Back to Journals » Nature and Science of Sleep » Volume 14
Effects of CPAP Treatment on Electroencephalographic Activity in Patients with Obstructive Sleep Apnea Syndrome During Deep Sleep with Consideration of Cyclic Alternating Pattern
Authors Chen S, Li Q, Zou X, Zhong Z, Ouyang Q, Wang M , Luo Y, Yao D
Received 13 July 2022
Accepted for publication 8 November 2022
Published 21 November 2022 Volume 2022:14 Pages 2075—2089
DOI https://doi.org/10.2147/NSS.S382305
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sarah L Appleton
Shuliang Chen,1,2,* Qi Li,1,* Xueliang Zou,3 Zhijun Zhong,1 Qian Ouyang,1 Mengmeng Wang,1 Yaxing Luo,1 Dongyuan Yao1
1Neurological Institute of Jiangxi Province and Department of Neurology, Jiangxi Provincial People’s Hospital and The First Affiliated Hospital of Nanchang Medical College, Jiangxi, People’s Republic of China; 2Queen Mary College, Nanchang University, Jiangxi, People’s Republic of China; 3Jiangxi Mental Hospital, Nanchang University, Jiangxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dongyuan Yao, Jiangxi Provincial People’s Hospital, 92 Aiguo Road, Nanchang, Jiangxi, 330006, People’s Republic of China, Tel/Fax +86-791-8689-5077, Email [email protected]
Objective: To investigate whether continuous positive airway pressure (CPAP) treatment would change EEG activities associated with cyclic alternating pattern (CAP subtype A1, A2, and A3) and non-CAP (NCAP) during non-rapid eye movement sleep stage 3 (N3) in patients with obstructive sleep apnea (OSA).
Methods: The effects of CPAP treatment on the percentages of sleep stage N3 occupied by the CAP and NCAP, power of EEG waves in the CAP and NCAP were examined in 18 patients with moderate-to-severe OSA undergoing polysomnographic recordings.
Results: Apnea and hypopnea index during sleep stage N3 was positively correlated with ratios of phases A2 and A3 duration to total phase A duration [Phase (A2+A3) /Phase A] and negatively correlated with phase A1/phase A. With CPAP treatment, percentages of sleep stage N3 occupied by total CAPs and subtypes A2 and A3, as well as CAP A2 and CAP A3 indexes were significantly decreased while percentages of sleep stage N3 occupied by NCAP (NCAP/N3) and CAP A1 index were significantly increased. In addition, CPAP treatment significantly decreased percentage of respiratory events associated CAPs and increased percentage of non-respiratory related CAPs. Moreover, absolute and relative delta power was significantly increased during phase A1, unchanged during phase A2 and phase B2, and significantly decreased during phases B1, A3 and B3. The absolute power of faster frequency EEG waves in CAPs showed a general trend of decrease. The absolute and relative power of delta waves with amplitudes ≥ 75 μV, but not < 75 μV, was significantly increased.
Conclusion: CPAP treatment improves the sleep quality in OSA patients mainly by increasing delta power and decreasing power of higher frequency waves during phase A1, and decreasing CAP A2 and A3 indexes as well as increasing NCAP/N3 and power of delta waves with amplitudes ≥ 75 μV during NCAP.
Keywords: obstructive sleep apnea, continuous positive airway pressure, polysomnographic recordings, non-rapid eye movement sleep, cyclic alternating pattern
Introduction
Obstructive sleep apnea (OSA) is a highly prevalent sleep-disordered breathing characterized by recurrent upper airway obstruction and collapse during sleep, and results in sleep fragmentation and intermittent hypoxemia.1 Airway obstruction during sleep may cause several changes, including cardiac electrophysiological disturbances, dramatic shift in intrathoracic pressure, and increases in sympathetic activities. The prevalence of OSA ranges from 13–33% in adult males and 6– 19% in adult females and increases with aging and body weight gain.2 Most OSA patients complain of dyspnea, shallow sleep, and nocturnal sleep snoring with apnea. Excessive daytime sleepiness (EDS) and daytime cognitive and behavioral deficits, such as disturbances in attention, memory, and alertness, have also been observed in OSA patients.3 Untreated OSA is associated with increases in nocturnal cardiovascular events, stroke, and heart attack.4–6 Studies have shown poor performance in mental behaviors and executive tasks in OSA patients, which is associated with the severity of hypoxemia, while EDS is associated with memory and attention deficits.2,7 Continuous positive airway pressure (CPAP) is still the treatment of choice for severe OSA, and it has been shown to improve sleep quality and EDS.8–10
The cyclic alternating pattern (CAP), a cyclic variation in electroencephalographic (EEG) activities within non-rapid eye movement (NREM) sleep, but not within REM sleep, is an EEG marker of unstable sleep and organized into sequences of successive cycles composed of two phases, A and B.11,12 Phase A involves phasic events allowing adaptive adjustments of ongoing states to internal and external stimuli and phase B refers to background fluctuation in cortical activities during CAP.13 Variations in respiratory activity, heart rate, and muscle tone during CAP increase during phase A and decrease during phase B.12 The CAP is related to cerebral activities during NREM sleep and is reciprocally influenced by ongoing autonomic and muscle activity, which might determine the pathophysiology of several sleep disorders and the effect of medication and continuous positive airway pressure (CPAP) treatment of OSA.13,14
The fraction of EEG activity without CAP for more than 60 s consecutively is scored as non-CAP (NCAP) and coincides with a condition of sustained physiological stability. CAP, as a prominent feature parameter in sleep, provides a powerful assessment of brief and frequent arousals. The vast majority of apneas were associated with CAPs. Apnea in all CAP-related respiratory events is strongly associated with phase B, while phase A is associated with respiratory compensation.14 Severe OSA patients were found to have fewer CAP subtype A1 and more CAP subtype A3, and CPAP treatment was found to increase the percentage of CAP subtype A1 and decrease the percentage of CAP subtype A3.11 Two-process model postulates that the propensity to sleep and sleepiness is controlled by the interaction of process S (homeostatic stress of sleep) and process C (circadian rhythm).15,16 Process S, representing sleep debt, increases during wakefulness and decreases during sleep. Slow wave activity (SWA) during NREM sleep is the main hallmark of process S during sleep17 and theta activity in waking is a marker of the rising limb of process S.16 Process C that promotes nighttime sleep and daytime alertness is influenced by the circadian pacemaker, the suprachiasmatic nucleus.18 Studies have shown that after 30 days of regular CPAP treatment (N30), regular homeostatic process S and ultradian rhythms such as REM–NREM sleep cycle and the waxing and waning oscillations of subtypes A2 and A3 in relation to the REM sleep periods occurred during N30 despite incomplete recovery of phase A1.19
Although OSA patients demonstrate both a delayed and reduced proportion of slow wave sleep (SWS) compared with non-OSA subjects, once they achieve SWS, sleep apnea and hypopnea markedly improve in most patients as SWS can modulate the propensity for upper airway collapse and help to achieve improved or near normal respiratory and arousal event frequencies.20 Previous studies have shown that EEG measurements could be used as biomarkers of cognitive performance and sleep quality in OSA patients21 and increased slow-wave activities (SWA) during sleep stage N3 correlated with improved procedural learning, memory process and faster reaction.22 With enough high-quality SWS, metabolic clearance and memory functions could benefit and help slowing the process of cognitive aging.23 During SWS, high-voltage slow waves rarely appear as isolated features, but in most cases they converge into collectives resulting in phase A of CAP subtype A1.11 CAP subtype A1 prevails in milder OSA patients, while CAP subtypes A2 and A3 predominate among moderate-to-severe OSA patients. The milder OSA patients also present higher sleep efficiency, and increased percentages of sleep stage N3 and REM sleep, as well as longer CAP sequences in sleep stage N3, while severe OSA patients spend more time in lighter sleep stages.24 It would be interesting to evaluate the effects of CPAP on EEG activities especially SWA associated with CAPs in comparison with those during NCAP in the patients with OSA. Therefore, the aim of this study was to determine whether CPAP treatment would change EEG activities associated with CAP subtypes A1, A2 and A3, and NCAP during sleep stage N3.
Methods
Subjects
Polysomnographic (PSG) recordings were performed on 18 male patients (mean± SEM: 52.78±1.93 years old, age range: 42–58 years old) with moderate-to-severe OSA and daytime sleepiness with the Epworth Sleepiness Scale score (ESS) > 10, without and with CPAP treatment in two separated nights. Before the experiment, the patients tried on the CPAP machine and the machine was programmed so the patient did not feel uncomfortable. Then, data recordings started and were kept continuously in the titration night until the patients woke up around 6–8 a.m. Power spectral density of EEG activities (0–30 Hz) during CAP and NCAP of NREM sleep stage 3 was carried out.
Inclusion criteria included: (1) 40–60 years old; (2) male gender; (3) AHI > 15; (4) no depression, stroke and other mental and neurological diseases; (5) no other sleep disorders, such as narcolepsy, insomnia, bruxism and restless leg syndrome; and (6) without taking drugs that affected sleep. The OSA patients who met the inclusion criteria underwent overnight PSG recording without and with CPAP in two separated nights.
Exclusion criteria included the presence of any causes of brain injury, neurological diseases, other primary or secondary lung diseases, and a history of alcohol and substance abuse. Patients who had used drugs that affected sleep or breathing, as well as any psychiatric medications within a month immediately before the experiments, were excluded from the study. Tea, caffeinated foods and beverages, and other stimulants were not permitted 24 hours prior to PSG recordings.
Polysomnographic Recordings
Overnight polysomnography was performed with a computerized recording system (Pro Fusion PSG 4 System, Compumedics Limited, Abbotsford, Australia) to record EEG (F4-M1, C4-M1, O2-M1, F3-M2, C3-M2, O1-M2), electrocardiographic (ECG), electrooculographic (EOG) activities, and electromyographic (EMG) activities from jaw muscles (mylohyoid and masseter), upper (bilateral flexor and extensor carpi radialis) and lower limb muscles (bilateral gastrocnemius and tibialis anterior muscle) as well as respiration via the thermistor, nasal pressure transducer and thoracoabdominal plethysmography. In addition, peripheral vascular oxygen saturation with percutaneous finger pulse oximetry and body position were also recorded.25,26 The study was performed using a Philips CPAP device coupled with a nasal mask (Philips BIPAP Auto767P, Bi-Flex mode, Pennsylvania, USA) which used an algorithm developed by the company with a tachometer and pressure generator to analyze airflow and provide a breathing curve synchronized with other monitored variables on a computerized sleep recording system. The maximum inhalation positive airway pressure (IPAP) was 12.42 ± 0.43 cmH2O and the maximum exhalation positive airway pressure (EPAP) was 9.14 ± 0.51 cmH2O.
Sleep was scored and analyzed according to the criteria established by the American Academy of Sleep Medicine (AASM).27 An obstructive sleep apnea episode was defined as a decrease by 90% in airflow from baseline with inspiratory efforts lasting at least 10 seconds, while hypopnea was defined as a decrease by 30% in airflow from respiratory baseline and a decrease in SpO2 by 3% or an arousal.27 The severity of OSAS was assessed by the number of sleep apnea and hypopnea per hour of sleep (AHI), hypoxemia was estimated with the minimum SpO2 and the duration of sleep with SpO2 <90%.
CAP Scoring
The CAP was scored according to the standards described by Terzano et al.28,29 The CAP sequence onset must be preceded by non-CAP (a continuous NREM sleep for > 60 s without CAP), with the following 3 exceptions: (1) before the first CAP sequence arising in NREM sleep; (2) after a wake to sleep transition; and (3) after a REM to NREM sleep transition.28,30 The CAP consisted of a phase A (phasic events) followed by a phase B (background EEG activities).
Phase A of the CAP was divided into three subtypes: A1, A2 and A3. Subtype A1 was characterized by EEG synchrony with high-amplitude slow waves (e.g. delta bursts, K-complex sequences, vertex sharp transients, and polyphasic bursts) and occupied more than 80% of the entire phase A1 duration. Subtype A2 sequence included EEG arousals and polyphasic bursts, and consisted of a mixture of slow and fast rhythms with 20–50% of phase A2 occupied by EEG desynchrony. Subtype A3 included K-alpha, EEG arousals, and polyphasic bursts and was dominated by rapid low-voltage rhythms with more than 50% of phase A3 occupied by EEG desynchrony. A movement artifact within a cyclic alternating pattern sequence was also classified as subtype A3. Each phase of CAP is 2–60 s in duration.11 The CAP parameters were defined as shown in Table 1. The effects of CPAP treatment on these parameters were tested in the OSA patients.
 |
Table 1 The Main Parameters of CAP and NCAP |
The EEG signals were digitized with the high-pass filter set at 0.01 Hz and the low-pass filter set at 35 Hz. Since EEG recordings from the frontal of the brain were more sensitive to assess the outcome and effectiveness of CPAP treatment, EEG activities recorded from F4 channel were chosen for detailed analysis.30 EEG waves contaminated movement and EMG artifacts were excluded before further analysis. A total of 24 second epochs in each NCAP (4 s epoch each, 0.2 s interval) were analyzed. Fast Fourier transform (FFT)-based power spectral analysis was performed by using the Brainstorm program version 2.0. (GNU GPLv2, McGill University, Montreal, QC, Canada) to analyze the CAPs (full length phase A and phase B) and 4 s epochs in the NCAP with 0.25 Hz resolution and cosine window smoothing.
The absolute and relative power of four specific frequency bands namely delta (0–3.99 Hz), theta (4–7.99 Hz), alpha (8–13 Hz), and beta (13.01–30 Hz) waves were calculated.3 Relative EEG power was determined as power of a given frequency band divided by the sum of absolute power across the four frequency bands, and it reflected the contribution of EEG activity of a given frequency band to the sum of absolute power.31–33 In addition, the delta waves with different amplitude (e.g. <75 µV, ≥75 µV) in NCAP were analyzed.
Statistical Analysis
Normality tests were performed for all variables before further statistical analysis. Values were expressed as mean ± standard error of the mean (SEM) if the variables were normally distributed; otherwise, values were expressed as median (minimum-maximum). The paired t-test or Wilcoxon signed-rank test were used wherever appropriate. Statistical analysis was performed using the SPSS Statistic 25.0 software package (IBM, Armonk, USA). P<0.05 was considered to be statistically significant.
Results
General Characteristics of Sleep Variables
As shown in Table 2, with CPAP treatment, the percentage of sleep stage N3 was significantly increased from 14.80 ± 1.91% without CPAP treatment to 21.30 ± 2.30% (P<0.05), duration of sleep stage N3 was significantly increased from 56.78 ± 7.75 min to 82.83 ± 9.64 min (P<0.01), while the percentage of sleep stage N1 was significantly decreased from 12.38 ± 1.14% to 9.21 ± 1.03%, the microarousal index was significantly decreased from 30.77 ± 2.32 to 11.82 ± 1.46 (P<0.01), arousal index was significantly decreased from 30.58 ± 2.30 to 11.91 ± 1.52 (P<0.001) and the PLMI was significantly decreased from 8.40 (1.00–29.30) to 1.10 (0.00–7.60) (P<0.001).
 |
Table 2 Sleep Variables in OSA Patients with and without CPAP Treatment |
The respiratory variables during sleep were significantly improved with CPAP treatment (Table 2). The minimal SpO2 was increased significantly from 75.50% (55.00–85.00%) to 90.00% (74.00–93.0%), and mean SpO2 was significantly increased from 94.50% (90.00–97.00%) to 96.0% (94.00–97.00%) (P<0.01). In contrast, AHI was significantly decreased from 44.00 (22.30–91.40) events/h to 1.70 (0.30–10.10) events/h, duration of apnea was significantly decreased from 27.00 (15.00–56.00) s to 17.00 (11.00–28.00) s (P<0.01) (Table 2).
Association of CAP with Respiratory Events
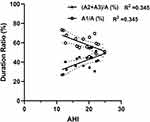
As shown in Table 3, a majority of CAP subtypes A2 and A3 was associated with OSA while most of CAP subtype A1 was not. The ratio of phase A1 duration to the duration of total phase As was negatively correlated with AHI in sleep stage N3, while the ratio of phase A2 and A3 duration to the duration of total phase As was positively correlated with AHI in sleep stage N3 (Figure 1). With CPAP treatment, the percentage of CAP subtype A1 associated with OSA events was significantly decreased but the percentage of CAP subtype A1 unrelated with OSA events was significantly increased. Moreover, the percentage of CAP subtypes A2 and A3 associated with OSA events was significantly decreased to almost zero but the percentage of CAP subtype A2 and A3 unrelated with OSA events was significantly increased (Table 3) although the total number of subtypes A2 and A3 was decreased (Table 4).
 |
Table 3 Changes of Subtypes of CAP with CPAP Treatment |
 |
Table 4 Changes in CAP Parameters with CPAP Treatment |
Among the 18 subjects, a predominantly fast low amplitude rhythm consisting of CAP A3 was observed during REM sleep in only 4 patients with OSA with a minimum SpO2 in the range of 55–58%. A total of 76.32% (74.48–81.40%) apneas occurred in relation to CAPs in the 4 patients and during CPAP, the percentage was decreased to 19.43% (13.63–26.30%).
Effects of CPAP Treatment on Main Parameters of CAP During NREM Sleep Stage 3
CPAP treatment significantly decreased the percentage of sleep stage N3 occupied by CAPs (CAP/N3) from 41.10 ± 3.01% to 32.98 ± 1.58% (P<0.05) and increased the percentage of sleep stage N3 occupied by NCAP (NCAP/N3) from 46.08 ± 2.14% to 50.46 ± 0.88% (P<0.05) (Table 4).
As shown in Table 4, the percentage of sleep stage N3 occupied by phase A (phase A/N3) was significantly decreased from 12.18% (6.78–31.76%) to 5.66% (2.74–23.54%) (P <0.01) and phase B/N3 was significantly decreased from 25.63 ± 1.04 to 21.84 ± 0.81 (P <0.05) with CPAP treatment. With CPAP treatment, the ratio of subtype A1 to all CAPs significantly increased from 58.79% (44.59–73.19%) to 71.39% (52.03–81.02%) (P<0.01) while the ratios of subtypes A2 and A3 to all CAPs were both significantly decreased (P<0.05). Similarly, subtype A1 index (the number of CAP A1 per hour of N3 sleep) significantly increased from 21.23 (6.43–45.33) to 27.35 (10.57–70.40) (P<0.05), while subtype A2 and A3 indexes were both significantly decreased (P<0.01). In addition, with CPAP treatment, the K complex index 1 (number of K complexes in CAP subtype A1 per hour of N3 sleep) significantly increased from 7.42 (0.00–28.00) to 10.16 (1.36–58.81) (P<0.05), but no changes in K complex indexes 2 and 3 (number of K complexes in CAP subtype A2 and A3 per hour of N3 sleep).
Effects of CPAP Treatment on EEG Power of CAP During NREM Sleep Stage 3
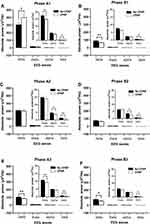
In phase A1, the absolute power of delta and theta waves was significantly increased while that of beta waves was significantly decreased and that of alpha waves was not significantly changed with CPAP treatment. In contrast, in phase A2, the absolute power of delta waves was not significantly changed while that of alpha and beta waves was significantly decreased with CPAP treatment. Moreover, in phase A3, the absolute powers of EEG waves (e.g. delta, theta, and beta waves) showed a general trend of decrease with CPAP treatment. With CPAP treatment, a general trend of decrease in the absolute power of delta waves in phase B (B1, B2, and B3) was found while those of other EEG waves were either significantly decreased or unchanged (Figure 2).
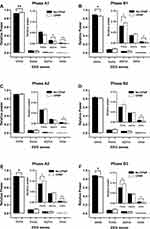
In phase A1, the relative power of delta waves was significantly increased, while that of other EEG waves showed a trend of decrease with CPAP treatment. In contrast, in phase A2, the relative power of delta waves was not significantly changed while that of beta waves was significantly decreased. In phase A3, the relative power of delta waves was decreased, while that of other EEG waves were decreased with CPAP treatment. With CPAP treatment, the relative power of delta waves was significantly decreased and that of theta waves was significantly increased in phase B1 and B3 while both were not significantly changed in phase B2. The relative power of beta waves, but not that of alpha waves was significantly decreased in all phase B (Figure 3).
Effects of CPAP on Duration Ratio and Absolute Power of EEG Waves During NCAP of NREM Sleep Stage 3
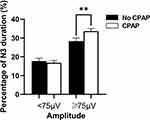
The percentage of duration of N3 sleep occupied by the segments containing EEG waves with amplitudes ≥75 μV, but not <75 μV, in the NCAP of N3 sleep was significantly increased by CPAP treatment (Figure 4).
The absolute and relative power of EEG waves did not show obvious changes with CPAP treatment (Figure 5). EEG data were divided into segments containing delta waves with amplitudes <75 μV and ≥75 μV to further examination of effects of CPAP treatment. The absolute and relative power of delta waves with amplitudes ≥75 μV was significantly increased, while absolute and relative power of delta waves with amplitudes <75 μV was not significantly changed with CPAP treatment (Figure 5).
Discussion
CPAP is the treatment of choice for OSA and is associated with many changes in OSA patients with the treatment. In the current study, we systematically examined the effects of CPAP on EEG activities in sleep stage 3 by focusing its effects on CAPs and found the ratio of CAP A1/all CAPs was significantly increased, power of delta waves in phase A1 and the power of waves ≥75 µV in NCAP were significantly increased while power of delta waves in phase B1, A3 and B3 was significantly decreased and there were no changes in power of waves <75 µV in NCAP and phase A2 and B2. These findings suggest that CPAP has different effects on different types of CAPs and on delta waves depending on their amplitudes in NCAP.
CAP is a marker of brain activity that occurs in conditions of reduced vigilance (sleep, coma, etc.), and contributes to arousal in both normal and pathological conditions.13 It is proposed to restore a steady cortical state through sustained oscillations and considered to be the main manifestation of sleep microstructure, which represents a local pillar of sleep quality just as sleep duration, intensity, and continuity.27,34 CPAP is the treatment of choice for OSA and can reverse the negative effects of OSA on sleep, shown as increases in N3 sleep, decreases in microarousals and improvements of sleep efficiency in addition to improvement of hypoxemia (Table 2). Furthermore, it was shown that CAP subtype A1 occurred more commonly than A2 and A3 during sleep stage N3 regardless of CPAP treatment (Table 4) and the ratio of phase A2 and A3 duration to total duration of phase As was found to be positively correlated with AHI in sleep stage N3, while the ratio of phase A1 duration to total duration of phase As was found to be negatively correlated with AHI in sleep stage N3 (Figure 1). Nevertheless, a majority of CAP subtypes A2 and A3 was associated with OSA while most of CAP subtype A1 was not, and more percentage of CAPs became no respiratory event related with CPAP treatment (Table 3). Since sleep apnea acts as an arousal stimulus to activate the patient’s autonomic nervous function, it is mainly used to generate EEG changes dominated by rapid low-voltage CAP A3 and mixed fast and slow rhythm CAP A2 to restore breathing after apnea. Most arousals during NREM sleep were found to be included in the CAPs, especially in subtype A2 or A3, and the extensive overlap between arousal and subtype A2 and A3 was obvious given the fact that 95% of subtype A3 and 62% of subtype A2 met the AASM criteria for arousal.23,36 CPAP treatment was found to reduce the occurrence of apnea and decreased CAP A2% and A3% in the current study, which indicates that arousals with CPAP treatment were decreased and sleep quality was improved in patients with OSA. This might be due to increases in stable sleep and decreases in AHI index as results of CPAP treatment. Indeed, CPAP treatment was found to be associated with increases in duration and percentage of sleep stage N3 and sleep efficiency, and decreases in arousal index and microarousal index in addition to improvement of respiration and hypoxemia in the current study (Table 2).
The specificity of CAP is mainly provided by phase A and all phase A subtypes have been shown to be capable of reinstatement of breathing, since respiration recovering after the offset of apneic period of OSA occurred with all phase A subtypes, with the strongest effect nonetheless noted during phase A3 subtype.11,14 Increased CAP can be induced by the conditions that induce vigilance instability such as noise, insomnia, interictal EEG paroxysms, nocturnal seizures, and periodic leg movements.35 While CAP phase A (especially A3) is always accompanied by respiratory compensation, this is due to the fact that the CAP phase A with a desynchronized pattern (i.e. predominant α and β-like activity) is strongly associated with stronger transient arousal and has a greater impact on neurovegetative function.28 It may represent the ability of the brain to control and maintain sleep in pathological environments. Each subtype of CAP A will be adjusted to the physiological or pathological conditions of the brain. Therefore, CAP phase A3 with a fast and low amplitude rhythm has the strongest excitation response and plays a major role in respiratory compensation in OSA condition. Whereas the CAP phase A1 with slow-wave mode and CAP phase A2 in hybrid mode rarely interrupted apnea and only resumed effective breathing when converted to the more powerful CAP phase A3. Most arousals during NREM sleep were found to be included in the CAPs, especially in subtype A2 or A3, and subtype A2 and A3 were positively correlated with light sleep and negatively correlated with deep sleep.23,36 Regular EEG oscillations in the transition from light sleep to deep stable sleep are basically expressed by the subtype A1, which is mainly composed of slow waves.11 Subtype A1 is usually predominant in patients with mild OSA while subtypes A2 and A3 dominate in moderate to severe OSA patients.24 Persistent mechanical and functional triggers such as hypoxia, hypercapnia, and respiratory effort gradually shift CAP from sleep protective (subtype A1) to arousal responses (subtype A2 and A3), leading to sleep fragmentation.16,37 The OSA patients with excessive sleepiness showed longer CAP time, higher CAP rate (especially subtype A2), greater number of CAP cycles and a lower mean duration of phase B than those without excessive sleepiness, but the number of arousals and arousal index did not differ significantly in the OSA patients with and without excessive daytime sleepiness although the number of arousals is often used to assess the level of sleep fragmentation.38
The current study showed that patients treated with CPAP had higher delta power in CAP phase A1, increased CAP A1% and reduced CAP A2% and A3%, suggesting that CPAP treatment reduced the patient’s arousal impulse and that CAP A2 and A3 played a less important role in restoring normal respiration and was switched to CAP A1 (lower frequency and higher amplitude). CPAP treatment reduced the occurrence of apnea as well as CAP A2% and CAP A3% in the current study, which further suggests the decreased arousals after CPAP treatment and improved sleep quality with CPAP treatment in OSA patients. The changes in CAP parameters (increase in A1% and decrease in A2% and A3%) and increased delta power in CAP phase A1 with CPAP treatment support the hypothesis that CPAP helps restore neurocognitive function. CAP parameters might provide a more appropriate evaluation for sleep.
CAP subtype A1 has been considered to be related to a deeper and more efficient slow wave sleep that facilitates processes of synaptic plasticity and phase-amplitude coupling. It was found in a previous study that there was a significant positive correlation between the phase A1 and neurocognitive tests that characterize frontal cognitive function (e.g. language fluency, memory process, and language learning).11 Since phase A1 mainly consists of slow waves (distributed in the frontal lobe area), its EEG shows a strengthened network structure associated with increased cognitive function, and fluctuations in delta waves during sleep stage N3 contribute to the consolidation of memory traces acquired during wakefulness.11,19,39 This suggests that the increase in delta power of phase A1 in OSA patients after CPAP will have a positive effect on patients’ daytime memory. K-complex is an important component of EEG transients associated with the termination of respiratory events, and the vast majority of K-complexes associated with respiratory events occur prior to event termination; the gradual increased delta power may reflect the gradual induction of evoked K complexes.40 Patients with CPAP treatment were shown to be associated with an increase in K-complexes with increased CAP subtype A1 (Table 4), which may indicate that the K-complex in phase A1 plays a defense or sleep protection function as previously reported.41 However, the K-complex indexes in CAP A2 and A3 were unchanged as K-complexes rarely occurred with CAP A2 and A3. These findings might indicate the patients’ sleep quality was improved.
CAP A2 is a type of CAPs happening between stable sleep associated with CAP A1 and unstable sleep associated with A3. CPAP did not significantly affect delta and theta power of CAP A2 and only decrease alpha and beta power of CAP A2 although the number of CAP A2 was decreased with CPAP treatment, which might also be associated with improvement of the patients’ sleep quality.
CAP subtype A3 is predominantly associated with cortical arousals and sleep fragmentation.11 In the current study, we have shown that both absolute delta, theta, and beta power of CAP subtype A3 were significantly decreased with CPAP while relative delta, beta power of CAP subtype A3 were decreased. This might be due to reduction of CAP A3, which commonly occurred with OSA, as a result of inhibition of the appearance of OSA with CPAP treatment. Once CAP A3 occurred with CPAP treatment, it showed less delta power and more power of higher frequency waves, it might be compensatory as it was less likely to occur. Cognitive performance appears to be inversely associated with the phase A2, whereas the phase A3 is inversely associated with planning and motor sequences.42 The increased A1% and the decreased A3% after treatment support the hypothesis that CPAP therapy helps restore neurocognitive function.
In healthy people, phase A in CAP may correspond to the very slow delta rhythm and higher frequency EEG waves, and these EEG activities are inhibited during phase B.43 Mean phase B duration was elevated in OSA patients, and fewer phase B seemed to have a protective effect on sleep segments.27,44 In the current study, it was found that percentage of total phase B of CAP occupying N3 sleep in duration was decreased with CPAP treatment (Table 4). Furthermore, the changes in powers of EEG waves during phase B1, B2, and B3 (Figures 3 and 5) associated with CPAP showed a general trend of inhibition of EEG wave activities especially delta waves with CPAP treatment, which might reflect an improvement of oscillation of EEG waves.
REM sleep is characterized by a lack of synchronized EEG, and phase A of REM sleep is dominated by fast low-amplitude rhythms (predominantly phase A3). Under normal circumstances, CAP does not occur during REM sleep. However, it is also possible to identify CAP consisting of phase A3 in REM sleep under conditions of extremely high frequency arousal, such as periodic REM sleep-related sleep apnea events.28 However, a predominantly fast low amplitude rhythm consisting of CAP A3 was observed in REM sleep of only four OSA patients among 18 subjects with a minimum SpO2 in the range of 55–58% during sleep stage N3, which may be because most patients with less severe OSA required to have CAP A3 during REM sleep in the current study.
NREM sleep is also characterized by prolonged stationary activities (i.e. NCAP) in addition to CAPs. NCAP is the tonic condition of sleep and controls subsystems that affect sleep mechanisms and is closely related to enabling them to achieve a global stable state in mutual equilibrium.11,33 In the current study, it was found that CPAP treatment was also associated with larger delta power and longer NCAP duration of sleep stage N3 with delta waves having an amplitude of 75 µV and higher (Figures 4 and 5) although the overall delta power was not changed during NCAP period of N3 sleep (Figure 5), which might suggest increased role of NCAP in stabilizing sleep by increasing NCAP/N3, and delta waves with amplitudes ≥75 μV with CPAP treatment.
In short, the current study has shown the changes in sleep microstructure in OSA patients during sleep stage N3 with CPAP treatment in consideration of CAP and NCAP and revealed that CPAP treatment could improve the sleep quality of OSA patients mainly by increasing delta power and decreasing higher frequency waves of CAP subtype A1, and decreasing the CAP subtype A2 and A3 indexes as well as increasing NCAP/N3 and power of delta wave with amplitudes ≥75 μV during NCAP. The CAP parameters may be appropriate arousal-related EEG markers for evaluation of sleep and can be used to assess improvement of sleep quality in OSA patients with CPAP treatment.
Limitation
In this study, sleep microstructures including CAPs and NCAP in patients with OSA without and with CPAP treatment were examined with some limitations. The number of subjects was relatively small and no healthy control group was included in the study. In addition, the study only focused on the effects of CPAP treatment on sleep stage N3 and no comparison between male and female patients was carried out.
Further studies are needed to examine the effects of CPAP treatment on CAPs and NCAP in sleep stage N3 and other sleep stages in large samples of male and female subjects separately.
Data Sharing Statement
The dataset supporting the conclusions of this paper is shown within the article.
Ethics Declarations
All experimental procedures and research protocols were approved by the Research Ethics Committee of Jiangxi Provincial People’s Hospital (No. 2019-014) according to the principles of the Declaration of Helsinki. Informed consents were obtained from all subjects and they could withdraw from the study at any time.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Jiangxi Provincial People’s Hospital Grant 2019-009, Natural Science Foundation of China Grant 81760202, and Jiangxi Provincial Natural Science Foundation Grant S2017ZRMSB2145.
Disclosure
The authors declare no conflicts of interest in relation to this study.
References
1. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. New Eng J Med. 1993;328(17):1230–1235. doi:10.1056/NEJM199304293281704
2. Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi:10.1016/j.smrv.2016.07.002
3. Morisson F, Lavigne G, Petit D, Nielsen T, Malo J, Montplaisir J. Spectral analysis of wakefulness and REM sleep EEG in patients with sleep apnoea syndrome. Eur Respir J. 1998;11(5):1135–1140. doi:10.1183/09031936.98.11051135
4. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. doi:10.1002/humu.21086
5. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. New Eng J Med. 2005;353:2034–2041. doi:10.1056/NEJMoa043104
6. Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–360. doi:10.1161/CIRCULATIONAHA.109.901801
7. Bédard MA, Montplaisir J, Richer F, Rouleau I, Malo J. Obstructive sleep apnea syndrome: pathogenesis of neuropsychological deficits. J Clin Exp Neuropsychol. 1991;13(6):950–964. doi:10.1080/01688639108405110
8. Sforza E, Krieger J. Daytime sleepiness after long-term continuous positive airway pressure (CPAP) treatment in obstructive sleep apnea syndrome. J Neurol Sci. 1992;110(1–2):21–26. doi:10.1016/0022-510x(92)90004-5
9. Meurice JC, Paquereau J, Neau JP, et al. Long-term evolution of daytime somnolence in patients with sleep apnea/hypopnea syndrome treated by continuous positive airway pressure. Sleep. 1997;20(12):1162–1166. doi:10.1093/sleep/20.12.1162
10. Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Deary IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(2):461–467. doi:10.1164/ajrccm.159.2.9803121
11. Parrino L, Ferri R, Bruni O, Terzano MG. Cyclic alternating pattern (CAP): the marker of sleep instability. Sleep Med Rev. 2012;16:27–45. doi:10.1016/j.smrv.2011.02.003
12. Terzano MG, Parrino L. Origin and significance of the cyclic alternating pattern (CAP). Sleep Med Rev. 2000;4(1):101–123. doi:10.1053/smrv.1999.0083
13. Parrino L, Terzano MG. Central nervous system arousals and cyclic alternating patterns. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia, PA: Elsevier; 2017:1576–1587.
14. Parrino L, Rausa F, Azzi N, Pollara I, Mutti C. Cyclic alternating patterns and arousals: what is relevant in obstructive sleep apnea? In memoriam mario giovanni terzano. Curr Opin Pulm Med. 2021;27(6):496–504. doi:10.1097/MCP.0000000000000825
15. Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1(3):195–204.
16. Borbély AA, Daan S, Wirz-Justice A, Deboer T. The two-process model of sleep regulation: a reappraisal. J Sleep Res. 2016;25(2):131–143. doi:10.1111/jsr.12371
17. Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14(6):557–568. doi:10.1177/074873099129000894
18. Daan S, Beersma DG, Borbély AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246(2 Pt 2):R161–83. doi:10.1152/ajpregu
19. Parrino L, Thomas RJ, Smerieri A, Spaggiari MC, Del Felice A, Terzano MG. Reorganization of sleep patterns in severe OSAS under prolonged CPAP treatment. Clin Neurophysiol. 2005;116(9):2228–2239. doi:10.1016/j.clinph.2005.05.005
20. Ratnavadivel R, Chau N, Stadler D, Yeo A, McEvoy RD, Catcheside PG. Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med. 2009;5(6):519–524. doi:10.5664/jcsm.27651
21. Dijk DJ. Regulation and functional correlates of slow wave sleep. J Clin Sleep Med. 2009;5(Suppl 2):S6–15. doi:10.5664/jcsm.5.2S.S6
22. Wichniak A, Geisler P, Brunner H, et al. Spectral composition of NREM sleep in healthy subjects with moderately increased daytime sleepiness. Clin Neurophysiol. 2003;114(8):1549–1555.
23. Terzano MG, Parrino L, Rosa A, Palomba V, Smerieri A. CAP and arousals in the structural development of sleep: an integrative perspective. Sleep Med. 2002;3(3):221–229. doi:10.1016/s1389-9457(02)00009-6
24. Gnoni V, Drakatos P, Higgins S, et al. Cyclic alternating pattern in obstructive sleep apnea: a preliminary study. J Sleep Res. 2021;30(6):e13350. doi:10.1111/jsr.13350
25. Lu J, Zhang Y, Han K, et al. Heart rate changes associated with rhythmic masticatory muscle activities and limb movements in sleep bruxers: preliminary findings. Cranio. 2021;39(1):47–57. doi:10.1080/08869634.2019.1578032
26. Han K, Wang C, Zhong Z, et al. Characterisation of the relationships between rhythmic masticatory muscle activities and limb movements in patients with sleep bruxism. J Oral Rehabil. 2019;46(5):399–408. doi:10.1111/joor.12760
27. Berry RB, Quan SF, Abreu AR, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.6. Darien, Illinois: American Academy of Sleep Medicine; 2020.
28. Terzano MG, Parrino L, Boselli M, Spaggiari MC, Di Giovanni G. Polysomnographic analysis of arousal responses in obstructive sleep apnea syndrome by means of the cyclic alternating pattern. J Clin Neurophysiol. 1996;13(2):145–155. doi:10.1097/00004691-199603000-00005
29. Parrino L, Grassi A, Milioli G. Cyclic alternating pattern in polysomnography: what is it and what does it mean? Curr Opin Pulm Med. 2014;20(6):533–541. doi:10.1097/MCP.0000000000000100
30. Terzano MG, Parrino L, Smerieri A, et al. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med. 2002;3(2):187–199. doi:10.1016/s1389-9457(01)00149-6
31. Parrino L, Smerieri A, Boselli M, Spaggiari MC, Terzano MG. Sleep reactivity during acute nasal CPAP in obstructive sleep apnea syndrome. Neurology. 2000;54(8):1633–1640. doi:10.1212/wnl.54.8.1633
32. Eskelinen V, Uibu T, Himanen SL. nCPAP treatment of obstructive sleep apnea increases slow wave sleep in prefrontal EEG. Clin EEG Neurosci. 2007;38(3):148–154. doi:10.1177/155005940703800311
33. Terzano MG, Parrino L, Fioriti G, Spaggiari MC, Piroli A. Morphologic and functional features of cyclic alternating pattern (CAP) sequences in normal NREM sleep. Funct Neurol. 1986;1(1):29–41.
34. Korkmaz S, Bilecenoglu NT, Aksu M, Yoldas TK. Cyclic Alternating pattern in obstructive sleep apnea patients with versus without excessive sleepiness. Sleep Disord. 2018;2018:8713409. doi:10.1155/2018/8713409
35. Terzano MG, Parrino L. Clinical applications of cyclic alternating pattern. Physiol Behav. 1993;54(4):807–813. doi:10.1016/0031-9384(93)90096-x
36. Parrino L, Smerieri A, Rossi M, Terzano MG. Relationship of slow and rapid EEG components of CAP to ASDA arousals in normal sleep. Sleep. 2001;24(8):881–885. doi:10.1093/sleep/24.8.881
37. Mutti C, Azzi N, Halasz P, Szucs A, Parrino L. Intra period CAP kinetics to stressful perturbation: a message from obstructive sleep apnea. Sleep Med. 2021;80:226–227. doi:10.1016/j.sleep.2021.01.060
38. Bennett LS, Langford BA, Stradling JR, Davies RJ. Sleep fragmentation indices as predictors of daytime sleepiness and nCPAP response in obstructive sleep apnea. Am J Respir Crit Care Med. 1998;158(3):778–786. doi:10.1164/ajrccm.158.3.9711033
39. Carnicelli L, Maestri M, Di Coscio E, et al. A longitudinal study of polysomnographic variables in patients with mild cognitive impairment converting to Alzheimer’s disease. J Sleep Res. 2019;28(5):e12821. doi:10.1111/jsr.12821
40. Thomas RJ. Arousals in sleep-disordered breathing: patterns and implications. Sleep. 2003;26(8):1042–1047. doi:10.1093/sleep/26.8.1042
41. Wauquier A, Aloe L, Declerck A. K-complexes: are they signs of arousal or sleep protective? J Sleep Res. 1995;4(3):138–143. doi:10.1111/j.1365-2869.1995.tb00162.x
42. Aricò D, Drago V, Foster PS, Heilman KM, Williamson J, Ferri R. Effects of NREM sleep instability on cognitive processing. Sleep Med. 2010;11(8):791–798. doi:10.1016/j.sleep.2010.02.009
43. Ferri R, Bruni O, Miano S, Plazzi G, Terzano MG. All-night EEG power spectral analysis of the cyclic alternating pattern components in young adult subjects. Clin Neurophysiol. 2005;116(10):2429–2440. doi:10.1016/j.clinph.2005.06.022
44. Parrino L, Ferri R, Zucconi M, Fanfulla F. Commentary from the Italian Association of Sleep Medicine on the AASM manual for the scoring of sleep and associated events: for debate and discussion. Sleep Med. 2009;10:799e808. doi:10.1016/j.sleep.2009.05.009
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.