Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Effects of early administration of insulin-like growth factor-1 on cognitive function in septic encephalopathy
Authors Yang Y, Liang S, Li Y, Gao F, Cao Y, Zhao X, Gao G, Li L
Received 14 October 2018
Accepted for publication 17 December 2018
Published 23 January 2019 Volume 2019:15 Pages 323—337
DOI https://doi.org/10.2147/NDT.S190845
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Yang Yang,1,* Shengru Liang,2,* Yuqian Li,1,* Fei Gao,1 Yuan Cao,3 Xiaoyu Zhao,4 Guodong Gao,1 Lihong Li1
1Department of Neurosurgery, Tangdu Hospital, Air Force Medical University, Xi’an, Shaanxi Province 710038, China; 2Department of Endocrinology, Xijing Hospital, Air Force Medical University, Xi’an, Shaanxi Province 710032, China; 3Department of Neurosurgery, Xijing Hospital, Air Force Medical University, Xi’an, Shaanxi Province 710032, China; 4Department of Neurosurgery, The 986th Hospital of Chinese People’s Libertation Army, Xi’an, Shaanxi Province 710054, China
*These authors contributed equally to this work
Background: Both protective and therapeutic functions of insulin-like growth factor-1 (IGF-1) in brain injury have been reported, but its effects on cognitive sequelae after septic encephalopathy (SE) remain unclear.
Materials and methods: This study was divided into three parts, and a septic model was built by cecal ligation and puncture (CLP). First, survival analysis was performed, and IGF-1’s effects on long-term cognition and depressive emotion were assessed. Second, the characteristics of IGF-1 function in cognition were evaluated. Finally, cytochrome C, caspase-9, tumor necrosis factor receptor (TNFR), and caspase-8 levels as well as cell apoptosis in the hippocampus were evaluated.
Results: IGF-1 did not reduce mortality or alleviate depressive symptoms in septic rats, but improved the memory of noxious stimulation and spatial learning and memory. These effects were observed only when IGF-1 was administered within 0–6 hours after CLP. Moreover, cytochrome C and caspase-9 expression levels were increased at 6 hours after CLP in the hippocampus, while TNFR and caspase-8 amounts were not increased until 12 hours after CLP. Cell apoptosis increased at 12 hours after CLP, but was inhibited by IGF-1.
Conclusion: Cognitive impairment in rats recovering from SE is associated with cell apoptosis in the hippocampus. Supplementation of IGF-1 reduces cell apoptosis by preventing the over-expression of cytochrome C and TNFR, and results in improved cognitive function. However, improvement only occurs when IGF-1 is administered at the early stage (within 6 hours) of sepsis. As cytochrome C activation occurs earlier than that of TNFR in this study, cytochrome C may be the main factor inducing apoptosis in early SE.
Keywords: septic encephalopy, memory, learning, IGF-1, cytochrome C, TNFR, apoptosis
Introduction
Sepsis is a systemic inflammation caused by severe infection.1–3 Although effective treatments, including administration of antibiotics, antithrombin, and β-2 agonists,4–7 fluid resuscitation and assessment of hemodynamics,8,9 and prophylaxis of stress ulcer and transfusion of red blood cells,10,11 have been adopted to reduce mortality in sepsis, many survivors still suffer from septic encephalopathy (SE), a neurological complication of sepsis characterized by short- and long-term symptoms.12–14 Long-term cognitive impairment and psychiatric disorders, such as memory deficits, learning disabilities, and depressive-like symptoms (not anxiety-like symptoms), can seriously lower the patient’s quality of daily life.12,15,16 Thus, the neurological sequelae caused by sepsis remain important medical problems, and new therapies targeted on them are urgently needed.
Studies indicate that two pathways, initiated by tumor necrosis factor receptor (TNFR) and cytochrome C, respectively, participate in the activation of cell apoptosis, including in hippocampal CA1 neurons.17,18 Thus, we hypothesized that the above neurological sequelae may be related to cell apoptosis, with TNFR and cytochrome C constituting the key factors promoting cell apoptosis in SE. IGF-1, mainly produced by the liver, has important functions on body inflammation and immunity and brain cell differentiation, proliferation, and maturation.19–23 Previous findings indicate that insulin-like growth factor-1 (IGF-1) may be an important anti-apoptosis factor.24 Administration of IGF-1 is associated with reduced oligodendrocyte apoptosis caused by a variety of insults.25–27 Guan et al found that intraventricular injection of IGF-1 reduces neuronal apoptosis in the hippocampus and has a potential therapeutic impact on hypoxic-ischemic brain injury.28 However, tissue IGF-1 amounts are mainly regulated by serum growth hormone (GH) levels and are decreased during inflammation, especially in sepsis, due to GH secretion and resistance.29,30 Upregulation of proinflammatory cytokines attenuates IGF-1 bioactivity by upregulating insulin-like growth factor binding proteins (IGFBPs), mainly IGFBP-1. Moreover, inflammation of the central nervous system (CNS) suppresses microglia-derived neuronal IGF-1 production.31 All these factors jointly attenuate the anti-apoptosis effect of IGF-1 and make brain cells more sensitive to apoptotic stimuli.32,33
As IGF-1 shows anti-apoptotic benefits and its concentration and bioactivity are decreased in sepsis, it may constitute a potential factor for SE treatment. In this study, the effects of IGF-1 on cognitive deficits and cell apoptosis in the hippocampus of septic rats were clarified. Then, we investigated whether the anti-apoptotic effect of IGF-1 is associated with cytochrome C and TNFR regulation.
Materials and methods
Animals and animal care
All studies were performed in accordance with National Institutes of Health guidelines and approved by the Animal Ethical Committee of Air Force Medical University. The male Sprague Dawley rats (n=435) ranging from 220 to 250 g were obtained from the laboratory animal center of Air Force Medical University. Rats were housed under a 12-hour light–dark cycle with free access to food and water.
Cecal ligation and puncture (CLP) surgery
Rats were deeply anesthetized with ketamine (90 mg/kg body weight) and xylazine (10 mg/kg body weight), and sepsis was induced by CLP as described previously.34 Briefly, lower quadrants of the abdomen were shaved and disinfected with alcohol. A longitudinal midline incision was then made, and the cecum was exteriorized from the abdominal cavity. Cecal contents were pushed to the distal side. Then, ligation was performed between the ileocecal valve and the distal cecum by using a 3-0 nylon filament. As the severity of sepsis is controlled by ligation at different sites between the ileocecal valve and the distal cecum, moderate sepsis was adopted in this study by ligation at the middle of the cecum followed by puncture from side to side at the middle of the ligated cecum. Small droplets of feces were extruded from both holes, and the cecum was then returned to the abdominal cavity. The peritoneum and the skin were closed by simple interrupted sutures. In the sham-operation group, a similar procedure was performed but without ligation and puncture of the cecum. All rats were returned to their cages and housed under a 12-hour light–dark cycle with free access to food and water.
Experimental groups and drug treatment
The study was divided into three parts and all rats were subcutaneously injected with saline (37°C, 5 mL/100 g) immediately and 12 hours after surgery. The first part, designed to evaluate the effect of IGF-1 on cognition of septic rats, was divided into five groups, that is sham-operative (n=15), antibiotic (n=30), antibiotic + IGF-1 (n=30), IGF-1 (n=30), and saline (n=30) groups. Rats in the antibiotic and antibiotic + IGF-1 groups were subcutaneously injected with ceftriaxone (Hoffman-La Roche Ltd., Basel, Switzerland) (3 mg/100 g) every 6 hours for 3 days. Rats in the antibiotic + IGF-1 and IGF-1 groups were subcutaneously injected with IGF-1 (ProsPec, Rehovot, Israel) (1 mg/kg) immediately and every 12 hours after surgery for 10 days (Figure S1A). The second part, also divided into five groups, was designed to study the feature of IGF-1’s effects on cognition of septic rats. Antibiotics were given to all rats, but IGF-1 was given at 0 hours (n=30), 6 hours (n=30), 12 hours (n=30), 24 hours (n=30), or 36 hours (n=30) after surgery in each group, respectively, and then supplied every 12 hours for 10 days (Figure S1B). The third part, designed to study the mechanisms of IGF-1’s function on cognition of septic rats, consisted of sham-operative (n=30), antibiotic (n=60), and antibiotic + IGF-1 (n=60) groups. Each group was divided into two subgroups, one for Western blot at 6 hours (n=10 in the sham-operation group; n=20 in both antibiotic and antibiotic + IGF-1 groups) and 12 hours (n=10 in the sham-operation group; n=20 in both antibiotic and antibiotic + IGF-1 groups) after surgery, and another for TUNEL and Nissl’s staining (n=10 in the sham-operation group; n=20 in both antibiotic and antibiotic + IGF-1 groups) 12 hours after surgery. Antibiotics were given immediately and then at 6 hours and 12 hours after surgery, respectively. IGF-1 (1 mg/kg) was given immediately after surgery in the sham-operated and antibiotic + IGF-1 groups (Figure S1C). The dosage selection of IGF-1 was based on previous studies in mice and rats.35,36
Behavioral trial
Behavioral tests were performed 10 days after surgery at 8:00–11:30 am with the instruments provided by the Department of Neurosurgery (Tangdu Hospital, Army Force Medical University, Xi’an, Shannxi, China). Tests were conducted and results were recorded by two colleagues who were blinded to the research.
Open-field tests
The open field was 40×60 cm and surrounded by 50 cm high walls made of black plywood. The ground floor was divided into nine equally spaced squares by black lines. After rats were fully accustomed to the experimental environment, they were placed in the middle of the floor and were allowed to move freely for 5 minutes (training session). Then all rats were put back into cages and at the same time on the second day, they received a similar training in another open field (testing session). The total number of crossings (horizontal activity) and rearings (standing upright), and the total time spent in the center and corner of the open field were recorded. At the end of each test, any olfactory cues on the surface of the arena were carefully eliminated.
The forced swimming test
A cylindrical tank made of transparent glass was used in this test. Rats cannot touch the bottom of the tank or escape when the tank was full of water. At the beginning of tests, water (23°C) was filled into the tank to a depth of 50 cm, and rats were placed in the water to swim for 15 minutes. Then rats were put back into cages and at the same time on the second day, they received a similar test for 5 minutes. In the second test, the periods of immobility, swimming, and struggling time were recorded and depressive-like behavior was evaluated by the immobility time. At the end of each test, water was replaced in order not to affect the behavior of the subsequent animals.
Morris water maze tests
A water maze pool full of opaque water and an escape platform (submerged 2.5 cm, not visible) was employed to evaluate the spatial learning and memory ability of rats. The escape platform was placed in one quadrant of the pool. Rats were randomly released on one side of the pool and 1 minute was given to find the platform. Once finding the platform, they were allowed to stay there for 30 seconds. All rats were trained four times per day for 4 consecutive days, and the time to reach the platform (latency) was recorded. The next day of the last training, rats started to swim from the same place without the platform. The number of “times” rats arrived at the quadrant within 1 minute was recorded.
Passive avoidance test
The apparatus consists of a light and a dark compartment (30×30×30 cm3) with a circular door on the wall between them. The floor of the dark compartment was electrifiable. Rats were fully accustomed to the experimental environment and then placed in the light room. When the rats stepped into the dark room (four paws), a 0.8 mA, 4.0-second scrambled foot shock was applied for 15 seconds and then rats were taken back to cages (training session). At the same time on the second day, rats received the same training (testing session), and the time they spent in the training and testing sessions to enter the dark room was recorded (latency). When rats did not enter the dark room for over 120 seconds, a time of 120 seconds was recorded.
Western blot analysis
Rats were killed by decapitation at 6 and 12 hours after CLP and hippocampus was immediately isolated on ice and stored at −80°C for further analysis. Samples were homogenized in lysis buffer containing phenylmethanesulfonyl fluoride for 30 minutes and then centrifuged (12,000 rpm) for 20 minutes. Protein quantitation was performed by using bicinchoninic acid protein assay kit (Beyotime, Shanghai, China). The samples were separated using 12% SDS-PAGE and then electrophoresis was adopted to transfer the samples to nitrocellulose membrane. Then, the membranes were blocked using 5% skim milk for 1 hour, and incubated overnight at 4°C with the appropriate primary rabbit anti-rat cytochrome C antibody (1:200; Protein Tech, Chicago, IL, USA), anti-rat caspase-9 antibody (1:300; Protein Tech), anti-rat TNFR1 antibody (1:300; Protein Tech), and anti-rat caspase-8 antibody (1:200; Protein Tech). After washing with Tris-buffered saline with Tween-20, membranes were incubated with horseradish peroxidase-conjugated appropriate secondary antibodies (1:2,000; Jackson, West Grove, PA, USA) for 1 hour. The membranes were washed and the blots were visualized using enhanced chemiluminescence reagents (EMD Millipore, Billerica, MA, USA) and Kodak (Fuji, Japan) radiography film. Bands were digitally scanned and analyzed by using ImageJ software, and the intensity signal of each band in each group was then recorded for statistical comparisons.
TUNEL staining
Rats were killed 12 hours after CLP. Brains were isolated and fixed in formalin for 48 hours. Tissues were embedded in paraffin and sliced into 5 μm sections. After deparaffinization and dexylenation, tissues were stained with fluorescein-dUTP for apoptotic cell nuclei according to the manufacturer’s instructions of In Situ Cell Death Detection Kit (Hoffman-La Roche Ltd). The nuclei were stained with DAPI (1:200; Thermo Fisher Scientific, Waltham, MA, USA) for 10 minutes. The ratio of apoptotic cells in five microscopic fields in the hippocampus region was evaluated manually by two colleagues blinded to the study by using fluorescent microscope (Olympus Corporation, Tokyo, Japan).
Nissl’s staining
To further exhibit the morphology of neuron and quantify it, Nissl’s staining was adopted. Neurons without apoptotic structural changes were counted, and criteria included the presence of a round, open, pale nucleus (not condensed and darkly stained) and granular Nissl staining of the cytoplasm. Early apoptosis of neuron was defined by nascent chromatin condensation into mini-masses, and end-stage apoptosis of neuron was characterized by the presence of at least two discrete, large round nuclear masses. According to these criteria, astrocytes, oligodendrocytes, and microglia were excluded from the counts. One percent toluidine blue was preheated to 50°C and then the samples for TUNEL staining were hydrated in toluidine blue for 20 minutes in a 56°C thermotank. Then they were washed in water and differentiated in 70% ethanol for 1 minute and 95% ethanol for 30 seconds. Then they were dehydrated in gradient ethanol and cleaned with xylene. Five microscopic fields in hippocampus were then selected under optical microscope (Olympus) to take count of the ratio of apoptotic neurons manually by two colleagues blinded to the study.
Statistical analysis
Statistical analysis was performed with SPSS Statistics (SPSS 20; SPSS, Inc, Chicago, IL, USA) and all values are presented as mean ± SD. The survival rate was calculated by the Kaplan–Meier method and compared by log-rank test. The comparisons among multiple groups were performed by one-way classification of ANOVA followed by Bonferroni’s test. The comparisons between two groups were performed by Student’s t-test. Nonparametric statistics were treated by Kruskal–Walls H-test followed by Nemenyi test. Differences were considered statistically different when P-values <0.05.
Ethics approval and informed consent
All studies were performed in accordance with National Institutes of Health guidelines and approved by the Animal Ethical Committee of Air Force Medical University.
Results
After 6 hours of surgery, all the animals administered CLP exhibited the symptoms of sepsis and/or SE, including malaise, chills, piloerection, reduced gross motor activity, tachypnea, and diarrhea. Meanwhile, rats in the sham-operated group showed no signs of sepsis or SE.
Animal survival
Lethality began as early as 12 hours after CLP. No animal died in the sham-operation group (n=15). In the antibiotic group (n=30), 15 animals died in the first 3 days, indicating a mortality rate of 50%. In the antibiotic + IGF-1 group (n=30), 17 animals died in the first 5 days, indicating a mortality rate of 53.7%. In the saline group (n=30), 25 animals died in the first 3 days, indicating a mortality rate of 83.3%. In the IGF-1 group (n=30), 24 rats died within 4 days, ie, a mortality rate of 80% (Figure 1).
Behavioral properties in the first part
Behavior trails were used to evaluate the effect of IGF-1 on cognitive impairment and emotional change during sepsis. Animals that died during the 10 days were excluded.
Open-field test
General behavior and habitual memory were tested in the open field. No differences were found in the number of crossings or rearings among groups in the training session, indicating that the ability of exploration and motion was similar among groups. When compared with their own data in training session, the number of crossings and rearings was reduced in sham-operation, antibiotic + IGF-1, and IGF-1 groups in the testing session, while no differences were found in antibiotic and saline groups. These findings indicated an impairment of memory in new environment (Figure 2).
Morris water maze results
Spatial learning and memory ability were evaluated by the Morris water maze test. On the first 2 days, no differences were found in time spent by the animals to reach the platform among the five groups. However, the antibiotic and saline groups spent more time reaching the platform on the third and fourth days compared with the sham-operation group, which indicated an impairment of learning ability and a protective effect of IGF on learning (Figure 3A). The number of times rats reached the target quadrant within 1 minute was recorded on the fifth day; the results showed that this number was lower in the antibiotic and saline groups compared with the sham-operation group, while no differences were found among sham-operation, antibiotic + IGF-1, and IGF-1 groups, or between the antibiotic and saline groups. These results indicated memory impairment during sepsis, which could be relieved by IGF-1 (Figure 3B).
Passive avoidance results
The memory of noxious stimulation was evaluated by the passive avoidance test. In the training session, rats spent similar times stepping into the dark compartment. Compared with their own data in the training session, rats in the sham-operation, antibiotic + IGF-1, and IGF-1 groups took more time to step into the dark compartment in the testing session, while no differences were found in the antibiotic and saline groups, indicating that IGF-1 could relieve the impairment of noxious memory after sepsis (Figure 4).
Emotional changes assessed by the forced swim test
The forced swim test was used to evaluate whether emotional changes occurred in septic rats. Compared with the sham-operative group, immobility time was prolonged in the remaining four groups administered CLP, with no significant differences among them. These findings indicated that septic rats exhibited depressive-like symptoms which might be caused by SE, and IGF-1 could not improve them (Figure 5).
Behavior properties in the second part
Behavior trials in this part were performed to investigate whether the protective effects of IGF-1 were time-dependent. A total of 10 days after CLP, there were 15, 11, 12, 14, and 12 rats left in the 0 hour, 6 hours, 12 hours, 24 hours, and 36 hours groups, respectively. Rats which died during the 10 days were excluded.
Open-field test results
No differences were found among groups in the number of crossings and rearings in the training session, indicating that the ability of exploration and motion was similar among groups. Compared with their own data in the training session, the number of crossings and rearings in the testing session was decreased in the 0-hour and 6-hour IGF-1 groups. However, no statistically significant differences were found in other groups. These findings indicated that administration of IGF-1 at 12, 24, or 36 hours after CLP could not protect memory in a new environment (Figure 6).
Morris water maze results
On the first 2 days of training, the time required by rats to reach the platform had no significant differences among the five groups. On the third and fourth days, animals in the 0-hour and 6-hour IGF-1 groups spent less time to reach the platform compared with the 12-hour, 24-hour, and 36-hour groups (Figure 7A). The numbers of times rats reached the target quadrant within 1 minute was recorded on the fifth day. The results showed that the number of times rats reached the target quadrant within 1 minute was higher in the 0-hour and 6-hour IGF-1 groups compared with the remaining three groups. No significant differences were observed among the 12-hour, 24-hour, and 36-hour IGF-1 groups or between the 0-hour and 6-hour IGF-1 groups (Figure 7B). These findings indicated that injection of IGF-1 at 12, 24, or 36 hours after CLP could not protect learning ability and spatial memory.
Passive avoidance test
Compared with training session, rats in testing session spent more time stepping into the dark compartment in 0-hour and 6-hour IGF-1 groups, while no differences were observed in 12-hour, 24-hour, and 36-hour IGF-1 groups. Besides, the time rats spent stepping into the dark compartment was similar among all groups in the training session. These results indicated that supplement of IGF-1 at 12, 24, or 36 hours after CLP could not improve memory of noxious stimulation (Figure 8).
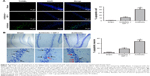
Cell apoptosis in the hippocampus
TUNEL staining showed that apoptotic rates in the antibiotic and antibiotic + IGF-1 groups were significantly higher than those of the sham-operation group, while the apoptotic rate in the antibiotic group was significantly higher than that of the antibiotic + IGF-1 group (Figure 9A). Nissl’s staining showed that neurons with normal morphology were predominant in the sham and antibiotic + IGF-1 groups, while neurons with early or end-stage apoptotic morphology were common in the antibiotic group. Moreover, the rates of neuronal apoptosis in the antibiotic and antibiotic + IGF-1 groups were significantly higher than that of the sham-operation group; meanwhile, the rate of neuronal apoptosis in the antibiotic group was significantly higher than that of the antibiotic + IGF-1 group (Figure 9B). These findings revealed the association of cell apoptosis inhibition in the hippocampus with memory and learning improvements during sepsis.
Protein quantification of cytochrome C and TNFR
As cell apoptosis in brain is tightly associated with the apoptotic pathway mediated by cytochrome C and TNFR,17,18 Western blot was performed to detect their expression in hippocampus. Results showed that 6 hours after CLP, the expression of cytochrome C and caspase-9 was higher in antibiotic group than in sham-operative and antibiotic + IGF-1 groups, while the expression of TNFR and caspase-8 was very low and had no differences among the three groups (Figure 10A). Twelve hours after CLP, the expression of TNFR and caspase-8 was increased and their expression was higher in antibiotic group than in sham-operative and antibiotic + IGF-1 groups. Moreover, the expression of cytochrome C and caspase-9 was still higher in antibiotic group (Figure 10B). These results indicated that both the pathways, initiated by cytochrome C and TNFR, participated in cell apoptosis during SE, but the activation of cytochorme C was earlier than TNFR. Moreover, IGF-1 could inhibit their expression.
Discussion
Although hippocampus is considered to be the center of learning and memory,37,38 the exact mechanisms of learning and memory remain unclear. Thus, treatment of learning and memory impairment remains a medical challenge. As a factor regulated by GH, previous reports have assessed the effect of IGF-1 on learning and memory impairment.39 Aging is considered to be the commonest cause of cognitive deficits.40,41 However, Erraji-Benchekroun et al found that it is not only aging but also age-related transcriptional changes of a variety of genes, including IGF-1, that are tightly related to cognitive deficits.42 A study by Bellar et al showed that intracerebroventricular injection of IGF-1 could alleviate cognitive deficits in elderly rats.43 These findings are supported by other studies indicating that adults with childhood-onset GH deficiency also exhibit memory deficits.44,45 Brain injury is another important cause of cognitive deficits, and IGF-1 has been reported to improve long-term cognition by reducing hypoxia-ischemia, oxidative-stress, and neuron apoptosis.46,47 Moreover, studies revealed the protective effect of IGF-1 on the brain undergoing ischemic injury in the context of age-related cerebrovascular diseases.48–50 Because SE is a common disease in the intensive care unit which frequently leads to learning and memory deficits, it is essential to develop new medicines or therapies to alleviate such deficits. The significance of the current study is that we were the first to investigate the effect of IGF-1 on the brain during sepsis, especially on cognitive and emotional changes.
As no previous study has assessed the role of IGF-1 in SE, we performed this research using a “whole to part” procedure, and divided it into several steps. First, the effects of IGF-1 on survival, and cognitive and emotional changes of septic rats were observed. As shown earlier, IGF-1 could improve learning and memory. Second, we evaluated whether the protective role of IGF-1 was time-dependent and preliminarily investigated the potential mechanism. The “whole to part” experimental design ensured a comprehensive, objective, credible, and accurate study.
We demonstrated for the first time that IGF-1 could protect spatial learning and memory, noxious memory, and memory in a new environment, which were impaired by sepsis, by inhibiting cell apoptosis in the hippocampus. Although Nissl’s staining indicated that neuron apoptosis is probably associated with memory and learning impairment, such conclusion cannot be definitely drawn without immunofluorescent staining using the neuron-specific nuclear protein. Moreover, we found that the anti-apoptotic role of IGF-1 might be associated with cytochrome C and TNFR downregulation. Interestingly, we also found that the protective effects of IGF-1 on spatial learning and memory, noxious memory, and memory in a new environment occurred only when the molecule was administered at the early stage of sepsis (less than 6 hours). These findings corroborated those reported by Barichello et al,51 who observed that oxidative stress in CNS is restricted to the earlier stage of sepsis, mainly before 6 hours. As oxidative stress is associated with mitochondrial permeability transition pore, the main regulator of cytochrome C release from the mitochondria to the cytoplasm,52 we believe that cognitive impairment is closely related to oxidative stress in CNS. In another study supporting our findings, Messaris et al demonstrated that mitochondrial-mediated apoptosis and cytochrome C release in CNS begin at the early stage (6–12 hours) of sepsis.17 As demonstrated earlier, it was the expression levels of cytochrome C and caspase-9 that were increased at 6 hours after surgery, but not of TNFR and caspase-8, and early injury of the hippocampus may be primarily caused by cytochrome C. Moreover, although IGF-1 was supplied during the whole study period, only the 0-hour and 6-hour IGF-1 groups showed improvement in learning and memory, indicating that cytochrome C expression at the early stage of sepsis plays a key role in hippocampal injury, and results in memory and learning impairment. Besides, mortality during sepsis was decreased by antibiotic treatment, and not IGF-1, as described previously.
Since IGF-1 is beneficial to cognitive function, selecting an appropriate time for drug administration seems important. In a study by Deijen et al, individuals with childhood-onset GH deficiency had improvement in memory deficit after GH administration for 6 consecutive months,53 indicating that although memory deficit development is a chronic process, it can be improved by GH when given for a certain period of time. Zhong et al found that administration of IGF-1 at 24 and 48 hours after hypoxia-ischemia could significantly reduce brain injury and improve long-term memory and cognition, but such effects were not observed with IGF-1 when administered after the first 2 days.46 In the current study, IGF-1 showed a protective effect on cognitive function when administered during the initial 6 hours after CLP, and no effect was observed in groups treated after 12 hours. In acute diseases such as SE and hypoxia-ischemia, cell apoptosis might be the main reason for learning and memory impairment; therefore, IGF-1 was provided early to relieve the impairment. In chronic diseases such as childhood-onset GH deficiency and aging-related cognitive deficits, learning and memory impairment is caused by IGF-1 and GH deficiencies, and these deficits can be reversed whenever IGF-1 is supplied.
This study had several limitations. First, as the study duration was shorter than that of other reports,12,54–56 the present findings only indicate that IGF-1 may protect from learning and memory impairment caused by sepsis. Further studies of longer duration (1 month or even longer) should be performed to evaluate the effect of IGF-1 on long-term cognition, as long-term cognitive impairment is common in patients recovering from sepsis. Second, as tolerance to sepsis and cecal contents are different among animals, it is difficult to achieve a uniform disease severity in models of sepsis. Third, since high mortality is associated with sepsis, the present sample size was not enough, especially in the saline group, which finally had only five survivors. Fourth, we only assessed cytochrome C and TNFR changes during sepsis, and whether other factors participated in hippocampal cell apoptosis deserves further attention. Fifth, as the forced swim test is mainly used in depressive disorders and associated treatments, the positive results in this study may only indicate that septic rats exhibit depressive-like symptoms which might be caused by SE; further studies should reveal the relationship between psychiatric disorders and SE. Finally, as DAPI is a fluorescent label for the nucleus of all kinds of cells, we could not distinguish apoptotic neurons from other apoptotic cells such as astrocytes and microglia; further studies are required to assess neuronal apoptosis by using the neuron-specific nuclear protein.
Conclusion
In conclusion, we demonstrate that IGF-1 can reduce cell apoptosis by preventing the over-expression of cytochrome C and TNFR, and lead to the improvement of cognitive function. Interestingly, the improvement only occurs with IGF-1 administered at the early stage of sepsis. As cytochrome C activation occurs earlier than that of TNFR, it may be the cause of apoptosis in early SE. As early IGF-1 administration relieves memory and learning impairment in SE, it may constitute a potential drug to improve cognitive function in septic patients.
Acknowledgments
The work was supported by the Department of Neurosurgery, Tangdu Hospital, Xi’an, China, and they provided all the experimental equipment for behavior tests. The work was also supported by the Department of Neurosurgery, Xijing Hospital, Xi’an, China, and they provided the experimental equipment for Western blot analysis and TUNEL staining. The authors thank Yuefan Yang, PhD, Department of Neurosurgery, Xijing Hospital, for his assistance in performing the Western blot analysis. The authors also thank Xiaoyan Chen, technician, Department of Neurosurgery, Xijing Hospital, for her technical guidance in performing TUNEL staining. We also thank Xingchen Zhao, PhD, Department of Genetics, Air Force Medical University, Xi’an, China, for his assistance in all the statistical work.
Disclosure
The authors report no conflicts of interest in this work.
References
Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. | ||
Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of Hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. | ||
Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348(2):138–150. | ||
Zhang D, Micek ST, Kollef MH. Time to appropriate antibiotic therapy is an independent determinant of postinfection ICU and hospital lengths of stay in patients with sepsis. Crit Care Med. 2015;43(10):2133–2140. | ||
Klompas M, Calandra T, Singer M. Antibiotics for Sepsis-Finding the equilibrium. JAMA. 2018;320(14):1433. | ||
Allingstrup M, Wetterslev J, Ravn FB, Møller A, Afshari A. Antithrombin III for critically ill patients. Cochrane Database Syst Rev. 2016;8(2):CD005370. | ||
Singh B, Tiwari AK, Singh K, et al. β2 agonist for the treatment of acute lung injury: a systematic review and meta-analysis. Respir Care. 2014;59(2):288–296. | ||
ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496–1506. | ||
Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. | ||
Krag M, Perner A, Wetterslev J, et al. Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive Care Med. 2015;41(5):833–845. | ||
Holst LB, Haase N, Wetterslev J, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371(15):1381–1391. | ||
Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. | ||
Widmann CN, Heneka MT. Long-term cerebral consequences of sepsis. Lancet Neurol. 2014;13(6):630–636. | ||
Salluh JI, Wang H, Schneider EB, et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. 2015;350(may19 3):h2538. | ||
Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60(6):1070–1077. | ||
Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012;8(10):557–566. | ||
Messaris E, Memos N, Chatzigianni E, et al. Time-dependent mitochondrial-mediated programmed neuronal cell death prolongs survival in sepsis. Crit Care Med. 2004;32(8):1764–1770. | ||
Zhan RZ, Wu C, Fujihara H, et al. Both caspase-dependent and caspase-independent pathways may be involved in hippocampal CA1 neuronal death because of loss of cytochrome c from mitochondria in a rat forebrain ischemia model. J Cereb Blood Flow Metab. 2001;21(5):529–540. | ||
Auernhammer CJ, Strasburger CJ. Effects of growth hormone and insulin-like growth factor I on the immune system. Eur J Endocrinol. 1995;133(6):635–645. | ||
Beck KD, Powell-Braxton L, Widmer HR, Valverde J, Hefti F. IGF1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron. 1995;14(4):717–730. | ||
Vicario-Abejón C, Yusta-Boyo MJ, Fernández-Moreno C, de Pablo F. Locally born olfactory bulb stem cells proliferate in response to insulin-related factors and require endogenous insulin-like growth factor-I for differentiation into neurons and glia. J Neurosci. 2003;23(3):895–906. | ||
Cao P, Maximov A, Südhof TC. Activity-dependent IGF-1 exocytosis is controlled by the Ca(2+)-sensor synaptotagmin-10. Cell. 2011;145(2):300–311. | ||
Feldman EL, Sullivan KA, Kim B, Russell JW. Insulin-like growth factors regulate neuronal differentiation and survival. Neurobiol Dis. 1997;4(3–4):201–214. | ||
Singleton JR, Dixit VM, Feldman EL. Type I insulin-like growth factor receptor activation regulates apoptotic proteins. J Biol Chem. 1996;271(50):31791–31794. | ||
Cao Y, Gunn AJ, Bennet L, et al. Insulin-like growth factor (IGF)-1 suppresses oligodendrocyte caspase-3 activation and increases glial proliferation after ischemia in near-term fetal sheep. J Cereb Blood Flow Metab. 2003;23(6):739–747. | ||
Lin S, Fan LW, Pang Y, Rhodes PG, Mitchell HJ, Cai Z. IGF-1 protects oligodendrocyte progenitor cells and improves neurological functions following cerebral hypoxia-ischemia in the neonatal rat. Brain Res. 2005;1063(1):15–26. | ||
Mason JL, Jones JJ, Taniike M, Morell P, Suzuki K, Matsushima GK. Mature oligodendrocyte apoptosis precedes IGF-1 production and oligodendrocyte progenitor accumulation and differentiation during demyelination/remyelination. J Neurosci Res. 2000;61(3):251–262. | ||
Guan J, Beilharz EJ, Skinner SJ, Williams CE, Gluckman PD. Intracerebral transportation and cellular localisation of insulin-like growth factor-1 following central administration to rats with hypoxic-ischemic brain injury. Brain Res. 2000;853(2):163–173. | ||
Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4(2):195–212. | ||
Lang CH, Fan J, Cooney R, Vary TC. IL-1 receptor antagonist attenuates sepsis-induced alterations in the IGF system and protein synthesis. Am J Physiol. 1996;270(3):E430–E437. | ||
Suh HS, Zhao ML, Derico L, Choi N, Lee SC. Insulin-like growth factor 1 and 2 (IGF1, IGF2) expression in human microglia: differential regulation by inflammatory mediators. J Neuroinflammation. 2013;10(1):37. | ||
Ross R, Miell J, Freeman E, et al. Critically ill patients have high basal growth hormone levels with attenuated oscillatory activity associated with low levels of insulin–like growth factor-I. Clin Endocrinol. 1991;35(1):47–54. | ||
Buckbinder L, Talbott R, Velasco-Miguel S, et al. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature. 1995;377(6550):646–649. | ||
Barichello T, Martins MR, Reinke A, et al. Cognitive impairment in sepsis survivors from cecal ligation and perforation. Crit Care Med. 2005;33(1):221–223. | ||
Saatman KE, Contreras PC, Smith DH, et al. Insulin-like growth factor-1 (IGF-1) improves both neurological motor and cognitive outcome following experimental brain injury. Exp Neurol. 1997;147(2):418–427. | ||
Contreras PC, Steffler C, Yu E, Callison K, Stong D, Vaught JL. Systemic administration of rhIGF-1 enhanced regeneration after sciatic nerve crush in mice. J Pharmacol Exp. 1995;274:1443–1449. | ||
Zeidman P, Maguire EA. Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat Rev Neurosci. 2016;17(3):173–182. | ||
García-García R, Cruz-Gómez ÁJ, Urios A, et al. Learning and memory impairments in patients with minimal hepatic encephalopathy are associated with structural and functional connectivity alterations in hippocampus. Sci Rep. 2018;8(1):9664. | ||
Yan H, Mitschelen M, Toth P, et al. Endothelin-1-induced focal cerebral ischemia in the growth Hormone/IGF-1 deficient Lewis dwarf rat. J Gerontol A Biol Sci Med Sci. 2014;69(11):1353–1362. | ||
Sonntag WE, Deak F, Ashpole N, et al. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci. 2013;5:27. | ||
Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5(2):87–96. | ||
Erraji-Benchekroun L, Underwood MD, Arango V, et al. Molecular aging in human prefrontal cortex is selective and continuous throughout adult life. Biol Psychiatry. 2005;57(5):549–558. | ||
Bellar D, Glickman EL, Juvancic-Heltzel J, Gunstad J. Serum insulin like growth factor-1 is associated with working memory, executive function and selective attention in a sample of healthy, fit older adults. Neuroscience. 2011;178:133–137. | ||
Clayton P, Gleeson H, Monson J, Popovic V, Shalet SM, Christiansen JS. Growth hormone replacement throughout life: insights into age-related responses to treatment. Growth Horm IGF Res. 2007;17(5):369–382. | ||
Arwert LI, Veltman DJ, Deijen JB, van Dam PS, Drent ML. Effects of growth hormone substitution therapy on cognitive functioning in growth hormone deficient patients: a functional MRI study. Neuroendocrinology. 2006;83(1):12–19. | ||
Zhong J, Zhao L, Du Y, Wei G, Yao WG, Lee WH. Delayed IGF-1 treatment reduced long-term hypoxia-ischemia-induced brain damage and improved behavior recovery of immature rats. Neurol Res. 2009;31(5):483–489. | ||
Wang J, Tang Y, Zhang W, et al. Insulin-like growth factor-1 secreted by brain microvascular endothelial cells attenuates neuron injury upon ischemia. FEBS J. 2013;280(15):3658–3668. | ||
Leinninger GM, Feldman EL. Insulin-like growth factors in the treatment of neurological disease. Endocr Dev. 2005;9:135–159. | ||
Mackay KB, Loddick SA, Naeve GS, Vana AM, Verge GM, Foster AC. Neuroprotective effects of insulin-like growth factor-binding protein ligand inhibitors in vitro and in vivo. J Cereb Blood Flow Metab. 2003;23(10):1160–1167. | ||
Schäbitz WR, Hoffmann TT, Heiland S, et al. Delayed neuroprotective effect of insulin-like growth factor-I after experimental transient focal cerebral ischemia monitored with MRI. Stroke. 2001;32(5):1226–1233. | ||
Barichello T, Fortunato JJ, Vitali AM, et al. Oxidative variables in the rat brain after sepsis induced by cecal ligation and perforation. Crit Care Med. 2006;34(3):886–889. | ||
Ghafourifar P, Schenk U, Klein SD, Richter C. Mitochondrial nitric-oxide synthase stimulation causes cytochrome c release from isolated mitochondria. Evidence for intramitochondrial peroxynitrite formation. J Biol Chem. 1999;274(44):31185–31188. | ||
Deijen JB, de Boer H, van der Veen EA. Cognitive changes during growth hormone replacement in adult men. Psychoneuroendocrinology. 1998;23(1):45–55. | ||
Semmler A, Frisch C, Debeir T, et al. Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exp Neurol. 2007;204(2):733–740. | ||
Yu X, Jia L, Yin K, Lv J, Yu W, Du H. Src is implicated in hepatic ischemia reperfusion-induced hippocampus injury and long-term cognitive impairment in young mice via NMDA receptor subunit 2A activation. Neuroscience. 2018;391:1–12. | ||
Freeman BD, Martins YC, Akide-Ndunge OB, et al. Endothelin-1 mediates brain microvascular dysfunction leading to long-term cognitive impairment in a model of experimental cerebral malaria. PLoS Pathog. 2016;12(3):e1005477. |
Supplementary material
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.