Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Effects of early HIV infection and combination antiretroviral therapy on intrinsic brain activity: a cross-sectional resting-state fMRI study
Authors Li R, Wang W, Wang Y, Peters S, Zhang X, Li H
Received 22 November 2018
Accepted for publication 6 March 2019
Published 10 April 2019 Volume 2019:15 Pages 883—894
DOI https://doi.org/10.2147/NDT.S195562
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Ruili Li,1,* Wei Wang,1,* Yuanyuan Wang,1,* Sönke Peters,2 Xiaodong Zhang,3 Hongjun Li1
1Department of Radiology, Beijing Youan Hospital, Capital Medical University, Beijing, 100069, People’s Republic of China; 2Clinic for Radiology and Neuroradiology, University Hospital of Schleswig-Holstein, Kiel 24105, Germany; 3Department of Radiology, Tianjin First Central Hospital, Tianjin, 300192, People’s Republic of China
*These authors contributed equally to this work
Objective: To investigate effects of early HIV infection and combination antiretroviral therapy (cART) on intrinsic brain activity by using amplitude of low-frequency fluctuation (ALFF) analysis.
Patients and methods: Forty-nine HIV patients, including 26 with cART (HIV+/cART+) and 23 treatment-naïve (HIV+/cART–), and 25 matched healthy controls (HCs) underwent resting-state functional magnetic resonance imaging examination. ALFF values were compared by using one-way ANOVA tests with Analysis of Functional NeuroImages (AFNI)’s 3dClustSim correction (voxel p<0.005, α<0.05). In addition, the ALFF values of brain regions that showed significant differences among the three groups were correlated with clinical and neuropsychological variables in both groups of patients by using Spearman correlation analysis.
Results: ANOVA analysis showed that statistic difference of ALFF values among three groups was located in the occipital cortex. Post hoc analysis showed a decrease in occipital ALFF value in HIV patients compared to HC, but showed no difference of occipital ALFF between HIV+/cART+ and HIV+/cART–. Additionally, compared with HC, HIV+/cART+ exhibited higher ALFF in the right caudate and frontoparietal cortex, and HIV+/cART- showed higher ALFF in the bilateral caudate. HIV+/cART+ demonstrated higher ALFF values in auditory cortex than HIV+/cART–. Moreover, ALFF values in the right occipital cortex were positively associated with CD4+/CD8+ ratio and executive function in HIV+/cART–.
Conclusion: Early HIV-infected individuals presented reduced spontaneous brain activity in the occipital cortex. cART appeared to be ineffective in halting the HIV-induced neurodegeneration but might delay the progression of neural dysfunction to some extent. ALFF might be a potential biomarker in monitoring the effects of HIV and cART on brain function.
Keywords: HIV, brain, cognitive function, highly active antiretroviral therapy, fMRI
Introduction
The HIV involves the central nervous system shortly after infection and may cause HIV-associated neurocognitive disorders (HANDs) in >50% of subjects over time.1 Although combination antiretroviral therapy (cART) has decreased the incidence of the severe form of HAND (HIV-associated dementia), the incidence of the milder forms (asymptomatic neurocognitive impairment [ANI] and mild neurocognitive disorder [MND]) persists with increasing tendency.2,3 The underlying neural traits of this persistence and the pathophysiology of cognitive dysfunction have not been fully elucidated.
The main method for detecting HIV-related cognitive dysfunction is neuropsychological testing. However, these tests are time-consuming and subjective,2,4 and they have no direct correlation with changes in brain structure and function. Additionally, pathophysiological changes have been shown to take place before changes in neuropsychological performance.5,6 Therefore, it is necessary to develop new methods to assess potential neurobiological changes in HIV patients, even before obvious everyday functional impairment could be detected.
Neuroimaging techniques provide a valuable noninvasive tool to assess the effects of HIV on brain function and might be helpful to understand the pathophysiology of HIV-associated neurodegeneration.7,8 In recent years, blood-oxygen-level-dependent (BOLD) functional magnetic resonance imaging (fMRI) is becoming a useful tool in assessing altered brain function in HIV patients. In particular, resting-state functional magnetic resonance imaging (rs-fMRI) allows investigation of brain function in the absence of any cognitive tasks,9 which makes it more suitable for assessing brain functional abnormalities.10–12 A body of rs-fMRI studies has claimed altered functional connectivity in HIV patients.10–18 Ann et al13 reported that functional connectivity between precuneus and prefrontal cortex decreased in HAND patients. Ortega et al14 found attenuated functional connectivity in cortico-striatal networks in cART-naïve chronic HIV-infected individuals (HIV+/cART–) with an average infection duration of 64.4 months. Moreover, altered functional connectivity of lateral occipital cortex network can be detected even in patients within 1 year of HIV infection.12 Further research regarding the effects of cART on brain networks showed that HIV+ individuals with cART (HIV+/cART+) had greater functional connectivity strength between the striatum and default mode network and ventral attention network than HIV+/cART–.14 Besides, HIV patients with detectable plasma viral load exhibited decreased functional connectivity within the salience network compared to patients with suppressed viral load.15 These studies confirmed that rs-fMRI was a sensitive technique to assess HIV-associated brain function changes before and after treatment, and cART had beneficial effects on cerebral function.
The abovementioned studies assessed the resting-state brain functional integration by using functional connectivity analysis. Amplitude of low-frequency fluctuations (ALFF), another vital rs-fMRI analysis method, can detect the amplitude of brain oscillation in the low-frequency range (0.01–0.08 Hz) and reflect brain functional segregation. Segregation refers to brain regions that interact to efficiently perform specific cognitive tasks, while integration relates to interactions between segregated regions to enable complex, integrative tasks.19 ALFF could measure the intensity of regional spontaneous brain activity by calculating the ALFF value of each voxel,20 which has been proven to be a reliable and reproducible rs-fMRI method and widely implemented in various neurological and psychiatric disorders.21–23 To date, a rare study has assessed the altered spontaneous brain activity in adult HIV patients by using ALFF algorithm. McIntosh et al24 investigated the effect of depression and chronic inflammation on patients chronically infected (>10 years) with HIV by using resting-state functional connectivity and ALFF. However, how does the spontaneous brain activity in early HIV patients (<one year) without neurological symptoms change and what role can the ALFF play in monitoring the effects of cART are not well understood yet.
Thus, in this study, we hypothesized that HIV patients suffered altered resting-state spontaneous brain activity, which could be detected by ALFF even in the early stage. Besides, ALFF might be an effective biomarker in monitoring the effect of cART on brain function. We used rs-fMRI with an ALFF algorithm to study spontaneous brain activity in early HIV patients and to monitor the treatment effect of cART.
Patients and methods
Subjects
This study was approved by the ethics committee of Beijing Youan Hospital and was conducted in accordance with the Declaration of Helsinki. Written informed consents were obtained from all participants. From March 2016 to April 2017, 49 patients with HIV (26 patients experiencing stable cART for at least 6 months [HIV+/cART+; female\male=2\24; mean age, 33.1±5.4 years]; 23 treatment-naïve HIV individuals [HIV+/cART–; female\male=1\22; mean age, 31.3±3.7 years]) were recruited from infectious disease outpatient clinic of Beijing Youan Hospital, Capital Medical University. HIV infection was confirmed by immunoassay western blot or through PCR. Clinical laboratory examinations (current plasma CD4+ cell count, CD4+/CD8+ ratio and plasma viral load) were performed prior to the MRI examination. Estimated disease course was determined according to patients’ self-reports on their risk behaviors and the history of cART was identified from either self-reports or medical records. All of the treated patients were administered with tenofovir + lamivudine + efavirenz in this study.
The inclusion criteria for patients were as follows: 1) 18–50 years old; 2) right hand dominant; 3) HIV positive; 4) HIV+/cART+ had experienced cART for at least 6 months with plasma viral load <40 copies/ml; 5) HIV+/cART– did not receive any sort of antiretroviral treatment. Exclusion criteria included: 1) age <18 years and >50 years old; 2) presence of any brain trauma, stroke, infection, or tumors according to medical history and conventional MRI; 3) apparent depression and anxiety (including those received antidepressant or antianxiety therapy) based on a detailed investigation of the patient’s neuropsychiatric symptoms; 4) any other major neurologic or psychiatric illness; 5) history of substance abuse; 6) head motion >1.0° or 1.0 mm during MR scanning. Twenty-five age-, sex-, and education-matched healthy control (HC) (female\male=1\24; mean age, 33.2±6.1 years) were recruited from the local community. The inclusion criteria were healthy individuals with age 18–50 years old and right hand dominant. The exclusion criteria for HC were the same as those of HIV patients.
Neuropsychological evaluation
Within 2 hrs before MRI scan, each patient received a battery of neuropsychological tests for HAND.2 Neuropsychological assessment includes 6 cognitive domains and a self-report of cognitive difficulties in everyday life. The activity of daily living scale was used as a questionnaire to assess changes in everyday functioning.4 The cognitive tests and their corresponding cognitive function are listed in Table 1. To further evaluate cognitive performance, comprehensive cognitive scores were created to assess the 6 cognitive domains. The raw scores for each test were converted to T scores and adjusted for age, gender, and years of education. If one cognitive domain included multiple cognitive tests, then averaged T score was calculated for that cognitive domain. ANI was considered by performance at least 1 SD below the mean of demographically adjusted normative scores in ≥2 cognitive domains without decreased everyday functioning.2 In case of ≥2 cognitive domains impairment with mild decreased everyday functioning, MND should be taking into account.2 Dementia was suspected in condition of overall neuropsychological impairment and severe decline in everyday functioning.2 Thirty patients in this study were diagnosed as non-HAND (cognitive intact: 19 patients; 1 impaired cognitive domain: 11 patients) and 19 patients were classified as ANI according to the Frascati criteria.2
 | Table 1 Neurocognitive scales and corresponding cognitive domains |
MR imaging data acquisition
Neuroimaging data were acquired by using a 3.0T MR scanner (Tim-Trio, Siemens, Erlangen, Germany) and a 32-channel phased-array head coil with foam pads to minimize head movement. All subjects were instructed to close eyes, stay still, not to think about anything particular, and not to fall asleep during the scan. rs-fMRI data were collected using a gradient echo sequence: TR/TE=2,000/30, FOV=224×224 mm, matrix=64×64, section thickness=3.5 mm, flip angle=90°, and voxel size =3.5×3.5×4.2 mm.3 Totally, 240 time points were obtained for each subject during the rs-fMRI scanning. Besides, the conventional MRI were also acquired by using T1WI (TR/TE=250/2.46) and T2-fluid-attenuated inversion recovery sequences (TR/TE/TI=8000/2370.9/97) for the exclusion of overt brain diseases.
Data preprocessing and ALFF calculation
Statistical parametric mapping software (SPM8; The FIL Methods Group, London, England) was applied for the preprocessing of rs-fMRI data in a standard manner. The first 10 time points were got rid of to allow for the equilibrium of the magnetic field and adaptation of the participant. Then, the remaining 230 time points were used for further slice timing, head motion correction, and spatial normalization with a resampled voxel size of 3×3×3 mm3 into standard Montreal Neurologic Institute space. None of the subjects exhibited head motion of translation >1.0 mm or rotation >1.0° during MRI acquisition. After that, the statistical maps were spatially smoothed with full-width at the half-maximum Gaussian kernel of 8 mm. ALFF analysis was performed using the rs-fMRI data analysis toolkit as previously described.20,25 All voxels from the time course were first converted to the frequency domain by using fast Fourier transform. The ALFF of each voxel was computed by averaging the square root of the power spectrum throughout 0.01 Hz–0.08 Hz. Finally, the ALFF values were z-score normalized.
Statistical analysis
Demographic, clinical, and neuropsychological data were analyzed with IBM SPSS 20.0 (IBM Inc. Armonk, NY, USA). The Kolmogorov–Smirnov test was performed to test data normal distribution. For variables with normal distribution, means ± SDs were present, and for data not normally distributed, medians with minimum, maximum, and IQR were present. Age was normally distributed, and one-way ANOVA was used to calculate the differences among the three groups, while education level was not normally distributed and we used Kruskal–Wallis test. CD4+ cell count, CD4+/CD8+ ratio, and neuropsychologic test scores were normally distributed, and independent two-sample t-test was used to compare the differences between the two patient groups, while disease course and duration of infection without medication were not normally distributed and Mann–Whitney U test was used. χ2 test was used to evaluate the sex distribution among the three groups and cognitive status between the two patient groups. Significant level was set as α=0.05.
rs-fMRI data were compared by using SPM8 (The FIL Methods Group). One-way ANOVA with sex, age, and education level as covariates was conducted to compare ALFF alterations among the three groups. Post hoc analysis was further performed for pairwise group comparisons. Multiple comparisons correction was performed with AFNI’s 3dClustSim by using mixed autocorrelation function (significance level: voxel p=0.005, α=0.05 with 5,000 Monte Carlo simulations).26
Subsequently, the normalized ALFF values of the clusters that showed significant differences among the three groups were extracted. Then, the correlations between the normalized ALFF values in two HIV+ groups (HIV+/cART– and HIV+/cART+) and the clinical variables and neuropsychologic tests scores were evaluated separately by Spearman correlation analysis with IBM SPSS 20.0 (p<0.05). In addition, we performed multiple regression analysis by using SPM8 with age, gender, and education level as covariates to assess the correlation between ALFF values with cognitive tests scores and clinical data (CD4+ T-cell count, CD4+/CD8+, plasma viral load, and duration of infection) with AFNI’s 3dClustSim correction (voxel p<0.005, α<0.05)
Results
Demographic, clinical, and neuropsychological data
Demographic, clinical, and neuropsychological data for the HIV+/cART+, HIV+/cART–, and HC are shown in Table 2. There were no significant differences in sex, age, and education level among the three groups. Significantly longer disease course was found in HIV+/cART+ compared to HIV+/cART–, but no significant difference existed for the duration of infection without medication in HIV+/cART+ and disease course in HIV+/cART–. The CD4+ cell counts in HIV+/cART+ were higher than HIV+/cART–, but the difference was not statistically significant. HIV+/cART+ had higher current CD4+/CD8+ ratio than HIV+/cART–. Plasma viral load was undetectable in HIV+/cART+. Cognitive status between the two HIV+ groups did not differ according to the neuropsychologic tests scores. ANI patients account for 46.2% (12/26) in HIV+/cART+ and 30.4% (7/23) in HIV+/cART–, others are non-HAND.
 | Table 2 Demographic, clinical, and neruopsychological data of the three groups |
Between-group comparison
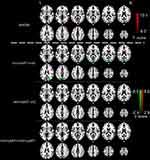
The one-way ANOVA results showed significant differences in ALFF value in the bilateral occipital cortices among the three groups. A pairwise comparison was performed for the post hoc analysis. Compared with HC, HIV+/cART+ displayed increased ALFF in the right frontoparietal cortex (middle cingulum cortex, middle frontal cortex, and postcentral cortex) and caudate, and reduced ALFF mainly in bilateral occipital cortex (bilateral superior and middle occipital cortex, left cuneus and postcentral cortex, right calcarine and precuneus). HIV+/cART– demonstrated increased ALFF in bilateral caudate and decreased ALFF in right occipital cortex (right lingual, calcarine and inferior occipital gyrus) compared to HC. Compared with HIV+/cART–, HIV+/cART+ showed higher ALFF values in auditory cortex (right superior temporal gyrus and supramarginal gyrus) (Figure 1 and Table 3).
 | Table 3 Regions showing ALFF differences among cART+, cART–, and healthy control subject groups |
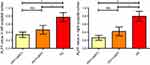
We further extracted the mean normalized ALFF value for each patient and control subject in the bilateral occipital cortex where altered ALFF was found among the three groups to generate a bar graph (Figure 2). HC had significantly higher ALFF values than either HIV+/cART+ or HIV+/cART–. ALFF values in HIV+/cART+ were slightly lower than that of HIV+/cART– without significant statistical difference.
Correlation analysis
Normalized ALFF values of bilateral occipital cortex where altered ALFF was found among the three groups were extracted and correlated to clinical variables and neuropsychological performance in HIV+ groups. Normalized ALFF values in the right occipital cortex were positively correlated with CD4+/CD8+ ratio and executive function in HIV+/cART– (r=0.59, p=0.003; r=0.69, p<0.001) (Figure 3). No significant relationships were observed between normalized ALFF values in bilateral occipital cortex and any clinical variables and neuropsychological scores in HIV+/cART+ group (all p>0.05). Additionally, we found no correlation between ALFF value and any cognitive tests scores and clinical data by performing multiple regression analysis with AFNI’s 3dClustSim correction (voxel p<0.005, α<0.05).
Discussion
In this study, we employed the ALFF algorithm to compare brain activity changes among HIV+/cART–, HIV+/cART+, and HC aiming to find alteration of ALLF in early-stage HIV patients and the potential value of ALFF in monitoring the cART therapy. We found that, compared with HC, HIV+/cART+ and HIV+/cART– showed reduced ALFF in occipital lobes. However, no ALFF difference was found in occipital cortex between HIV+/cART+ and HIV+/cART–. Moreover, ALFF values of right occipital cortex were positively correlated with CD4+/CD8+ ratio and executive function in HIV+/cART–. Besides, in comparison with HC, HIV+/cART+ showed higher ALFF mainly in the right frontoparietal lobe and caudate, and HIV+/cART– showed higher ALFF in bilateral caudate. In addition, compared with HIV+/cART–, HIV+/cART+ showed higher ALFF in the right superior temporal gyrus and supramarginal gyrus.
We found reduced ALFF in the occipital cortex in both HIV+/cART+ and HIV+/cART– compared to HC, which indicated that the occipital cortex might be a vital brain region related to early HIV infection with or without cART. Recently, lines of evidence from multimodal MRI indicated that the occipital cortex did get involved in neuropsychological impairment of HIV patients. Based on rs-fMRI studies, it is claimed that HIV-infected individuals had decreased functional connectivity in occipital cortex.12,27 Wang et al12 found decreased functional connectivity in lateral occipital cortex in patients within 1 year of HIV infection, rather than other brain networks. Moreover, Egbert et al27 reported that occipital lobe was still affected by HIV more profoundly than other brain networks even years after infection. Besides, Ances et al28 demonstrated significant reductions in rs-cerebral blood flow in both visual cortex and lenticular nuclei in recent HIV-infected individuals by using arterial spin labeling MRI. Additionally, previous structural studies found corresponding altered brain structure of occipital cortex in HIV-infected patients. Aylward et al29 reported progressive thinning of the grey matter in posterior cortices in HIV dementia and Filippi et al30 found compromised structural integrity of parieto-occipital white matter tracts in patients with HIV in a Diffusion Tensor Imaging
Compared to HIV+/cART–, HIV+/cART+ displayed a trend of a lower ALFF value in the occipital cortex, but the differences were not statistically significant. Boban et al36 presented that there were no significant differences in MRS parameters between HIV+/cART– and HIV+/cART+ in HIV patients with longer duration of infection and longer cART history compared to our study. There were two possible explanations for the discrepancy. The first possible explanation was that the disease course in HIV+/cART– was shorter than that of HIV+/cART+ in this study. Recent studies have reported that there is a tendency of brain function damage progression due to continued neuroinflammation and glial activation with the prolongation of HIV infection.37,38 Second, unlike HIV, many antiretroviral drugs did not effectively penetrate the blood‒brain barrier, and the brain had been recognized as a sanctuary for HIV causing long-term damage.39,40 cART and disease course of HIV infection may have opposite effects on brain function changes. In this study, the effect of cART might not prevail over the effect of disease duration. HIV+ individuals might continue to experience neurological impairment due to the virus despite effective plasma virological control and normal CD4+ T-cell counts. In this study, the duration before medication of HIV+/cART+ was comparable to the disease course of HIV+/cART–, and HIV+/cART+ displayed higher CD4+ T-cell counts and undetectable plasma viral load after an average of 30 months of treatment. Alternatively, our results showed that although the ALFF value of the occipital lobe was not significantly improved compared with the untreated group, there was no further deterioration. The findings might imply that in early stage of HIV infection, cART appeared to be ineffective in halting the HIV-associated neurodegeneration but might delay the progression of brain dysfunction to a certain extent. In terms of rs-functional connectivity studies, there was no consensus on how the cART affected the functional connectivity in HIV+ yet, probably due to various patient cohorts. Thomas et al11 found that treatment had no effect on rs-functional connectivity. Several studies observed increased functional connectivity in cortico-striatal and default mode network after cART.14,41 Notably, one preliminary longitudinal study claimed increased functional connectivity in visual pathways after a follow-up of average 30 months in patients with highly active antiretroviral therapy.42
Apart from that, compared to HC, we observed increased ALFF in the right caudate and frontoparietal lobe in HIV+/cART+ and in the bilateral caudate in HIV+/cART–. Previous fMRI studies indicated the involvement of fronto-striatal circuitry in HIV-infected patients.43 Prior studies using other imaging modalities also reported that caudate was implicated in HIV+.17,27,44–48 Ances et al found reduced blood flow and volume in caudate in HIV patients with neurocognitive impairments.44 HIV-related brain structure studies identified that the most serious atrophy was seen frequently in the caudate.45,46 Caudate atrophy was even noted in HIV patients without neurocognitive impairments.27 Thames et al47 found increased activation in caudate in HIV patients during a phonemic fluency task. Furthermore, abnormal functional connectivity of the caudate to the prefrontal cortex was documented in HIV patients in response to task demands.48 Another rs-fMRI study found functional connectivity reduction between the dorsolateral prefrontal cortex and the dorsal caudate in non-HNAD HIV patients.17 It was unclear whether the increased ALFF in caudate reflected an advantage or a deficit. It seems that the increased ALFF in the caudate and frontoparietal lobe in HIV patients might take part in the reorganization of brain function in HIV infection.
It is worth noting that increased ALFF was found in the right superior temporal gyrus and supramarginal gyrus in HIV+/cART+, compared with HIV+/cART–. Superior temporal gyrus, a part of Wernicke area, is a primary auditory center, which is responsible for the auditory processing. Supramarginal gyrus, an auditory language center, is recruited for spatial positioning and language functions. Otolaryngological symptoms have been reported in HIV patients.49–51 The incidence of hearing loss in HIV/AIDS patients ranges from 20% to 40%.52 Hearing impairment may be due to the direct effects of HIV,53 and the use of cART or other potential ototoxic drugs.54,55 Previous studies have shown that HIV could affect the central auditory system.56,57 There is a report that HIV-positive women perform more poorly in auditory processing, relative to HIV-negative women.58 Compared to HC, HIV-positive adults presented approximately 34% higher rate of hearing loss and also had higher pure-tone thresholds at all frequencies measured.52 HIV individuals had poorer results in pure-tone and high-frequency audiometry, suggesting impairment in the peripheral auditory pathway; in addition, HIV+/cART+ presented a higher proportion of alterations compared with HIV+/cART–.59 Campanini et al54 emphasized cART drugs might lead to higher rates of hearing impairment due to their ototoxicity. Although cART has enormous benefits of restoring overall health and life quality,60 it may be a confounding factor of HIV-related brain function and may have adverse effects on hearing. None of the HIV patients in the present study had hearing loss symptoms, which might be related to the early stage of HIV infection in this current study. In accordance with our results, we speculate that the function of auditory-related brain cortex might be affected due to potential impairment in the peripheral auditory pathway. As this study was cross-sectional, longitudinal studies should be designed to examine the effect of HIV and cART on hearing to confirm our assumption.
Furthermore, we found that ALFF values in the right occipital cortex were positively correlated with CD4+/CD8+ ratio in HIV+/cART–. The abnormality of ALFF values was related to reduced spontaneous neural activity. Clinically, a lower CD4+/CD8+ ratio has been represented as immunosenescence.61 This might suggest that immunosenescence among the early HIV+ patients without therapy would accelerate the reduction of spontaneous neural activity in the right occipital cortex. Occipital cortex is associated with visual learning, and prefrontal cortex is involved in executive function,62 whereas we found that reduced ALFF values in the right occipital cortex were positively associated with declined executive function in HIV+/cART–. This suggests that abnormal spontaneous neural activity of right occipital cortex in HIV+/cART– might contribute to changes in executive function. Executive function difficulty was not unique to frontal lobe damage, and it had been shown in previous studies that executive function impairment was associated with functional and structural abnormalities in the prefrontal, parietal, occipital, temporal cortex, caudate, and insula.48,63 This suggests that frontal lobe, the most advanced part of brain development, is highly interconnected with the other brain regions, such as temporal lobe, occipital lobe, and parietal lobe. Therefore, functional change in these regions will present difficulty in executive function specific to the frontal lobe. In HIV+ individuals on cART, we found that ALFF values in the bilateral occipital cortex were not significantly correlated with any clinical variables or cognitive performance. Some previous studies also demonstrated that there was no significant relationship between neuroimaging and HIV-related laboratory values and neuropsychological performance.11,14 These results suggest the possibility that HIV-related laboratory indicators and cognitive performance have been affected by medication with the introduction of cART.
Limitations
There are several limitations to this study. First, we acknowledge that the results were obtained from a scant number of patients, which might lower the statistical power. Further studies with larger cohorts are needed to confirm our findings. Second, neuropsychological evaluation in HCs was not conducted. Future studies should include more detailed information in control subjects. Third, this study only focuses on male homosexual transmission HIV patients in China; thus, the findings could not be generalized to other ways of HIV transmission or other countries. Fourth, perfusion MRI had not been performed. It was necessary to combine perfusion with rs-fMRI in a cohort of HIV-infected patients to investigate the effect of perfusion on BOLD dependent signal in future study. Fifth, this study was limited by cross-sectional design. Longitudinal rs-fMRI studies in HIV-infected patients before and after cART are warranted. However, all the patients (HIV+/cART– and HIV+/cART+) were enrolled from Beijing city, and there was no difference in duration of infection before medication, which could diminish the bias induced from a cross-sectional design to some extent.
Conclusion
In summary, our study indicated that early HIV patients showed reduced spontaneous brain activity in the occipital visual cortex. cART appeared to be ineffective in halting the HIV-induced accelerated neurodegenerative process but may delay the progression of neural dysfunction to some extent. Another finding was that HIV+/cART+ had higher spontaneous brain activity in auditory cortex, and cART drugs might affect the central auditory processing; thus a longitudinal study needs to be designed and performed in order to investigate the effect of HIV and cART on brain function over time. ALFF appeared to have the potential for detecting altered spontaneous brain activity in HIV patients and for monitoring the therapeutic effect of cART.
Acknowledgments
This paper was supported by the National Natural Science Foundation of China (No. 81771806, 81701679), Capital Medical University Research and Incubation Funding (No. PYZ2017124), and Beijing Municipal Administration of Hospitals Incubating Program (No. PX2016036). The authors would like to express deep appreciation to all anonymous reviewers for their comments.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Roberts TK, Buckner CM, Berman JW. Leukocyte transmigration across the blood-brain barrier: perspectives on neuro AIDS. Front Biosci. 2010;15(2):478–536. doi:10.2741/3631
2. Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi:10.1212/01.WNL.0000287431.88658.8b
3. Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi:10.1007/s13365-010-0006-1
4. Gandhi NS, Skolasky RL, Peters KB, et al. A comparison of performance-based measures of function in HIV-associated neurocognitive disorders. J Neurovirol. 2011;17(2):159–165. doi:10.1007/s13365-011-0023-8
5. Garvey LJ, Pavese N, Politis M, et al. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. Aids. 2014;28(1):67–72. doi:10.1097/01.aids.0000432467.54003.f7
6. Jessen Krut J, Mellberg T, Price RW, et al. Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PLoS One. 2014;9(2):e88591. doi:10.1371/journal.pone.0088591
7. Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13(11):976–986. doi:10.1016/S1473-3099(13)70269-X
8. Holt JL, Kraft-Terry SD, Chang L. Neuroimaging studies of the aging HIV-1-infected brain. J Neurovirol. 2012;18(4):291–302. doi:10.1007/s13365-012-0114-1
9. Zhang D, Raichle ME. Disease and the brain’ s dark energy. Nat Rev Neurol. 2010;6(1):15–28. doi:10.1038/nrneurol.2009.198
10. Thomas JB, Brier MR, Ortega M, Benzinger TL, Ances BM. Weighted brain networks in disease: centrality and entropy in human immunodeficiency virus and aging. Neurobiol Aging. 2015;36(1):401–412. doi:10.1016/j.neurobiolaging.2014.06.019
11. Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM. Pathways to neurodegeneration: effects of HIV and aging on resting-state functional connectivity. Neurology. 2013;80(13):1186–1193. doi:10.1212/WNL.0b013e318288792b
12. Wang X, Foryt P, Ochs R, et al. Abnormalities in resting-state functional connectivity in early human immunodeficiency virus infection. Brain Connect. 2011;1(3):207–217. doi:10.1089/brain.2011.0016
13. Ann HW, Jun S, Shin NY, et al. Characteristics of resting-state functional connectivity in HIV-associated neurocognitive disorder. PLoS One. 2016;11(4):e0153493. doi:10.1371/journal.pone.0153493
14. Ortega M, Brier MR, Ances BM. Effects of HIV and combination antiretroviral therapy on cortico-striatal functional connectivity. Aids. 2105;29(6):703–712. doi:10.1097/QAD.0000000000000611
15. Guha A, Wang L, Tanenbaum A, et al. Intrinsic network connectivity abnormalities in HIV-infected individuals over age 60. J Neurovirol. 2016;22(1):80–87. doi:10.1007/s13365-015-0370-y
16. Herting MM, Uban KA, Williams PL, et al. Default mode connectivity in youth with perinatally acquired HIV. Medicine (Baltimore). 2015;94(37):e1417. doi:10.1097/MD.0000000000000874
17. Ipser JC, Brown GG, Bischoff-Grethe A, et al. HIV infection is associated with attenuated frontostriatal intrinsic connectivity: a preliminary study. J Int Neuropsychol Soc. 2015;21(3):203–213. doi:10.1017/S1355617715000156
18. Janssen MAM, Hinne M, Janssen RJ, et al. Resting-state subcortical functional connectivity in HIV-infected patients on long-term cART. Brain Imaging Behav. 2017;11(5):1555–1560. doi:10.1007/s11682-016-9632-4
19. Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13(5):336–349. doi:10.1038/nrn3214
20. Zang YF, He Y, Zhu CZ, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91. doi:10.1016/j.braindev.2006.07.002
21. Fu Z, Tu Y, Di X, et al. Characterizing dynamic amplitude of low-frequency fluctuation and its relationship with dynamic functional connectivity: an application to schizophrenia. Neuroimage. 2018;180(Pt B):
22. Li P, Ding D, Ma XY, Zhang HW, Liu JX, Zhang M. Altered intrinsic brain activity and memory performance improvement in patients with end-stage renal disease during a single dialysis session. Brain Imaging Behav. 2018. doi:10.1007/s11682-018-9828-x
23. Luo S, Qi RF, Wen JQ, et al. Abnormal intrinsic brain activity patterns in patients with end-stage renal disease undergoing peritoneal dialysis: a resting-stage functional MR imaging study. Radiology. 2016;278(1):181–189. doi:10.1148/radiol.2015141913
24. McIntosh RC, Paul R, Ndhlovu LC, et al. Resting-state connectivity and spontaneous activity of ventromedial prefrontal cortex predict depressive symptomology and peripheral inflammation in HIV. J Neurovirol. 2018;24(5):616–628. doi:10.1007/s13365-018-0658-9
25. Qi R, Zhang L, Wu S, et al. Altered restingstate brain activity at functional MR imaging during the progression of hepatic encephalopathy. Radiology. 2012;264(1):187–195. doi:10.1148/radiol.12111429
26. Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI clustering in AFNI: false-positive rates redux. Brain Connect. 2017;7(3):152–171. doi:10.1089/brain.2016.0475
27. Egbert AR, Biswal B, Karunakaran K, et al. Age and HIV effects on resting state of the brain in relationship to neurocognitive functioning. Behav Brain Res. 2018;344:20–27. doi:10.1016/j.bbr.2018.02.007
28. Ances BM, Sisti D, Vaida F, et al. Resting cerebral blood flow: a potential biomarker of the effects of HIV in the brain. Neurology. 2009;73(9):702–708. doi:10.1212/WNL.0b013e3181b59a97
29. Aylward EH, Brettschneider PD, McArthur JC, et al. Magnetic resonance imaging measurement of gray matter volume reductions in HIV dementia. Am J Psychiatry. 1995;152(7):987–994. doi:10.1176/ajp.152.7.987
30. Filippi CG, Ulug AM, Ryan E, Ferrando SJ, van Gorp W. Diffusion tensor imaging of patients with HIV and normal-appearing white matter on MR images of the brain. AJNR Am J Neuroradiol. 2001;22(2):277–283.
31. Küper M, Rabe K, Esser S, et al. Structural gray and white matter changes in patients with HIV. J Neurol. 2011;258(6):1066–1075. doi:10.1007/s00415-010-5883-y
32. Cui Y, Jiao Y, Chen YC, et al. Altered spontaneous brain activity in type 2 diabetes: a resting-state functional MRI study. Diabetes. 2014;63(2):749–760. doi:10.2337/db13-0519
33. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi:10.1093/brain/awl004
34. Mwanza JC, Nyamabo LK, Tylleskär T, Plant GT. Neuro-ophthalmological disorders in HIV infected subjects with neurological manifestations. Br J Ophthalmol. 2004;88(11):1455–1459. doi:10.1136/bjo.2004.044289
35. Roelfsema PR, de Lange FP. Early visual cortex as a multiscale cognitive blackboard. Annu Rev Vis Sci. 2016;2:131–151. doi:10.1146/annurev-vision-111815-114443
36. Boban J, Kozic D, Turkulov V, et al. HIV-associated neurodegeneration and neuroimmunity: multivoxel MR spectroscopy study in drug-naïve and treated patients. Eur Radiol. 2017;27(10):4218–4236. doi:10.1007/s00330-017-4772-5
37. Ferguson D, Clarke S, Berry N, Almond N. Attenuated SIV causes persisting neuroinflammation in the absence of a chronic viral load and neurotoxic antiretroviral therapy. Aids. 2016;30(16):2439–2448. doi:10.1097/QAD.0000000000001178
38. Gendelman HE, Gelbard HA. Adjunctive and long-acting nanoformulated antiretroviral therapies for HIV-associated neurocognitive disorders. Curr Opin HIV AIDS. 2014;9(6):585–590. doi:10.1097/COH.0000000000000111
39. Whitehead N, Potterton J, Coovadia A. The neurodevelopment of HIV-infected infants on HAART compared to HIV-exposed but uninfected infants. AIDS Care. 2014;26(4):497–504. doi:10.1080/09540121.2013.841828
40. Ellero J, Lubomski M, Brew B. Interventions for neurocognitive dysfunction. Curr HIV/AIDS Rep. 2017;14(1):8–16. doi:10.1007/s11904-017-0346-z
41. Zhuang Y, Qiu X, Wang L, et al. Combination antiretroviral therapy improves cognitive performance and functional connectivity in treatment-naïve HIV-infected individuals. J Neurovirol. 2017;23(5):704–712. doi:10.1007/s13365-017-0553-9
42. Corrêa DG, Zimmermann N, Ventura N, et al. Longitudinal evaluation of resting-state connectivity, white matter integrity and cortical thickness in stable HIV infection: preliminary results. Neuroradiol J. 2017;30(6):535–545. doi:10.1177/1971400917739273
43. Plessis SD, Vink M, Joska JA, Koutsilieri E, Stein DJ, Emsley R. HIV infection and the fronto-striatal system: a systematic review and meta-analysis of fMRI studies. Aids. 2014;28(6):803–811. doi:10.1097/QAD.0000000000000151
44. Ances BM, Roc AC, Wang J, et al. Caudate blood flow and volume are reduced in HIV+ neurocognitively impaired patients. Neurology. 2006;66(6):862–866. doi:10.1212/01.wnl.0000203524.57993.e2
45. Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr. 2012;59(5):469–477. doi:10.1097/QAI.0b013e318249db17
46. Becker JT, Sanders J, Madsen SK, et al. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav. 2011;5(2):77–85. doi:10.1007/s11682-011-9113-8
47. Thames AD, Sayegh P, Terashima K, et al. Increased subcortical neural activity among HIV+ individuals during a lexical retrieval task. Neurobiol Dis. 2016;92(Pt B):175–182. doi:10.1016/j.nbd.2015.10.017
48. Melrose RJ, Tinaz S, Castelo JMB, Courtney MG, Stern CE. Compromised fronto-striatal functioning in HIV: an fMRI investigation of semantic event sequencing. Behav Brain Res. 2008;188(2):337–347. doi:10.1016/j.bbr.2007.11.021
49. Heinze B, Swanepoel DW, Hofmeyr LM. Systematic review of vestibular disorders related to human immunodeficiency virus and acquired immunodeficiency syndrome. J Laryngol Otol. 2011;125(9):881–890. doi:10.1017/S0022215111001423
50. Kallail KJ, Downs D, Scherz J, Sweet D, Zackula RE. Prevalence of communication disorders in HIV-infected adults. J Int Assoc Provid AIDS Care. 2014;13(1):8–11. doi:10.1177/2325957413510608
51. Torre P
52. Van der Westhuizen Y, de Swanepoel W, Heinze B, Hofmeyr LM. Auditory and otological manifestations in adults with HIV/AIDS. Int J Audiol. 2013;52(1):37–43. doi:10.3109/14992027.2012.721935
53. Khoza K, Ross E. Auditory function in a group of adults infected with HIV/AIDS in Gauteng, South Africa. S Afr J Commun Disord. 2002;49:17–27.
54. Campanini A, Marani M, Mastroianni A, Cancellieri C, Vicini C. Human immunodeficiency virus infection: personal experience in changes in head and neck manifestations due to recent anti-retroviral therapies. Acta Otorhinolaryngol Ital. 2005;25(1):30–35.
55. Reyes-Contreras L, Silva-Rojas A, Ysunza-Rivera A, Jimenez-Ruiz G, Berruecos-Villalobos P, Romo-Gutierrez G. Brainstem auditory evoked response in HIV-infected patients with and without AIDS. Arch Med Res. 2002;33(1):25–28.
56. Matas CG, Leite RA, Magliaro FC, Goncalves IC. Audiological and electrophysiological evaluation of children with acquired immunodeficiency syndrome (AIDS). Braz J Infect Dis. 2006;10(4):264–268.
57. Matas CG, Silva SM, Marcon Bde A, Gonçalves IC. Electrophysiological manifestations in adults with HIV/AIDS submitted and not submitted to antiretroviral therapy. Pro Fono. 2010;22(2):107–113.
58. Heilman KJ, Harden ER, Weber KM, Cohen M, Porges SW. Atypical autonomic regulation, auditory processing, and affect recognition in women with HIV. Biol Psychol. 2013;94(1):143–151. doi:10.1016/j.biopsycho.2013.06.003
59. Matas CG, Angrisani RG, Magliaro FC, Segurado AA. Audiological manifestations in HIV-positive adults. Clinics (Sao Paulo). 2014;69(7):469–475.
60. Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8(1):33–44. doi:10.1038/nrn2040
61. Sainz T, Serrano-Villar S, Diaz L, et al. The CD4/CD8 ratio as a marker T-cell activation, senescence and activation/exhaustion in treated HIV-infected children and young adults. Aids. 2013;27(9):1513–1516. doi:10.1097/QAD.0b013e32835faa72
62. Jahanshad N, Valcour VG, Nir TM, et al. Disrupted brain networks in the aging HIV+ population. Brain Connect. 2012;2(6):335–344. doi:10.1089/brain.2012.0105-Rev
63. Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci. 2012;16(1):17–26. doi:10.1016/j.tics.2011.11.007
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.