Back to Journals » Clinical Ophthalmology » Volume 17
Effects of Custom-Designed Soft Contact Lenses on Irregular Astigmatism Correction in Patients with Keratoconus
Authors Nishida T, Kojima T, Ogi S, Kataoka T, Isogai N, Yoshida Y, Nakamura T
Received 11 May 2023
Accepted for publication 6 July 2023
Published 27 July 2023 Volume 2023:17 Pages 2149—2162
DOI https://doi.org/10.2147/OPTH.S420940
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Tomoya Nishida, Takashi Kojima, Saaya Ogi, Takahiro Kataoka, Naoki Isogai, Yoko Yoshida, Tomoaki Nakamura
Nagoya Eye Clinic, Nagoya, Japan
Correspondence: Takashi Kojima, Nagoya Eye Clinic, Namiyose-cho 24-14, COLLECT MARK kanayama 2F atsuta-ku, Nagoya, 456-0003, Japan, Tel +81- 52-872-0490, Fax +81- 52-872-0491, Email [email protected]
Purpose: To evaluate the efficacy of YOUSOFT soft contact lenses in correcting irregular astigmatism and prescription results of patients with keratoconus.
Patients and Methods: The retrospective observational study included 55 eyes (mean age, 32.2 ± 10.6 years; 36 men and 6 women) of 42 patients with keratoconus who tried YOUSOFT for rigid gas permeable (RGP) lens intolerance. Average keratometry, corneal astigmatism, and maximum keratometry were 49.4 ± 5.2 diopters (D), 3.7 ± 2.1 D, and 57.3 ± 8.2 D, respectively. Patients were divided into YOUSOFT prescription and non-prescription cases, wherein the prescription rates were calculated. YOUSOFT visual acuity was compared with spectacle-corrected distance visual acuity (CDVA) and RGP lens-CDVA.
Results: YOUSOFT was prescribed to 28 out of 42 patients (prescription rate 67%). In the YOUSOFT prescription cases, YOUSOFT-CDVA (logMAR − 0.04; 95% confidence interval [CI]: − 0.08 to 0.00) was significantly better than spectacle-CDVA (logMAR 0.23; 95% CI: 0.08 to 0.38; P < 0.0001), whereas YOUSOFT-CDVA (logMAR − 0.03; 95% CI: − 0.08 to 0.03) did not significantly differ from the RGP lens-CDVA (logMAR − 0.02; 95% CI: − 0.08 to 0.04; P = 0.856).
Conclusion: YOUSOFT was effective in correcting irregular corneal astigmatism, suggesting that it is highly effective in patients with RGP lens intolerance.
Keywords: rigid gas-permeable contact lens intolerance, YOUSOFT soft contact lens, visual acuity, prescription rate
Introduction
Keratoconus develops in the teenage years or early adulthood and causes progressive changes in the corneal shape, including thinning and protrusion. The pathogenesis is supposedly a combination of abnormal collagen production and degradation caused by genetic abnormalities and environmental factors, including atopic dermatitis, contact lens wear, and allergies, resulting in corneal fragility and thinning.1,2 However, the specific mechanism is still unknown.
When keratoconus becomes severe, correcting the refractive error with glasses or soft contact lenses becomes difficult. Consequently, correcting the visual acuity with rigid gas permeable contact lenses is necessary. However, when the keratoconus becomes severe, the wear time is limited due to rigid gas permeable lens intolerance, such as foreign body sensation and pain caused by wearing the rigid gas permeable lens, and the quality of life deteriorates.3 Further, surgical treatment such as corneal transplantation is indicated if vision correction with contact lenses is poor, or it is difficult to wear rigid gas permeable lenses.4
For patients with rigid gas permeable lens intolerance, various methods—including the piggyback contact lens system, scleral lenses, hybrid contact lenses, and other special soft contact lenses—may be attempted, and various reports support the clinical efficacy of these lenses.5–11
KeraSoft (Bausch & Lomb Inc., Rochester, NY), a hydrogel material, is a soft contact lens designed for keratoconus. Previous reports showed that KeraSoft obtained better visual acuity than spectacle correction.12 Other reports also showed that patients with keratoconus could achieve equivalent visual acuity and quality of life with KeraSoft as they would with rigid gas permeable lenses.13
YOUSOFT (TOMEY CONTACT LENS CO., LTD. Nagoya, Japan) is a soft contact lens made of hydrogel material with a lens diameter and central thickness of 14.5 mm and 0.4 mm, respectively, having a prism ballast design. Although there have been many reports on KeraSoft,12–15 studies regarding the clinical outcomes of YOUSOFT are limited.16
Previous reports have shown that the corrected visual acuity of YOUSOFT was better than the uncorrected visual acuity before the wear; however, the effect of YOUSOFT in correcting irregular astigmatism has not yet been clarified. In this study, we compared the corrected visual acuity of YOUSOFT with that of spectacle-corrected visual acuity in patients with keratoconus to evaluate the effectiveness of YOUSOFT in correcting irregular astigmatism. The results of prescribing YOUSOFT for patients with keratoconus with rigid gas permeable lens intolerance were also examined.
Materials and Methods
Patients
Consecutive patients with keratoconus who visited the Nagoya Eye Clinic between April 2020 and July 2022 and requested a YOUSOFT prescription due to rigid gas permeable lens intolerance were studied retrospectively. Of these patients, both the final YOUSOFT prescription and non-prescription cases were also studied (Supplementary Materials).
Finally, 55 eyes of 42 consecutive patients (mean age 32.2 ± 10.6 years; 36 men and 6 women) were enrolled. Of these, 20, 2, and 3 eyes were post-corneal crosslinking, post-intrastromal corneal ring insertion, and post-cataract surgery, respectively.
The diagnosis of keratoconus was made by a corneal specialist using slit lamp microscopic findings and corneal shape analysis. For corneal shape analysis, corneal topography (TMS4: TOMEY, Nagoya, Japan) and anterior segment optical coherence tomography (CASIA2: TOMEY, Nagoya, Japan) were used.
The severity of keratoconus was classified based on the Amsler–Krumeich classification.17 Keratoconus severity classification was stage 1 (mild group) for 25 eyes, stage 2 (moderate group) for 22, stage 3 (severe group) for 3, and stage 4 (severe group) for 5. The average observation period was 5.6 (1–19) months after the YOUSOFT prescription.
This study was approved by the Institutional Review Board of the Nagoya Eye Clinic (2021-50) and conducted in accordance with the Declaration of Helsinki. Because this study was retrospective, an opt-out method, rather than a consent form, was approved; patient data were anonymized after collection. The correspondence table between study IDs and patient information was kept on a password-protected hospital computer.
YOUSOFT
YOUSOFT is a hydrogel material soft contact lens (Group II). The materials include N,N-dimethylacrylamide, N-vinylpyrrolidone, methyl methacrylate, alkyl methacrylate compounds, and ethylene glycol dimethacrylate. The water content is 80%, the oxygen permeability is 44×10−11 (cm2/sec) [mLO2/(mL x mmHg)], and the light transmittance is ≥90%. The lens has a prism-ballast design.
The lens has a diameter of 14.5 mm, a central thickness of 0.4 mm, and a base curve of 7.8–8.8 mm (0.2 mm step) in six steps. Correctable spherical power is + 30.0 diopters to −30.0 diopters (0.25 diopter step), astigmatism power is −0.25 diopters to −6.0 diopters (0.25 diopter step), and astigmatism axis is 5° to 180° (5° step).
YOUSOFT Fitting Procedure
YOUSOFT was fit according to the manufacturer’s recommendation. Briefly, the base curve of the trial lens was first selected according to the severity of the keratoconus; 8.2 mm was selected for mild-to-moderate cases and 8.0 mm for severe cases. Next, the movement of the trial lens was assessed immediately after wearing it. If the trial lens moved down by 2 mm or more when looking upward, the base curve of the trial lens was changed to a one-step steep lens. Also, if the lens was stuck with almost no movement, the base curve of the trial lens was changed to a one-step flat lens. Additionally, if there was no problem with the lens movement immediately after wearing it, patients wore it for another 15 min to make the lens fit on the cornea, and after 15 min of wearing the lens, lens movement was examined again. Further, when there was no problem with the lens movement, the lens power was adjusted by objective and subjective refraction measurements, and the visual acuity was confirmed while wearing YOUSOFT. Finally, the cornea specialist made a final confirmation of the lens fitting under a slit lamp microscope and prescribed it if the patient desired.
Visual Acuity Measurements
First, spectacle-corrected distance visual acuity was measured. Subsequently, corrected distance visual acuity was measured while wearing YOUSOFT (YOUSOFT-corrected distance visual acuity). For patients who were rigid gas permeable lens users, their corrected distance visual acuity under rigid gas permeable lens wear (rigid gas permeable lens-corrected distance visual acuity) was also measured.
Evaluation Items
The following items were evaluated: 1) YOUSOFT prescription rate and reasons for non-prescription; 2) comparison of patient backgrounds between those with YOUSOFT prescription and non-prescription; 3) comparison of YOUSOFT-corrected distance visual acuity with spectacle-corrected distance visual acuity; 4) comparison of YOUSOFT-corrected distance visual acuity with rigid gas permeable lens-corrected distance visual acuity; 5) YOUSOFT visual acuity according to the keratoconus severity; and 6) complications due to YOUSOFT use.
Anterior Segment Optical Coherence Tomography Parameters
The following six parameters were used for corneal shape and aberration evaluation: average keratometry (diopter); average corneal refractive power of keratometric power at 3 mm diameter by axial power map, corneal astigmatism (diopter); corneal astigmatism of keratometric power at 3 mm diameter by axial power map, maximum keratometry (diopter); maximum keratometric power in the 10 mm diameter region by axial power map, anterior asymmetry component (diopter); asymmetry component within 6 mm diameter of the anterior surface of the cornea obtained by Fourier analysis, posterior asymmetry component (diopter); asymmetric component within 6 mm diameter of the posterior surface of the cornea obtained by Fourier analysis, total higher-order aberrations (diopter); and total higher order aberrations within 6 mm diameter cornea.
Statistical Analyses
The Wilcoxon signed-rank test was performed to compare YOUSOFT-corrected distance visual acuity with spectacle-corrected distance visual acuity. The Mann–Whitney U-test was used to compare YOUSOFT prescription and non-prescription cases. SPSS (ver. 24; IBM Japan, Tokyo) was used for statistical analyses. A P-value of <5% was considered statistically significant.
The sample size was calculated using G*Power (Universität Düsseldorf, Germany) as follows: the smallest number of samples in which there was a significant difference between YOUSOFT CDVA and RGP CDVA in the prescription case in the Wilcoxon signed-rank test was 33 (effect size 0.7, 90% power, 95% confidence level). The overall minimum number of cases required was calculated to be 47, and approximately 70% of cases in the preliminary study were available for YOUSOFT prescriptions.
Results
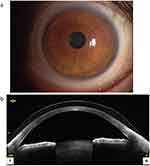
A representative slit-lamp biomicroscopy photograph and an anterior segment optical coherence tomography image of an eye wearing YOUSOFT are shown in Figure 1.
Table 1 shows the demographic characteristics of patients included in this study.
 |
Table 1 Demographics of the Study Population |
YOUSOFT Prescription Rate
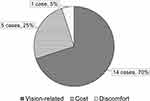
Twenty-seven of the 28 patients had been using YOUSOFT for more than 1 month without any problems. One patient discontinued the use of YOUSOFT due to unstable vision. Among non-prescription cases, 14 patients did not choose YOUSOFT because they could see better with rigid gas permeable or scleral lenses (Figure 2).
Comparison of YOUSOFT Prescription Cases with Non-Prescription Cases
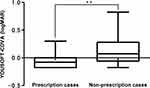
The results of the comparison between the YOUSOFT prescription and non-prescription cases are shown in Table 2. In the YOUSOFT prescription cases, YOUSOFT-corrected distance visual acuity (logMAR −0.04; 95% confidence interval [CI]: −0.08 to 0.00) was significantly better than that in the YOUSOFT non-prescription cases (logMAR 0.12; 95% CI: 0.01 to 0.23; P = 0.003) (Figure 3). No significant differences in other parameters were found between the prescription and non-prescription cases.
 |
Table 2 Comparison of Patient Backgrounds Between YOUSOFT Prescription and Non-Prescription Cases |
Comparison of YOUSOFT-Corrected Distance Visual Acuity and Spectacle-Corrected Distance Visual Acuity
In the YOUSOFT prescription cases, YOUSOFT-corrected distance visual acuity (logMAR −0.04; 95% CI: −0.08 to 0.00) was significantly better than spectacle-corrected distance visual acuity (logMAR 0.23; 95% CI: 0.08 to 0.38; P < 0.0001) (Figure 4). Similarly, in the YOUSOFT non-prescription cases, YOUSOFT-corrected distance visual acuity (logMAR 0.12; 95% CI: 0.01 to 0.23) was significantly better than spectacle-corrected distance visual acuity (logMAR 0.24; 95% CI: 0.08 to 0.42; P = 0.004) (Figure 5).
Comparison of YOUSOFT-Corrected Distance Visual Acuity with Rigid Gas Permeable Lens-Corrected Distance Visual Acuity
Of the cases in which rigid gas permeable lens-corrected distance visual acuity was measurable, YOUSOFT-corrected distance visual acuity was compared with rigid gas permeable lens-corrected distance visual acuity in the YOUSOFT prescription (12 eyes) and non-prescription (12 eyes) cases, respectively. YOUSOFT-corrected distance visual acuity (logMAR −0.03; 95% CI: −0.08 to 0.03) and rigid gas permeable lens-corrected distance visual acuity (logMAR −0.02; 95% CI: −0.08 to 0.04; P = 0.856) were not significantly different in the YOUSOFT prescription cases (Figure 6a). However, in the YOUSOFT non-prescription cases, rigid gas permeable lens-corrected distance visual acuity (logMAR −0.06; 95% CI: −0.11 to 0.00) was significantly better than YOUSOFT-corrected distance visual acuity (logMAR 0.10; 95% CI: 0.00 to 0.20; P = 0.011) (Figure 6b).
YOUSOFT-Corrected Distance Visual Acuity According to Keratoconus Severity
Cases were divided by the severity of keratoconus: 25, 22, and 8 eyes in the mild, moderate, and severe groups, respectively (Table 3). The YOUSOFT-corrected distance visual acuity according to severity is shown in Figure 7.
 |
Table 3 Demographics by the Severity of YOUSOFT Prescription Cases |
In the mild group, there were 16 eyes in which YOUSOFT-corrected distance visual acuity was improved over spectacle-corrected distance visual acuity. Seven eyes showed no change, and two eyes experienced worsening.
In the moderate group, there were 21 eyes in which YOUSOFT-corrected distance visual acuity was improved over spectacle-corrected distance visual acuity, and one eye showed no change.
In the severe group, YOUSOFT-corrected distance visual acuity was better than spectacle-corrected distance visual acuity in all cases.
Complications Due to the Use of YOUSOFT
Eight patients complained of dryness during YOUSOFT wear. Since no particular corneal epithelial damage was observed, preservative-free hyaluronate ophthalmic solution 0.1% (NIHON TENGANYAKU KENKYUSYO CO. LTD., Tokyo, Japan) was prescribed, and lenses were continued to be worn. No corneal edema or neovascularization was observed during the follow-up.
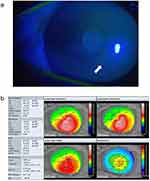
Representative Cases Where YOUSOFT Was Highly Useful
Among the cases in this current study, a representative case in which YOUSOFT was useful is shown in Figure 8. The patient was a 60-year-old female who had cataract surgery. The average corneal refractive power, maximum corneal refractive power, and corneal astigmatism were 51.3 diopters, 58.3 diopters, and 3.6 diopters, respectively. The keratoconus severity was stage 2. She originally used a rigid gas permeable lens; however, because of the corneal epithelial damage caused by the rigid gas permeable lens and the pain caused by wearing it, YOUSOFT was requested. The spectacle-corrected distance visual acuity (logMAR) was 0.7, and YOUSOFT-corrected distance visual acuity (logMAR) improved to 0.05.
In this case, because of the ocular dryness caused by wearing YOUSOFT, a preservative-free hyaluronate ophthalmic solution of 0.1% was prescribed, and the YOUSOFT use was continued.
Discussion
It has been reported that both the visual acuity and wearing comfort in keratoconus eyes with rigid gas permeable lens intolerance were good for YOUSOFT lenses.16 In this report, only patients who eventually started using YOUSOFT were evaluated. To assess the refractive correction capability of YOUSOFT, we examined all patients with keratoconus who tried a YOUSOFT prescription in our clinic, including prescription and non-prescription cases.
The effect of correcting irregular astigmatism was examined by comparing the visual acuity corrected by YOUSOFT with that corrected by spectacles. Additionally, we evaluated the effect of correcting irregular astigmatism by comparing rigid gas permeable lens-corrected visual acuity with YOUSOFT-corrected visual acuity.
In this study, YOUSOFT visual acuity was better than spectacle visual acuity in both prescription and non-prescription cases. The lens thickness of YOUSOFT is designed to be 0.4 mm, which is thicker than a standard soft contact lens. This lens thickness may reduce irregular corneal astigmatism and provide better vision than spectacle correction. However, when comparing YOUSOFT visual acuity between the prescription and non-prescription cases, YOUSOFT visual acuity was better in the prescription cases. YOUSOFT prescription and non-prescription cases showed no significant differences in anterior segment optical coherence tomography parameters. However, corneal astigmatism greater than 6.0 diopters in YOUSOFT prescription cases was observed in 2 eyes (6%), compared with 6 eyes (30%) in non-prescription cases (data not shown). Since the amount of astigmatism that can be corrected with YOUSOFT is approximately 6.0 diopters, there were many cases of under-correction of astigmatism in the non-prescribed cases. Therefore, it is likely that YOUSOFT visual acuity was poorer in the YOUSOFT non-prescription cases than in the YOUSOFT prescription cases.
Of the 28 patients who were followed for more than 1 month after the prescription, 27 continued to wear YOUSOFT without problems. In a previous report, three patients (15%) discontinued wearing YOUSOFT.16 Factors that led to the discontinuation of YOUSOFT wear were dissatisfaction with visual acuity in two patients and cumbersome handling of the lenses in one patient.16 The YOUSOFT contact lenses have a larger diameter than regular soft contact lenses. Therefore, a large eyelid opening is required when wearing YOUSOFT contact lenses, making them somewhat difficult to wear. Patient dropout is expected, especially in Asian patients, due to a narrow palpebral fissure. However, in our study, almost all patients with a YOUSOFT prescription could use it without any problems, and there was one dropout during the follow-up period. At our clinic, we take the time to teach patients how to wear and care for their lenses until they are comfortable with them; this may be the reason for the low dropout rate. The YOUSOFT visual acuity of the patient who discontinued YOUSOFT wear in our study was good, with a logMAR of −0.08. However, this patient stopped wearing YOUSOFT due to unstable vision caused by lens movement. Thus, when prescribing YOUSOFT in the future, it is advisable to check vision by wearing YOUSOFT for an extended period rather than judging only on visual acuity.
In this study, YOUSOFT was prescribed to nearly two-thirds of the patients, while one-third did not want a YOUSOFT prescription. YOUSOFT-corrected distance visual acuity and rigid gas permeable lens-corrected distance visual acuity were not significantly different in the YOUSOFT prescription cases. However, YOUSOFT non-prescription cases had poorer YOUSOFT visual acuity than rigid gas permeable lens visual acuity. Rigid gas permeable lenses can correct irregular corneal astigmatism with a tear lens created by lens wear. Rigid gas permeable lenses have also been reported to compress the cornea when worn and correct anterior and posterior corneal regular and irregular astigmatism.18 Conversely, the deformation effect on corneal shape when wearing soft contact lenses was reported to be less than that of rigid gas permeable lenses.19 YOUSOFT may be less effective in correcting corneal regular and irregular astigmatism than rigid gas permeable lenses because it is made of soft contact lens material. Therefore, future studies on the effectiveness of YOUSOFT and rigid gas permeable lenses in correcting regular and irregular astigmatism in a large number of cases will be necessary.
When examined by the severity of keratoconus, YOUSOFT visual acuity improved more than spectacle visual acuity in almost all severe and moderate cases. However, in the mild cases, YOUSOFT visual acuity was worse than spectacle visual acuity in two eyes (8%). The two eyes with reduced YOUSOFT visual acuity (0.10) had spectacle acuities of 0.00 and −0.08. These two cases were subsequently prescribed rigid gas permeable lenses. In the future, more efficient prescriptions might be possible if it becomes feasible to predict whether or not YOUSOFT visual acuity will improve relative to spectacle visual acuity based on corneal shape.
Kerasoft, a soft contact lens for keratoconus, is reportedly useful in rigid gas permeable lens intolerance cases.12–14 Two types of Kerasoft are available: one with hydrogel material and a thin one with silicone hydrogel material. The front surface of the Kerasoft lens is an aspheric or aspheric toric prismatic ballast design. Contrastingly, YOUSOFT is a soft contact lens with a prismatic ballast design made of hydrogel material. The diameter of the Kerasoft lens can be manufactured in three levels: 14.0 mm, 14.5 mm, and 15.0 mm. However, YOUSOFT has only one type of lens diameter—14.5 mm. The first choice for Kerasoft’s trial lenses is calculated using corneal topography and a special calculator; therefore, lens selection is somewhat complicated. Meanwhile, YOUSOFT lens selection is simple, as the lens only needs to be selected from six base curve levels according to the disease severity. However, the base curve range of YOUSOFT is only 7.80–8.80 mm, while the base curve range of Kerasoft is 7.40–9.40 mm; thus, Kerasoft has a wider base curve range. Additionally, Kerasoft spherical power can be produced from +30.0 diopters to −30.0 diopters, and corneal astigmatism power can be produced from −0.5 diopters to −15.0 diopters. Although YOUSOFT spherical power can also be produced from +30.0 diopters to −30.0 diopters, its corneal astigmatism power can be produced from −0.25 diopters to −6.0 diopters. Therefore, Kerasoft may be able to correct more severe keratoconus and astigmatism than YOUSOFT. However, since there is a large variation in keratoconus between each case, future comparative studies using the same cases are needed.
There are some limitations to this study. First, we were unable to collect the corneal endothelial cell density data from all patients before and after the YOUSOFT prescription. The oxygen transmissibility of YOUSOFT is low, which may affect corneal endothelial cell density and corneal neovascularization after long-term use. Additionally, the follow-up period of this study is relatively short, with a mean observation period of 5.6 months following the YOUSOFT prescription. In this study, the only complication caused by YOUSOFT was the complaint of dryness; however, it is necessary to monitor the long-term course of dry eye disease. Additionally, we could not compare the visual acuity and wearing comfort between YOUSOFT and rigid gas permeable lenses, nor could we examine changes in the quality of life. Therefore, it is necessary to evaluate these factors using questionnaires and other methods in the future. This study was conducted immediately after the introduction of YOUSOFT in our clinic; therefore, as prescription experience increases, even better results may be obtained. In addition, our study used the Amsler-Krumeich classification to evaluate the severity of keratoconus. However, Belin et al reported a new severity grading system for keratoconus (ABCD keratoconus grading system) that uses the posterior corneal shape in addition to the anterior corneal shape.20 In our study, we used a grading system based solely on the anterior corneal shape. Therefore, the results may differ if the ABCD keratoconus grading system is used. In the future, the shape of the posterior corneal surface should also be evaluated and classified using the ABCD keratoconus grading system.
Conclusions
In conclusion, although there are a few cases of poorly corrected visual acuity compared with rigid gas permeable lenses, the results suggest that YOUSOFT is effective in correcting irregular astigmatism in keratoconus patients with rigid gas permeable lens intolerance.
Abbreviations
RGP, rigid gas permeable; D, diopter; CDVA, corrected distance visual acuity; CI, confidence interval.
Data Sharing Statement
All data generated or analyzed during this study are included in the manuscript and its Supplementary Materials. Further enquiries can be directed to the corresponding author.
Study Approval Statement
This retrospective study was approved by the Institutional Review Board of the Nagoya Eye Clinic (2021-50). The study was performed in accordance with the tenets of the Declaration of Helsinki.
Consent to Participate Statement
An opt-out method was approved, and the need for obtaining written informed consent was waived.
Acknowledgments
This study was orally presented at the Annual Meeting of the Japan Orthoptic Congress, held in Tokyo, Japan, on November 20–21, 2021.
Author Contributions
All authors made a significant contribution to the work reported, including either the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors did not receive any funding relevant to this study.
Disclosure
Dr. Kojima reports receiving personal fees from Carl Zeiss Meditec, Johnson & Johnson, SEED, Staar Surgical, Santen Pharmaceutical, Otsuka Pharmaceutical, and Alcon Japan, outside of the submitted work. Dr. Kojima also has a patent (2019-045345) licensed to Takashi Kojima. Dr. Nakamura reports receiving personal fees from Staar Surgical, Santen Pharmaceutical, Otsuka Pharmaceutical, Carl Zeiss Meditec, and Johnson & Johnson, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Karolak JA, Gajecka M. Genomic strategies to understand causes of keratoconus. Mol Genet Genomics. 2017;292(2):251–269. doi:10.1007/s00438-016-1283-z
2. Gordon-Shaag A, Millodot M, Shneor E, Liu Y. The genetic and environmental factors for keratoconus. BioMed Res Int. 2015;2015:795738. doi:10.1155/2015/795738
3. Wu Y, Tan Q, Zhang W, et al. Rigid gas-permeable contact lens related life quality in keratoconic patients with different grades of severity. Clin Exp Optom. 2015;98(2):150–154. doi:10.1111/cxo.12237
4. Koppen C, Kreps EO, Anthonissen L, Van Hoey M, Dhubhghaill SN, Vermeulen L. Scleral lenses reduce the need for corneal transplants in severe keratoconus. Am J Ophthalmol. 2018;185:43–47. doi:10.1016/j.ajo.2017.10.022
5. Sengor T, Kurna SA, Aki S, Ozkurt Y. High Dk piggyback contact lens system for contact lens-intolerant keratoconus patients. Clin Ophthalmol. 2011;5:331–335. doi:10.2147/OPTH.S16727
6. Zadnik K, Barr JT, Edrington TB, et al. Baseline findings in the collaborative longitudinal evaluation of keratoconus (CLEK) study. Invest Ophthalmol Vis Sci. 1998;39(13):2537–2546.
7. Ozcan SC, Ozcan DO. Effects of a new-generation hybrid contact lens on visual performance and vision-related quality of life in patients with keratoconus. Arq Bras Oftalmol. 2023;86(1):7–12. doi:10.5935/0004-2749.20230001
8. Kibet Yego W, Moodley VR. Visual acuity and refractive error improvement in keratoconic patients: a low-income context management perspective. Clin Optom. 2020;12:113–122. doi:10.2147/OPTO.S258905
9. Nau AC. A comparison of Synergeyes versus traditional rigid gas permeable lens designs for patients with irregular corneas. Eye Contact Lens. 2008;34(4):198–200. doi:10.1097/ICL.0b013e31815c859b
10. Hashemi H, Shaygan N, Asgari S, Rezvan F, Asgari S. ClearKone-Synergeyes or rigid gas-permeable contact lens in keratoconic patients: a clinical decision. Eye Contact Lens. 2014;40(2):95–98. doi:10.1097/ICL.0000000000000016
11. Kloeck D, Koppen C, Kreps EO. Clinical outcome of hybrid contact lenses in keratoconus. Eye Contact Lens. 2021;47(5):283–287. doi:10.1097/ICL.0000000000000738
12. Nicula C, Nicula D, Suciu C. Results in keratoconus correction with Kerasoft 3 contact lenses. Rom J Ophthalmol. 2020;64(2):122–127. doi:10.22336/rjo.2020.23
13. Yildiz EH, Erdurmus M, Elibol ES, Acar B, Vural ET. Contact lens impact on quality of life in keratoconus patients: rigid gas permeable versus soft silicone-hydrogel keratoconus lenses. Int J Ophthalmol. 2015;8(5):1074–1077. doi:10.3980/j.issn.2222-3959.2015.05.38
14. Uçakhan OO, Bayraktutar BB. KeraSoft 3 contact lenses in corneal ectasia. Eye Contact Lens. 2014;40(6):390–394. doi:10.1097/ICL.0000000000000092
15. Fernandez-Velazquez FJ. Kerasoft IC compared to rose-K in the management of corneal ectasias. Cont Lens Anterior Eye. 2012;35(4):175–179. doi:10.1016/j.clae.2012.02.005
16. Hiraoka T, Kiuchi G, Hiraoka R, Oshika T. Clinical performance of a custom-designed soft contact lens in patients with keratoconus and intolerance to rigid contact lenses. Jpn J Ophthalmol. 2022;66(4):350–357. doi:10.1007/s10384-022-00924-1
17. Alió JL, Shabayek MH. Corneal higher order aberrations: a method to grade keratoconus. J Refract Surg. 2006;22(6):539–545. doi:10.3928/1081-597X-20060601-05
18. Koh S, Inoue R, Maeda N, et al. Corneal tomographic changes during corneal rigid gas-permeable contact lens wear in keratoconic eyes. Br J Ophthalmol. 2022;106(2):197–202. doi:10.1136/bjophthalmol-2020-317057
19. Tyagi G, Collins MJ, Read SA, Davis BA. Corneal changes following short-term rigid contact lens wear. Cont Lens Anterior Eye. 2012;35(3):129–136. doi:10.1016/j.clae.2012.01.006
20. Belin MW, Kundu G, Shetty N, Gupta K, Mullick R, Thakur P. ABCD: a new classification for keratoconus. Indian J Ophthalmol. 2020;68(12):2831–2834. doi:10.4103/ijo.IJO_2078_20
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.