. Argiriadou H, Papagiannopoulou P, Foroulis CN, et al. Intraoperative infusion of S(+)-ketamine enhances post-thoracotomy pain control compared with perioperative parecoxib when used in conjunction with thoracic paravertebral ropivacaine infusion. J Cardiothorac Vasc Anesth. 2011;25:455–461. doi:10.1053/j.jvca.2010.07.011
24. Kim JG, Lee HB, Jeon SB. Combination of Dexmedetomidine and Ketamine for Magnetic Resonance Imaging Sedation. Front Neurol. 2019;10:416. doi:10.3389/fneur.2019.00416
25. Chu Q, Zhu K, Bai Y, et al. A single low dose of dexmedetomidine efficiently attenuates esketamine-induced overactive behaviors and neuronal hyperactivities in mice. Front Hum Neurosci. 2021;15:735569. doi:10.3389/fnhum.2021.735569
26. Ewertz M, Jensen AB. Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncol. 2011;50(2):187–193. doi:10.3109/0284186X.2010.533190
27. Bozymski KM, Crouse EL, Titus-Lay EN, et al. Esketamine: a novel option for treatment-resistant depression. Ann Pharmacother. 2020;54(6):567–576. doi:10.1177/1060028019892644
28. Glue P, Medlicott NJ, Harland S, et al. Ketamine’s dose-related effects on anxiety symptoms in patients with treatment refractory anxiety disorders. J Psychopharmacol. 2017;31(10):1302–1305. doi:10.1177/0269881117705089
29. Zarate CA, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi:10.1001/archpsyc.63.8.856
30. Duman RS, Li N, Liu R-J, et al. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62(1):35–41. doi:10.1016/j.neuropharm.2011.08.044
31. Carpi A, Nicolini A, Antonelli A, et al. Cytokines in the management of high risk or advanced breast cancer: an update and expectation. Curr Cancer Drug Targets. 2009;V9N8:888–903. doi:10.2174/156800909790192392
32. Venetsanakos E, Beckman I, Bradley J, et al. High incidence of interleukin 10 mRNA but not interleukin 2 mRNA detected in human breast tumours. r J Cancer. 1997;V75N12:1826–1830.
33. Deegan CA, Murray D, Doran P, et al. Anesthetic technique and the cytokine and matrix metalloproteinase response to primary breast cancer surgery. Reg Anesth Pain Med. 2010;V35N6:490–495. doi:10.1097/AAP.0b013e3181ef4d05
34. Helmy SA, Wahby MA, El-Nawaway M. The effect of anesthesia and surgery on plasma cytokine production. Anaesthesia. 1999;54(8):733–738. doi:10.1046/j.1365-2044.1999.00947.x
35. Al-Hashimi M, Scott S, Thompson JP, Lambert D. Opioids and immune modulation: more questions than answers. Br J Anaesth. 2013;111(1):80–88. doi:10.1093/bja/aet153
36. Welters ID, Feurer MK, Preiss V, et al. Continuous S-(+)-ketamine administration during elective coronary artery bypass graft surgery attenuates pro-inflammatory cytokine response during and after cardiopulmonary bypass. Br J Anaesth. 2011;106(2):172–179. doi:10.1093/bja/aeq341
37. Bravo-Cuéllar A, Romero-Ramos JE, Hernández-Flores G, et al. Comparison of two types of anesthesia on plasma levels of inflammatory markers. Cir Cir. 2007;V75N2:99–105.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.