Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Effects of Atorvastatin Therapy on Postoperative Delirium After Malignant Tumor Surgeries in Older Adults
Authors Wan R, Cai S, Pan D, Yang W, Zhou R
Received 28 January 2022
Accepted for publication 24 March 2022
Published 19 April 2022 Volume 2022:18 Pages 915—923
DOI https://doi.org/10.2147/NDT.S360332
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Rong Wan,1 Shengwei Cai,2 Dejian Pan,2 Weilin Yang,2 Rengui Zhou2
1Department of Quality Management, The 904th Hospital of Joint Logistic Support Force (Anhui Medical University Affiliated Wuxi Clinical College), Wuxi, 214044, People’s Republic of China; 2Department of Oncology, The 904th Hospital of Joint Logistic Support Force (Anhui Medical University Affiliated Wuxi Clinical College), Wuxi, 214044, People’s Republic of China
Correspondence: Rong Wan, Department of Quality Management, The 904th Hospital of Joint Logistic Support Force (Anhui Medical University Affiliated Wuxi Clinical College), Xing Yuan North Road 101, Wuxi, 214044, People’s Republic of China, Tel +86 051085142023, Fax +86 051085142601, Email [email protected]
Introduction: Postoperative delirium (POD) is one of the prevalent and potentially fatal clinical conditions, leading to high disability and mortality in older patients, as well as increased duration of hospital stay and more hospitalization expenses. There were no effective drugs in the clinical management of POD, and an absence of evidence-based medicine concerning the treatment of POD.
Materials and Methods: The present study explored whether atorvastatin (Ato) can decrease the occurrence rate of POD. The present research included patients over the age of 60 who were hospitalized to critical care units (ICUs) following surgery for malignant tumors. Patients received Ato (40mg/day) or placebos utilizing a computer-based random sequencing (in a 1:1 ratio). The primary outcome measure was the occurrence of delirium within the first seven days following surgery.
Results: A total of 230 individuals were classified into two groups, namely the placebo group (n=123) and the Ato group (n=107). Patients belonging to two groups had similar baseline clinical data, and there were no statistically significant differences between them. The occurrence of delirium in the Ato group was remarkably reduced unlike the case in the placebo group. 30-day all-cause mortality did not vary significantly between the two groups. Pulmonary infection and Bedsore were significantly decreased in the Ato group than the placebo group, there were no statistically significant differences between the two groups in rhabdomyolysis and abnormal liver enzymes. In terms of medication responses, there were no statistically significant differences between the two groups. Ato patients had remarkably shortened hospital stays and spent remarkably less on hospitalization expenditures in comparison with those in the placebo group.
Conclusion: The findings from the present research indicated that Ato can decrease the occurrence of delirium following surgical operation of malignant tumors among elderly patients, it also can reduce the duration of hospitalization, hospital cost, and post-surgical complications, but not improve 30-day all-cause mortality.
Registration Number: ChiCTR-IPR-17011984.
Keywords: atorvastatin, 30-day all-cause mortality, postoperative delirium, RCT, outcome
Introduction
Delirium is a frequent and potentially deadly clinical condition whose characteristics include the presence of transitory organic mental illness, acute brain malfunction, alterations in cognitive function, and disorientation. It may result in long-term cognitive damage, a higher rate of disability, and death, as well as the prolonged duration of time spent in hospital and greater healthcare expenditures.1 Delirium is known to occur in up to 74% of severely sick individuals.2 According to a recent survey, around 60–80% of elderly persons admitted to the hospital suffered from delirium.3 Delirium is identified in over 20% of patients admitted to the intensive care unit (ICU).4 In recent times, as the population has aged, the proportion of elderly patients undergoing surgery has risen, with the prevalence of postoperative delirium (POD) in this population reaching as high as 46% in one study.5 Every year in the United States, over 2.6 million persons over the age of 65 suffer from delirium, resulting in an approximately annual healthcare cost exceeding US$164 billion. Furthermore, delirium, especially POD, has the potential to elevate the long-term risk of developing dementia or even death.6 The management of delirium in elderly individuals has evolved into a significant problem during the last two decades. According to Tmimi7 POD has been recorded in 41% of patients undergoing heart surgery. Korc-Grodzicki8 discovered that delirium developed in 19% of people with cancer, according to his findings. There are currently no medications available for the management or protection against delirium. Notably, the majority of medications now available in clinics have no specific impact on delirium, and there is also a scarcity of evidence-based medicine in the field of delirium therapy.
Statins, which are enzyme antagonists of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, are currently frequently applied in the treatment of cardiovascular illnesses as cholesterol-lowering drugs, and they have been shown to reduce the risk of heart attack and stroke. Several pleiotropic impacts of statins have been demonstrated, including cerebral vasospasm, oxidative stress, and anti-inflammatory actions, as well as the ability to prevent the aggregation of platelets.9–11 Our earlier studies also revealed that statins could attenuate brain edema as well as early brain injury under their neuroprotective properties. Statins also help mitigate cerebral vasospasm and facilitate structure and function remodeling of vascular endothelium via the mechanism of protecting autoregulation of cerebral vessels.12,13 Page 14 was the first to describe a prospective cohort study of data from sequential ICU patients throughout August 2011 to February 2012. In their study, they recruited 151 patients who were on statins and 319 patients who were not taking statins. The findings revealed that continuous statin medication in severely sick populations in the United Kingdom exhibited a more dramatic therapeutic impact on delirium. Nonetheless, according to the results of an extremely significant and authoritative randomized controlled trial (NCT00979121 and NCT00719446), which included 568 patients (293/275) at 35 hospitals in the United States and demonstrated no merit of rosuvastatin in terms of alleviating delirium or cognitive disorder in the ICU throughout a 12-month follow-up period.15
Atorvastatin (Ato) was a statin drug that exerted neuroprotection and anti-delirium in previous studies.9,10,16 However, because of the unique nature of patients receiving surgery for treating malignant tumors, the efficacy of Ato in treating delirium remains unclear. As a consequence, the present research investigated whether Ato therapy may help to minimize POD and enhance patient outcomes following malignant tumor removal.
Methods
Study Design
A placebo-controlled, parallel-arm, double-blind, randomized, experiment was carried out in Jiangsu from January 2016 to December 2019. A total of 319 senior individuals were evaluated over this period. To determine if the intervention is superior, the present research was conducted. The Clinical Research Ethics Committees of the Anhui Medical University Affiliated Wuxi Clinical College and the Wuxi Clinical College endorsed the methodology used in the present research (2015-YXLL-021), and was in accordance with the Declaration of Helsinki. The protocol for the research was subjected to the approval granted by the Ethics Committees of all the collaborating centers. Those patients whose competence could be demonstrated by their comprehensive awareness of time, place, and person, along with their comprehension of the investigator’s explanation of the trial, or through other means from their next of kin or legally authorized representatives, were asked to obtain written informed consent for the study. Following malignant tumor surgical intervention. In addition, patients were allocated at random (1:1) who were administered 40 mg/day of Ato or placebos for seven days following procedure (Figure 1). Oral administration of ato or placebos was performed for seven days following malignant tumor resection. The last check-up was performed 30 days following the procedure.
 |
Figure 1 Study design. Abbreviations: Ao, atorvastatin; CAM, confusion assessment method; CAM-ICU, confusion assessment method for the intensive care unit. |
Patients Enrolled in the Study and Sample Selection Procedures
Patients were included in the present research if they were over 60 years of age and underwent malignant tumor surgical procedures in the ICU. The following were the criteria for inclusion: (1) Over the age of 60; (2) Could be randomly assigned to receive either Ato or a placebo during seven days after the surgical intervention; and (3) Malignant tumor excision was performed under general anesthesia and patients were hospitalized to the ICU. The exclusion criteria were as follows: (1) Patients who are unlikely to be salvaged upon admission; (2) Hypercholesterolemia in conjunction with diabetes; (3) Injuries to the brain or neurosurgery; (4) neurological disorders; (5) Patients having a history of mental health problems or epilepsy; (6) Anomalies of the liver and kidneys; (7) Multiple organ dysfunction; and (8) other explanations were discovered by researchers.
Randomization and Concealment
With the aid of the SPSS software (version:14.0) (SPSS Institute, Hefei, Anhui Medical University), permuted-block randomization was carried out based on a computer system that used an allotment list to produce random numbers (in a one-to-one ratio). This was carried out by a statistician who was not a member of the research team to maintain the integrity and blinding of the research. The outcomes of the random sampling process were enclosed in prenumbered envelopes and kept at the location of the research till the study’s conclusion was reached. The patients included in the present research were given 40 mg/day of Ato or placebos randomly for 7 days after undergoing a malignant tumor resection (Figure 1). The study medicines were delivered by a research nurse following the random assignment sequence. Both the research participants and the patients were unaware of which medicine was being applied in the trial. In the event of an emergency, such as acute hepatic failure, two experts might recommend that the treatment allotment be unmasked and that the study medicine be adjusted or discontinued if required, according to the protocol. All of the occurrences were recorded in detail. Then, we acquired information on patients’ demographics, medical histories, and pertinent investigation findings.
Outcome Assessment
All clinical and imaging data were subjected to assessment by a masked independent diagnostic and assessment committee. This committee included two researchers that were trained before the start of the present research and did not engage in the clinical care of patients. The primary outcome measure was the occurrence of delirium during the first seven days following surgery. Approximately 24 hours following the surgical procedure, the first signs of POD were assessed. Subsequently, the condition was assessed twice daily (from 6 to 8 am and from 6 to 8 pm). Delirium was determined utilizing the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) and the Confusion Assessment Method (CAM). A total of four characteristics of delirium were identified by both CAM and CAM-ICU: (1) Acute commencement of alterations in mental state or a course that fluctuates; (2) Inattentiveness; (3) Incoherent thought processes; and (4) Consciousness has been altered. Concerning patients with a diagnosis of delirious, they exhibited the above criteria 1 and 2, as well as 3 or 4. The second set of outcomes comprised all-cause 30-day death.
Safety Evaluation and Postoperative Complications
We kept track of the length of time spent in the intensive care unit, the frequency of non-delirium postoperative problems, and the costs incurred during hospitalization. The most prevalent adverse effects of Ato include rhabdomyolysis and abnormal liver enzymes, which were also the most severe. Pulmonary infection was confirmed by chest computed tomography and sputum culture; Bedsore was confirmed by physical examination after two doctors and nurses; Rhabdomyolysis was diagnosed by blood biochemistry, muscle enzyme levels detection, and clinical manifestation; Abnormal liver enzymes was diagnosed by liver-function tests. Finally, we check the related index every two days over the first 14 days.
Postoperative Hospital Stay and Hospitalization Costs
As previously reported in clinical trials, patients having delirium had remarkably longer post-surgical hospital stays and higher hospitalization expenses. Thus, the present research examined the difference in the overall duration of hospital stay and healthcare costs among patients belonging to the two groups.
Statistical Analysis
Data from the baseline as well as outcome assessments were input into the database by a research nurse. The information was gathered on handwritten forms and stored in a digital database that was password secured. All continuous data are expressed as the mean ± standard deviation. The analyses of statistical data were performed using the SPSS software (version: 14.0) (SPSS, Inc., Chicago, USA). For categorical data, Spearman correlations and independent two-sample t-tests were performed to determine their significance. Comparisons for the categorical data for the two subgroups were conducted with the aid of Fisher’s exact t-test. Comparisons of the continuous or ordinal data for the two groups were performed utilizing the Mann–Whitney U-test. P < 0.05 was established as the threshold for statistical significance.
Results
319 elderly patients were evaluated between January 2016 and December 2019. A sum of 230 participants was given Ato (n=107) or a placebo (n=123) treatment in a random manner. There were no cases of opening-blindness observed throughout the research period. Furthermore, no statistically significant differences were discovered in terms of the baseline data between the two subgroups (Table 1). None of the patients were lost to follow-up throughout the present research. The eventual intention-to-treat analysis incorporated all of the patients (Figure 2). The concluding appointment with the last randomly selected patient took place on February 15, 2020.
 |
Table 1 Demographic and Baseline Characteristics of the Study Population in the Two Groups |
 |
Figure 2 Trial profile. |
The Endpoint and Clinical Outcomes
The total incidence of delirium following malignant tumor surgical intervention in older patients was 19.6% (45/230), according to the findings. At 7 days postoperatively, the delirium incidence was found to be 11.2% (12/107) in the Ato group and 26.8% (33/123) in the placebo control group. As opposed to the Ato group, the placebo group exhibited a remarkably greater delirium occurrence rate, with a significant difference (p =0.003). After 30 days, the all-cause mortality rate in the Ato group was 1.9% (2/107), whereas it was 4.1% (5/123) in the placebo control group, with no statistically significant difference (p =0.454, Table 2).
 |
Table 2 Comparison of Endpoint-Clinical Outcomes Between the Two Groups |
Postoperative Complications
There was 29 (23.6%) case occurred pulmonary infection in the control group, while there were 14 (13.1%) cases of pulmonary infection in the Ato group, which were improved after drug treatment. There was 18 (14.6%) case of bedsore in the control group, while there were 6 (5.6%) cases of bedsore in the Ato group. The incidence of pulmonary infection and bedsore in the Ato group was remarkably diminished as opposed to that in the placebo group (p<0.05, Table 3). Furthermore, no significant difference was identified between the two groups in the possible side effects of Ato induced rhabdomyolysis (0 vs 2.8%, p=0.099) and abnormal liver enzymes (27.6% vs 38.3%, p=0.085, Table 3). No blind cases were opened during the treatment period.
 |
Table 3 Comparison of Postoperative Complications Between the Two Groups |
Postoperative Hospital Stay and Hospitalization Costs
In the Ato group, the average duration of stay was 16.5 days, whereas the value for the placebo group was 17.7 days, with a statistically significant difference (p =0.01). The mean hospitalization expenditure of the Ato group was 61,000 RMB, which was much less as opposed to the placebo control group’s cost of 68,000 RMB, with a statistically significant difference (p =0.01, Table 4).
 |
Table 4 Comparison of Secondary Endpoint Between the Two Groups |
Discussion
According to the findings of the current investigation, preventive low-dose Ato oral following surgical procedure may greatly reduce the occurrence of POD in older patients undergoing malignant tumor surgical intervention. Moreover, it shortened the duration of hospital stays and the expenditure of hospitalization. In this case, postoperative consequences, such as liver failure, remained unchanged. However, the abnormal liver enzymes of patients in the Ato treatment group was elevated in contrast with that in the placebo group, and this should be taken into account because the synergistic effect after the combined application of antibiotics in some patients may lead to an increase in the proportion of patients with abnormal liver function.
According to the findings, the total incidence of POD in elderly people with malignant tumors was 19.6%, which was consistent with earlier reports.7 Crawford17 indicated that the incidence of post-surgical delirium was 7.5% among patients with head and neck oral cancer. In the adult brain tumor resection, the overall incidence was 14.78%, including 50.76% hyperactive delirium, 41.67% hypoactive delirium, and 7.57% mixed delirium.18 Ishibashi-Kanno19 also reported that 33.3% of oral tumors after reconstructive surgery. The specific cause of POD in elderly individuals who underwent surgery for a malignant tumor remained uncertain. The results in our prior literature analyses demonstrated multiple causes that may lead to POD (Figure 3).13 Previous research has shown that delirium is correlated with increased mortality, higher rates of disability, prolonged hospitalizations, impaired functional recovery, and long-term cognitive deterioration in individuals above the age of 70.20 Furthermore, POD may increase the financial burden placed on patients and their families, as well as affect inpatient bed turnover and the length of time spent in the hospital. As a result, it was critical to reduce the frequency of POD to a great extent. According to recent meta-analyses of the literature that were founded on multicenter randomized controlled trials, no effective and safe medication can prevent and treat delirium in severely sick adults.21
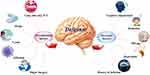 |
Figure 3 Etiology of delirium. |
In the present study, Ato intervention was used to reduce the incidence rate of POD in older patients with malignant tumors. There are still many controversies in clinical studies in anti-delirium effection of statin. According to the findings of an earlier retrospective analysis that included 284,158 consecutive patients over the age of 65 undergoing elective surgical treatments, the result was the exact opposite of ours that statin administration may increase the risk of POD in aged patients.22 A systematic review and meta-analysis also showed a disappointing result that statin did not prevent delirium in n critically ill and cardiac surgery patients.23 Furthermore, statins have been reported to aggravate POD among elderly patients undergoing elective surgery.22 While, most of these studies were retrospective or small size study, and their conclusions were not reliable. In contrast, a single-center retrospective study reported that pre-operative statins treatment may remarkably reduce the incidence of POD in vascular surgery patients, where the specific mechanism may be associated with inflammation.24 TK25 also reported a similarly results that continuous perioperative statin use may be decrease the risk of delirium after TKA, simvastatin was the most effective statin for POD prevention. According to Lee’s26 findings, preoperative statin might remarkably decrease the occurrence of POD in patients undergoing vascular surgical treatment.
The following were some of the study’s limitations: (1)Patients were not called back for study visits for a full evaluation of their quality of life in the context of activities of daily living (ADL), because the researchers obtained important baseline and endpoint data. (2)All individuals were evaluated and recruited after being admitted to the intensive care unit, and there was no baseline delirium evaluation with cognitive function assessment. (3)In the present research, Ato was administered in a single dosage. It is possible that the standard low dosage might not be effective. (4)The present research should have examined clinical parameters including pain, the daily incidence of delirium, subjective sleep quality, and the duration of time spent before extubation.
Conclusion
The findings of the present research suggest that Ato treatment may help to minimize the risk of POD following malignant tumor resection surgery. Furthermore, it resulted in a considerable reduction in hospitalization expenditures as well as hospitalization duration. It is still controversial if the good benefits given by this innovative application of Ato will lead to better long-term results for the participants. To completely grasp the prospective application of Ato in postoperative patients, more research with individuals who have had previous procedures and received varied dosages is required.
Data Sharing Statement
The datasets used and/or analysed during the current study, included redacted study protocol, redacted statistical analysis plan, and individual participant data supporting the results reported are available from the corresponding authors on reasonable request.
Ethics Statement
The Anhui Medical University Affiliated Wuxi Clinical College Clinical Research Ethics Committees granted its approval for the research procedure (2015-YXLL-021). All participating centers’ Ethics Committees approved the study procedures. Those patients whose competence could be established by their accurate orientation for time, place, and person, along with their comprehension of the investigator’s explanation of the trial, were asked to provide written informed consent for the present research.
Acknowledgments
We hereby express our gratitude to Jiangsu Brilliant Biological Technology Co., Ltd. for providing technical and linguistic help.
Funding
Modified Medium and Long Catheter Insertion T202018.
Disclosure
All authors hereby declare that there are no conflicting interests in this work.
References
1. Gleason LJ, Schmitt EM, Kosar CM, et al. Effect of delirium and other major complications on outcomes after elective surgery in older adults. JAMA Surg. 2015;150(12):1134–1140. doi:10.1001/jamasurg.2015.2606
2. Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32(4):955–962. doi:10.1097/01.Ccm.0000119429.16055.92
3. Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513–1520. doi:10.1097/CCM.0b013e3181e47be1
4. Mather JF, Corradi JP, Waszynski C, et al. Statin and its association with delirium in the medical ICU. Crit Care Med. 2017;45(9):1515–1522. doi:10.1097/ccm.0000000000002530
5. Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–39. doi:10.1056/NEJMoa1112923
6. Redelmeier DA, Manzoor F, Thiruchelvam D. Association between statin use and risk of dementia after a concussion. JAMA Neurol. 2019;76(8):887–896. doi:10.1001/jamaneurol.2019.1148
7. Al Tmimi L, Verbrugghe P, Van de Velde M, et al. Intraoperative xenon for prevention of delirium after on-pump cardiac surgery: a randomised, observer-blind, controlled clinical trial. Br J Anaesth. 2020;124(4):454–462. doi:10.1016/j.bja.2019.11.037
8. Korc-Grodzicki B, Sun SW, Zhou Q, et al. Geriatric assessment as a predictor of delirium and other outcomes in elderly patients with cancer. Ann Surg. 2015;261(6):1085–1090. doi:10.1097/sla.0000000000000742
9. Chen J, Li M, Zhu X, et al. Atorvastatin reduces cerebral vasospasm and infarction after aneurysmal subarachnoid hemorrhage in elderly Chinese adults. Aging. 2020;12(3):2939–2951. doi:10.18632/aging.102788
10. Chen J, Zhang C, Yan T, et al. Atorvastatin ameliorates early brain injury after subarachnoid hemorrhage via inhibition of pyroptosis and neuroinflammation. J Cell Physiol. 2021;236(10):6920–6931. doi:10.1002/jcp.30351
11. Chen JH, Wu T, Xia WY, et al. An early neuroprotective effect of atorvastatin against subarachnoid hemorrhage. Neural Regen Res. 2020;15(10):1947–1954. doi:10.4103/1673-5374.280326
12. Chen JH, Wu T, Yang LK, et al. Protective effects of atorvastatin on cerebral vessel autoregulation in an experimental rabbit model of subarachnoid hemorrhage. Mol Med Rep. 2018;17(1):1651–1659. doi:10.3892/mmr.2017.8074
13. Chen J, Wang Y, Hu X, et al. The role of statins in the management of delirium: recent advances. CNS Neurol Disord Drug Targets. 2020. doi:10.2174/1871527319666200720111318
14. Page VJ, Davis D, Zhao XB, et al. Statin use and risk of delirium in the critically ill. Am J Respir Crit Care Med. 2014;189(6):666–673. doi:10.1164/rccm.201306-1150OC
15. Needham DM, Colantuoni E, Dinglas VD, et al. Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis-associated acute respiratory distress syndrome: an ancillary study to a randomised controlled trial. Lancet Respir Med. 2016;4(3):203–212. doi:10.1016/S2213-2600(16)00005-9
16. Sohrevardi S, Nasab F, Mirjalili M, et al. Effect of atorvastatin on delirium status of patients in the intensive care unit: a randomized controlled trial. Arch med sci. 2021;17(5):1423–1428. doi:10.5114/aoms.2019.89330
17. Crawford JE, Zubair F, Baniulyte G, et al. Postoperative delirium in patients with head and neck oral cancer in the West of Scotland. Br J Oral Maxillofac Surg. 2021;59(3):353–361. doi:10.1016/j.bjoms.2020.08.116
18. Chen H, Jiang H, Chen B, et al. The incidence and predictors of postoperative delirium after brain tumor resection in adults: a cross-sectional survey. World Neurosurg. 2020;140:e129–e139. doi:10.1016/j.wneu.2020.04.195
19. Ishibashi-Kanno N, Takaoka S, Nagai H, et al. Postoperative delirium after reconstructive surgery for oral tumor: a retrospective clinical study. Int J Oral Maxillofac Surg. 2020;49(9):1143–1148. doi:10.1016/j.ijom.2020.01.018
20. Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi:10.1056/NEJMoa1301372
21. van den Boogaard M, Slooter AJC, Bruggemann RJM, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium the REDUCE Randomized Clinical Trial. JAMA. 2018;319(7):680–690. doi:10.1001/jama.2018.0160
22. Redelmeier DA, Thiruchelvam D, Daneman N. Delirium after elective surgery among elderly patients taking statins. Cmaj. 2008;179(7):645–652. doi:10.1503/cmaj.080443
23. Vallabhajosyula S, Kanmanthareddy A, Erwin P, Esterbrooks D, Morrow L. Role of statins in delirium prevention in critical ill and cardiac surgery patients: a systematic review and meta-analysis. J Crit Care. 2017;37:189–196. doi:10.1016/j.jcrc.2016.09.025
24. Mu DL, Zhang DZ, Wang DX, et al. Parecoxib supplementation to morphine analgesia decreases incidence of delirium in elderly patients after hip or knee replacement surgery: a Randomized Controlled Trial. Anesth Analg. 2017;124(6):1992–2000. doi:10.1213/ane.0000000000002095
25. Oh T, Park H, Shin H, Jeon Y, Do S, Hwang J. The role of perioperative statin use in the prevention of delirium after total knee replacement under spinal anesthesia. J Arthroplasty. 2018;33(12):3666–3671.e1. doi:10.1016/j.arth.2018.08.022
26. Lee DS, Lee MY, Park CM, Kim DI, Kim YW, Park YJ. Preoperative statins are associated with a reduced risk of postoperative delirium following vascular surgery. PLoS One. 2018;13(3):e0192841. doi:10.1371/journal.pone.0192841
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
