Back to Journals » Drug Design, Development and Therapy » Volume 17
Effects of Empagliflozin on Gut Microbiota in Heart Failure with a Preserved Ejection Fraction: The Design of a Pragmatic Randomized, Open-Label Controlled Trial (EMPAGUM)
Authors Guan XQ, Wang CH, Cheng P, Fu LY, Wu QJ, Cheng G, Guan L, Sun ZJ
Received 11 January 2023
Accepted for publication 1 May 2023
Published 18 May 2023 Volume 2023:17 Pages 1495—1502
DOI https://doi.org/10.2147/DDDT.S404479
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yan Zhu
Xue-Qing Guan,1 Chuan-He Wang,1 Peng Cheng,1 Ling-Yu Fu,2 Qi-Jun Wu,3– 5 Gong Cheng,1 Lin Guan,1 Zhi-Jun Sun1
1Department of Cardiology, Shengjing Hospital of China Medical University, Shenyang, 110021, People’s Republic of China; 2Department of Clinical Epidemiology and Evidence-Based Medicine, The First Affiliated Hospital, China Medical University, Shenyang, 110021, People’s Republic of China; 3Department of Clinical Epidemiology, Shengjing Hospital of China Medical University, Shenyang, 110021, People’s Republic of China; 4Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, 110021, People’s Republic of China; 5Clinical Research Center, Shengjing Hospital of China Medical University, Shenyang, 110021, People’s Republic of China
Correspondence: Zhi-Jun Sun, Department of Cardiology, Shengjing Hospital of China Medical University, No. 39 of Huaxiang Road, Tiexi District, Shenyang, 110021, People’s Republic of China, Tel +86 02496615, Email [email protected]
Abstract: Although empagliflozin has been recommended for individuals with heart failure, its effects on heart failure with preserved ejection fraction (HFpEF) remain uncertain from a physiopathological standpoint. The metabolites produced by gut microbiota have been shown to have a crucial role in the development of heart failure. Sodium-glucose cotransporter-2 inhibitors (SGLT2) have been shown to change the make-up of the gut microbiota in rodent studies. There is mixed evidence from similar studies investigating whether or not SGLT2 can affect the microbiota in the human gut. This trial is a pragmatic, randomized, open-label controlled study with empagliflozin as an intervention. We will enroll 100 patients with HFpEF and randomly assign them to one of two groups to receive either empagliflozin or a placebo. Patients in the Empagliflozin group will be given 10 mg of the drug daily, while those in the Control group will not be given empagliflozin or any other SGLT2. The purpose of the trial is to validate the changes that occur in gut microbiota in patients with HFpEF who take empagliflozin and to investigate the function of gut microbiota and their metabolites in the process.
Keywords: derived metabolite, gut microbiota, heart failure, HF, myocardial fibrosis, SGLT2, short-chain fatty acids, SCFAs, sodium-glucose cotransporter-2 inhibitor
Introduction
Heart failure (HF) is a complex clinical disease characterized by symptoms and indications caused by any anatomical or functional impairment of ventricular filling or blood ejection.1 As one of the major cardiac disorders, HF has a significant morbidity and mortality rate around the world. According to data from a multicenter observational study, the combined endpoint of mortality or HF hospitalization after one year was 36% for acute heart failure (AHF) and 14.5% for chronic heart failure (CHF).2 There were 1.2 million heart failure hospitalizations in the United States in 2017, involving 924,000 patients. This represents a 26% increase over the last decade, and the figure is expected to rise more in the future.1 Heart failure can be categorized into several phenotypes based on the left ventricular ejection fraction (LVEF), with HFpEF accounting for more than half of all patients.3 HFpEF is a complex biological process that includes systemic inflammation, epicardial adipose tissue buildup, cardiac fibrosis, and vascular dysfunction, among other metabolic pathways.4–6 However, the mechanisms and therapeutic strategies for it require additional experimental data.7
Intestinal flora is a complex collection of microbial communities that are intimately linked to the human body and participate in a variety of biological processes.8 Patients with heart failure frequently have decreased cardiac output as well as peripheral circulation congestion. In this circumstance, intestinal ischemia and edema cause a disruption in gut flora, resulting in an overproduction of inflammatory agents and toxic metabolites, which accelerates the HF process.9–11 However, new evidence suggests that gut metabolites play a role in various HF pathways. Some of them (such as SCFAs) have potential protective effects, whereas others (uremic toxins) have the inverse effect.12
Empagliflozin, a novel type of hypoglycemic medication, is an SGLT2 that blocks glucose reabsorption by the kidneys. As a result, more glucose is excreted in the urine, decreasing blood glucose levels. However, multiple research have revealed other biological effects of SGLT2. SGLT2 has been shown in animal models to reduce sodium consumption, inhibit NO production, enhance cardiac energy metabolism, and regulate inflammation.13,14 The EMPEROR-preserved trial is a large-scale Phase III clinical trial examining the effect of empagliflozin on the prognosis of patients with HFpEF. According to current research, empagliflozin may be effective in this type, with a 21% reduction in the key composite endpoint of time to HF hospitalization or cardiovascular mortality.15 However, the mechanisms by which SGLT2 works on HF are not completely understood. Recent studies have found that SGLT2 impacts the intestinal microbiota in animal models.16,17
Given the growing importance of intestinal microbiota in HF and the effects of SGLT2 on intestinal microbiota and HF, we are conducting a clinical trial to assess the effects of empagliflozin on gut microbiota in patients with HFpEF under real-world conditions, and this paper describes the study’s design (NCT05584319).
Aim of the Study
The purpose of this study is to investigate the effects of empagliflozin on intestinal microbiota and associated metabolites in individuals with HFpEF.
Study Oversight
The trial is a pragmatic, randomized, open-label controlled study with empagliflozin as an intervention. We will enroll 100 patients with HFpEF and divide them into two groups. Patients in the Empagliflozin group will receive 10 mg of empagliflozin daily, whereas patients in the Control group will not receive empagliflozin or any other SGLT2.
Study Participants and Inclusion/Exclusion Criteria
The trial will enroll 100 participants with HFpEF between November 2022 and October 2024. After signing the informed consent form, patients who meet the inclusion criteria will be randomly assigned to either the Empagliflozin Group or the Control Group (Table 1).
 |
Table 1 Inclusion/Exclusion Criteria for the Trial |
Study Process and Follow-Up
The study is a prospective open-label randomized controlled trial conducted at Shengjing Hospital in Shenyang, China. After a week of screening, participants who meet the inclusion criteria and sign the informed consent form will be randomly assigned to either the Empagliflozin or the Control groups. Randomization will be conducted using RANDOM.ORG (an online randomization website). Participants having a randomized figure between 1–50 will be assigned to the Empagliflozin group, while the others will be assigned to the Control group.
The observation course will continue for 6 months, during which time efficacy and safety assessments will be carried out. Laboratory investigations, imaging examinations, physical examinations, and questionnaires will be used to assess the patients’ prognosis at both the beginning and end of the trial. The following are the specifics:
Laboratory Examinations
Various biomarkers, including but not limited to C-reactive protein (CRP) and soluble suppression of tumorigenicity 2 (ST2); end-organ functional assessments (liver function, renal function tests), and serum level of N-terminal prohormone of B-type natriuretic peptide (NT-proBNP) will be evaluated. NT-proBNP will be evaluated using the Hiscl-5000 high-sensitivity chemiluminescence immunoanalyzer (Japan) by Hissen Micron, and sST2 will be assessed using the Shanghai Meilian Co., Ltd. enzyme-linked immunosorbent assay (ELISA). Liver function, renal function tests, and other biochemical tests will be measured and rechecked in the Shengjing Hospital core laboratory using an automated analyzer (AU5800; Beckman Coulter, Inc., Carlsbad, CA, United States). At baseline and every six months, participants will provide feces samples in the hospital, which will subsequently be frozen at −80 °C until further use. In the relevant laboratory, gut microbiomes will be tested using 16S rRNA sequencing.
Imaging Examinations
ECG and echocardiography will be utilized to assess the patients’ cardiac function in this study (Philips Diagnostic Ultrasound System and Transducers, USA; Iricon Aricon Digital ECG Machine for ECG-12C, China).
Physical Examinations
The New York Heart Association functional class, in conjunction with other physical examinations, will be used to evaluate patients’ heart function.
Questionnaires
The Kansas City Cardiomyopathy Questionnaire (KCCQ) will be used to assess patients’ quality of life in relation to heart failure, and the Food Frequency Questionnaire-25 (FFQ25) will be used to assess patients’ nutrition status.
Investigators will keep track of the relevant syndrome for the entire six months, and all patients will be followed up by phone on a monthly basis. MACE events such as hospital readmission, all causes of mortality, other concomitant diseases, and drug conditions will be noted (Figure 1).
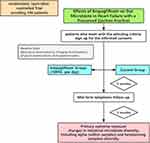 |
Figure 1 Trial procedure. |
Outcome Measures
Primary Outcome Measure
The primary outcome will be changes in gut microbiota diversity, including alpha (within samples) and beta (across samples) diversity. Furthermore, using general linear models, the effects of empagliflozin as an intervention on changes in gut flora abundance will be studied, with changes in OUT abundance as the outcome and empagliflozin intervention as the exposure. The specifics are as follows:
The CTAB/SDS method will be used to extract total genomic DNA from the samples. The 16s rRNA genes will next be amplified and purified using the AxyPrepDNA Gel Extraction Kit (AXYGEN). The quality of sequencing libraries will be evaluated using the Qubit@ 2.0 Fluorometer (Thermo- Scientific) and the Agilent Bioanalyzer 2100 system. Finally, the library will be sequenced using an Illumina NovaSeq6000 platform to generate 250bp paired-end reads.
Sequence analysis will be performed using the UPARSE software package and the UPARSE-OUT and UPARSE-OTUref algorithms to recognize and compare the biological characteristics of the samples. Alpha (within samples) and beta (across samples) diversity will be analyzed using in-house Perl scripts. Sequences with ≥ 97% similarity will be allocated to the same OTUs. The STAMP software will be used to confirm differences in individual taxonomic abundances between the two groups.
Aside from changes in OTU abundance in patients, other indicators such as inflammation factors will be investigated to detect correlations between numerous biomarkers and gut flora altered by empagliflozin, with potential confounders such as age, gender, and BMI being addressed.
Secondary Outcome Measures
Sample Size Calculation
Since no clinical trials have been undertaken on how SGLT2 impacts the gut microbiome in patients with HF, determining the probable effect sample size is difficult. Experiments on mice and rats administered sGLT2 revealed alterations in the gut microbiome composition, supporting the hypothesis that SGLT2 can influence gut flora.17,18 Furthermore, with a sample size of 69 and a 6-week intervention duration, Vijay et al discovered that fiber or omega-3 intervention relates to changes in the gut microbiota and relative metabolites. In contrast, no significant changes in gut microbiome alpha diversity or composition were observed in a 12-week treatment study with dapagliflozin for patients with type 2 diabetes.19 As a result, the inconsistent experimental results make estimating the sample size more challenging.
As empagliflozin has been licensed for clinical use by the Food and Drug Administration (FDA), this trial is designed as a pragmatic randomized controlled trial (pRCT) to assess drug efficacy in real-world settings. This trial also includes adaptive designs, and the efficiency of the outcomes will be assessed during the process to ensure the sample size is effective.
Discussion
Chronic systemic inflammatory conditions in patients with HF trigger the release of inflammatory factors and signaling molecules such as IL-6 and neprilysin, which results in water-sodium retention.20,21 As a result, the blood supply to the digestive tract is interrupted, leading to increased permeability of the gut mucosa and flora displacement.9 Previous clinical studies suggest that patients with HFpEF may have altered gut flora and an elevated inflammatory status.11,20 Huang et al discovered a rise in the number of microbiota linked with inflammation, such as Enterococcus and Lactobacillus, whereas an anti-inflammatory effect (Lachnospira butyricicoccus and others) was related with a reduction.22 Gut flora fractions such as LPS and related metabolites enter the circulation through the compromised intestinal mucosal barrier in HF, triggering additional inflammatory stimuli and worsening HF.23
Furthermore, accumulating evidence suggests that metabolites generated from gut microbiota, including short chain fatty acids (SCFAs), trimethylamine N-oxide (TMAO), secondary bile acids (BAs), and uremic toxins, contribute in various pathways relevant to the HF development process.24–30 SCFAs, among other metabolites, have been shown to protect against HF. SCFAs are produced by gut microbiota (Bacteroides, Bifidobacterium, and Faecalibacterium) as byproducts of dietary fiber fermentation to supply energy sources.31 SCFAs are required for the maintenance of the intestinal mucosal barrier and are linked to the promotion of intestinal immune activity. A growing number of studies indicate that SCFAs are involved in a variety of processes. SCFAs were reported to improve post-infarction cardiac repair by triggering CX3CR1+ monocyte infiltration in the peri-infarct zone.32 According to Pluznick et al, SCFAs (acetic acid/propionic acid) reduce myocardial fibrosis and restrict ventricular remodeling.33
Empagliflozin is a sodium-glucose transporter 2 inhibitor that inhibits glucose reabsorption in the kidney, hence excreting excess glucose from the urine and lowering blood glucose levels, and has been used as a novel form of hypoglycemic medication. However, as the research continues, the impacts of SGLT2 will be extended. Empagliflozin had good efficacy in HFpEF among 5988 patients in the EMPEROR-preserved trial, lowering composite endpoint events by 21%.34 In laboratory and clinical trials, SGLT2 was reported to be capable of alleviating inflammation induced by adipose tissue, reducing EAT volume, and attenuating cardiac fibrosis in both atrial and ventricular tissues.35,36 However, the specific mechanisms for this have yet to be discovered. Recent studies in mice and rats suggest that SGLT2 may influence the composition of the gut microbiome.18,37
Prior studies on how gut flora and metabolites affect HF, as well as the unknown mechanism of SGLT2, led to the following questions: What’s the connection between SGLT2 and gut flora? Is there a link between SGLT2, gut flora, and heart failure? The ongoing study aims to evaluate changes in gut microbiota in patients using empagliflozin who do not have a lower ejection fraction and to investigate the role of gut microbiota and their metabolites in the process. The study will look at the differences in gut microbiota abundance and the diversity of gut flora in individuals with HFpEF who have been taking empagliflozin for 6 months. Significantly, sST2, a fibrosis index, will be evaluated in the trial along with serum SCFA levels and gut microbiota producing SCFAs (such as Bacteroides, Bifidobacterium, and Faecalibacterium) to see if SGLT2 could alleviate cardiac fibrosis via gut microbiota.
The discovery of a prior clinical trial involving 69 healthy participants who were given omega-3 or inulin filters in SCFAs generating gut microbiomes strengthens the chances of reaching the intended goal. Changes in gut microbiota and SCFAs were shown to be linked with cardiovascular risk factors and cytokines in this particular trial.38 There were no significant changes in the gut microbiome alpha diversity or composition in a double-blind randomized trial of 44 patients with type 2 diabetes who were given dapagliflozin for 12 weeks.19 Due to the obvious discrepancy between clinical trials and laboratory studies,17,18,37 it is critical to determine if SGLT2 can alter the gut microbiome.
Furthermore, this study will look at how empagliflozin affects the gut flora of patients with HFpEF, as well as fibrosis-related factor (sST2), serum SCFAs, and SCFA-producing microbiota. Based on the information presented above, a putative SGLT2-gut-HF axis could be established. As a result, future study into the SGLT2 and gut microbiota processes may yield some concise new approaches for the clinical treatment of HFpEF. Furthermore, this trial aims to have a longer observation time of 6 months by combining the pRCT approach with other methods such as adaptive design to improve the efficiency of the outcomes.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki (as was revised in 2013). This controlled trial was approved by the Ethics Committee of the Shengjing Hospital of China Medical University (No. 2022PS999K), and registered in the Clinical Trial Register website (https://clinicaltrials.gov/ct2/home, NCT05584319, 10-8-2022). Informed consent will be obtained from all subjects involved in the study.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Heidenreich PA, Bozkurt B, Aguilar D, et al. AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022;2022(145):e876–e894. doi:10.1161/CIR.0000000000001062
2. Crespo-Leiro MG, Anker SD, Maggioni AP, et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail. 2016;18:613–625. doi:10.1002/ejhf.566
3. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591–602. doi:10.1038/nrcardio.2017.65
4. Franssen C, Chen S, Unger A, et al. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4:312–324. doi:10.1016/j.jchf.2015.10.007
5. van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur J Heart Fail. 2018;20:1559–1566. doi:10.1002/ejhf.1283
6. Rush CJ, Berry C, Oldroyd KG, et al. Prevalence of coronary artery disease and coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. JAMA Cardiol. 2021;6:1130–1143. doi:10.1001/jamacardio.2021.1825
7. Villalba-Orero M, Garcia-Pavia P, Lara-Pezzi E. Non-invasive assessment of HFpEF in mouse models: current gaps and future directions. BMC Med. 2022;20:349. doi:10.1186/s12916-022-02546-3
8. Maddox J. Immunology made accessible. Nature. 1984;310(5974):183. doi:10.1038/310183a0
9. Pasini E, Aquilani R, Testa C, et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. 2016;4:220–227. doi:10.1016/j.jchf.2015.10.009
10. Cui X, Ye L, Li J, et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci Rep. 2018;8:635. doi:10.1038/s41598-017-18756-2
11. Beale AL, O’Donnell JA, Nakai ME, et al. The gut microbiome of heart failure with preserved ejection fraction. J Am Heart Assoc. 2021;10:e020654. doi:10.1161/JAHA.120.020654
12. Pluznick JL, Protzko RJ, Gevorgyan H, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A. 2013;110:4410–4415. doi:10.1073/pnas.1215927110
13. Nikolaou PE, Mylonas N, Makridakis M, et al. Cardioprotection by selective SGLT-2 inhibitors in a non-diabetic mouse model of myocardial ischemia/reperfusion injury: a class or a drug effect? Basic Res Cardiol. 2022;117:27. doi:10.1007/s00395-022-00934-7
14. Crea F. The far-reaching beneficial effects of sodium-glucose co-transporter 2 inhibitors in heart failure. Eur Heart J. 2022;43:2907–2910. doi:10.1093/eurheartj/ehac437
15. Packer M, Butler J, Zannad F, et al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR-preserved trial. Circulation. 2021;144:1284–1294. doi:10.1161/CIRCULATIONAHA.121.056824
16. Lee DM, Battson ML, Jarrell DK, et al. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc Diabetol. 2018;17:62. doi:10.1186/s12933-018-0708-x
17. Yang M, Shi F-H, Liu W, et al. Dapagliflozin modulates the fecal microbiota in a type 2 diabetic rat model. Front Endocrinol. 2020;11:635. doi:10.3389/fendo.2020.00635
18. Hata S, Okamura T, Kobayashi A, et al. Gut microbiota changes by an SGLT2 inhibitor, luseogliflozin, alters metabolites compared with those in a low carbohydrate diet in db/db mice. Nutrients. 2022;14(17):3531. doi:10.3390/nu14173531
19. van Bommel EJM, Herrema H, Davids M, Kramer MHH, Nieuwdorp M, van Raalte DH. Effects of 12-week treatment with dapagliflozin and gliclazide on faecal microbiome: results of a double-blind randomized trial in patients with type 2 diabetes. Diabetes Metab. 2020;46:164–168. doi:10.1016/j.diabet.2019.11.005
20. Chia YC, Kieneker LM, van Hassel G, et al. Interleukin 6 and development of heart failure with preserved ejection fraction in the general population. J Am Heart Assoc. 2021;10:e018549. doi:10.1161/JAHA.120.018549
21. Packer M. Leptin-aldosterone-neprilysin axis: identification of its distinctive role in the pathogenesis of the three phenotypes of heart failure in people with obesity. Circulation. 2018;137:1614–1631. doi:10.1161/CIRCULATIONAHA.117.032474
22. Huang Z, Mei X, Jiang Y, Chen T, Zhou Y. Gut microbiota in heart failure patients with preserved ejection fraction (GUMPTION study). Front Cardiovasc Med. 2021;8:803744. doi:10.3389/fcvm.2021.803744
23. Yuzefpolskaya M, Bohn B, Nasiri M, et al. Gut microbiota, endotoxemia, inflammation, and oxidative stress in patients with heart failure, left ventricular assist device, and transplant. J Heart Lung Transplant. 2020;39:880–890. doi:10.1016/j.healun.2020.02.004
24. Vijay A, Astbury S, Le Roy C, Spector TD, Valdes AM. The prebiotic effects of omega-3 fatty acid supplementation: a six-week randomised intervention trial. Gut Microbes. 2021;13. doi:10.1080/19490976.2020.1863133
25. Tayyeb JZ, Popeijus HE, Mensink RP, Konings MCJM, Mokhtar FBA, Plat J. Short-chain fatty acids (except hexanoic acid) lower NF-kB transactivation, which rescues inflammation-induced decreased apolipoprotein A-I transcription in HepG2 cells. Int J Mol Sci. 2020;21:5088. doi:10.3390/ijms21145088
26. Vallance HD, Koochin A, Branov J, et al. Marked elevation in plasma trimethylamine-N-oxide (TMAO) in patients with mitochondrial disorders treated with oral l-carnitine. Mol Genet Metab Rep. 2018;15:130–133. doi:10.1016/j.ymgmr.2018.04.005
27. Trøseid M, Ueland T, Hov JR, et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015;277:717–726. doi:10.1111/joim.12328
28. Wang G, Kong B, Shuai W, Fu H, Jiang X, Huang H. 3,3-Dimethyl-1-butanol attenuates cardiac remodeling in pressure-overload-induced heart failure mice. J Nutr Biochem. 2020;78:108341. doi:10.1016/j.jnutbio.2020.108341
29. Mayerhofer CCK, Ueland T, Broch K, et al. Increased secondary/primary bile acid ratio in chronic heart failure. J Card Fail. 2017;23:666–671. doi:10.1016/j.cardfail.2017.06.007
30. Chen Y, Zelnick LR, Huber MP, et al. Association between kidney clearance of secretory solutes and cardiovascular events: the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis. 2021;78(2):226–235.e1. doi:10.1053/j.ajkd.2020.12.005
31. Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi:10.1136/gut.28.10.1221
32. Tang TWH, Chen H-C, Chen C-Y, et al. Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation. 2019;139:647–659. doi:10.1161/CIRCULATIONAHA.118.035235
33. Kelly CJ, Zheng L, Campbell EL, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi:10.1016/j.chom.2015.03.005
34. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi:10.1056/NEJMoa2107038
35. Lee H-C, Shiou Y-L, Jhuo S-J, et al. The sodium-glucose co-transporter 2 inhibitor empagliflozin attenuates cardiac fibrosis and improves ventricular hemodynamics in hypertensive heart failure rats. Cardiovasc Diabetol. 2019;18:45. doi:10.1186/s12933-019-0849-6
36. Requena-Ibáñez JA, Santos-Gallego CG, Rodriguez-Cordero A, et al. Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF: from the EMPA-TROPISM study. JACC Heart Fail. 2021;9:578–589. doi:10.1016/j.jchf.2021.04.014
37. Oh TJ, Sul WJ, Oh HN, et al. Butyrate attenuated fat gain through gut microbiota modulation in db/db mice following dapagliflozin treatment. Sci Rep. 2019;9:20300. doi:10.1038/s41598-019-56684-5
38. Vijay A, Astbury S, Panayiotis L, et al. Dietary interventions reduce traditional and novel cardiovascular risk markers by altering the gut microbiome and their metabolites. Front Cardiovasc Med. 2021;8:691564. doi:10.3389/fcvm.2021.691564
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
