Back to Journals » Journal of Pain Research » Volume 15
Effects of a Digital Musculoskeletal Acute Care Program on Chronic Pain Prevention: An Observational Study with Nonparticipant Comparison Group
Authors Hong M , Topete M, Yang M, Bailey JF
Received 5 August 2022
Accepted for publication 10 October 2022
Published 17 November 2022 Volume 2022:15 Pages 3605—3613
DOI https://doi.org/10.2147/JPR.S385134
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alaa Abd-Elsayed
Mindy Hong,1 Melanie Topete,1 Manshu Yang,2 Jeannie F Bailey3
1Hinge Health, Inc, San Francisco, CA, USA; 2Department of Psychology, University of Rhode Island, Kingston, RI, USA; 3Department of Orthopaedic Surgery, University of California San Francisco, San Francisco, CA, USA
Correspondence: Mindy Hong, Hinge Health, Inc, 455 Market Street, Suite 700, San Francisco, CA, 94105, USA, Email [email protected]
Background: There is limited research on whether digital interventions can prevent acute or subacute pain from developing into chronic pain. This observational study’s primary objective examined whether chronic pain was more likely to be prevented in digital acute MSK program participants than nonparticipants. An exploratory objective was time to pain relief for program participants versus nonparticipants.
Patients and Methods: The intervention group participants conducted video visits with physical therapists and were recommended exercise therapies and educational articles delivered through an app dedicated to addressing musculoskeletal (MSK) needs. This study used a multidimensional approach incorporating pain, function, depression, and anxiety scores to determine whether chronic pain prevention was achieved at 12 weeks. Descriptive analyses, unadjusted, and adjusted logistic regression were conducted. Time-to-event analysis was performed to compare the time to pain relief between groups.
Results: A total of 171 participants (intervention: 75, nonparticipants: 96) with baseline and 3, 6, and 12 week follow-up data were included in the sample. Baseline mean (SD) age was 44.2 (11.8) years and mean VAS pain was 43.3 (22.9), out of 100. Results showed significantly higher odds of achieving chronic pain prevention at 12 weeks in the intervention participants versus nonparticipants. After adjusting for age, pain region, registration month, number of weeks of pain experienced, and healthcare service use at 12 weeks, 20.5% of the intervention group and 5.5% of the nonparticipant group achieved chronic pain prevention. At 91 days, the probability of developing chronic pain was 77.7% for nonparticipants and 46.5% for intervention participants (p< 0.001; Log rank test).
Conclusion: A digital acute MSK program may help to prevent chronic pain from developing among those with acute and subacute MSK needs. Study results also suggest that program participants achieve chronic pain relief sooner compared to nonparticipants.
Keywords: acute, chronic, musculoskeletal, pain, prevention, telemedicine
Introduction
Approximately 1.7 billion people have musculoskeletal (MSK) conditions worldwide, making it the biggest contributor to years lived with disability.1 MSK-related conditions, which include back and neck pain, osteoarthritis, rheumatoid arthritis, and gout, account for 19.1%, 17.1%, 10.6%, and 25.7% of all years lived with disability (YLD) in Europe, Asia, Africa, and the United States, respectively.2 In the United States alone, 100 million adults experience chronic pain, which can greatly affect an individual’s function, emotional and social well being, economics, and overall quality of life (QOL).3,4 Additionally, MSK conditions are one of the highest contributors to healthcare costs in the United States.5 Chronic MSK pain is estimated to cost $560 to $635 billion per year with a loss of productivity,6 accounting for 30% of missed work days in 2018.7 One determinant to diagnosing MSK pain is the length of pain experienced. Acute pain is defined as pain that has been present for 4 weeks or less. Subacute pain lasts between 4 and 12 weeks, and chronic pain lasts for 12 weeks or more.8
Acute pain that is not resolved early can develop into chronic pain. The transition from acute to chronic pain is complex and the mechanisms of this transition are not clearly defined.9 However, a systematic review shows a median of 26% of patients with acute low back pain (LBP) transitioned to chronic LBP.10 In another study, 32% of adults progressed from acute LBP to chronic LBP11 after 6 months, and 24–87% of adults with acute LBP had a recurrence within 1 year.12 The transition is also prevalent in youth, where 36% of children and adolescents progress from acute to persistent MSK pain.13
Although acute and chronic pain have differences with regard to time frame and pathophysiology, both share similarities with associated risk factors, such as genetics and psychological components. Additionally, both acute and chronic pain share similar treatment principles to decrease pain, improve functional mobility, and address potential psychological factors. Studies show that poorly managed acute pain is associated with a high prevalence of chronic pain and that treatment timing and type are imperative for chronic pain prevention.14 As a result, it is essential to mitigate the risk of this transition, as chronic pain can lead to declines in overall QOL, increases in healthcare utilization and costs, and potentially ineffective surgeries.9
Digital health interventions have been shown to improve pain and functional disability in the management of acute, subacute, and chronic MSK conditions.15 In a previous study, we found that participants of a digital acute MSK program showed significantly more pain and functional improvement by 12 weeks, compared to nonparticipants.16 However, to our knowledge, there have not been any studies evaluating whether digital interventions can resolve acute pain and prevent chronic conditions from developing. Due to its multifaceted and subjective nature, pain is often a complicated and difficult outcome to measure. Research has shown that using pain scores alone are inaccurate in evaluating overall improvement in acute treatment plans, in that one-dimensional pain ratings are unable to accurately capture the impact of pain on other aspects of life, such as function, sleep quality, or mental health.17 As a result, multidimensional pain measurement strategies, which combine biopsychosocial and functional factors in addition to pain scores, are recommended.14 By using a multidimensional pain measurement strategy, researchers are able to capture and incorporate multiple concepts of pain, including perceptions of pain relief, function, and psychological experiences (eg anxiety and depression).18
Thus, the primary objective of this study was to use a multidimensional measurement strategy consisting of pain, function, and psychosocial variables, to examine whether the transition to chronic pain was prevented at 12 weeks for participants of a digital acute MSK program against a nonparticipant comparison group. An exploratory objective was to compare the time to pain relief between the acute and nonparticipant groups. Findings of this study will contribute to the limited evidence base about the role of digital health in preventing acute MSK pain from developing into chronic pain.
Methods
A complete description of the study methods can be found in the previously published study.15 An abridged version is presented here.
Study Design
An observational, prospective cohort study comparing prevention of chronic pain in digital MSK acute program participants (herein, intervention group) to nonparticipants was conducted.
Acute Program
Developed by physical therapists (PTs), the objective of the acute program was to address participants’ acute or subacute MSK pain through virtual consultations, app-guided exercise therapy sessions, and education. Participants were able to access the acute program app through tablets or smartphones.
Participants started the acute program with a video visit with a licensed PT, who would learn more about the participant’s history and goals. The PT guided the participant through a series of movement tests to evaluate their current level of function. After the visit, the PT provided the participant with a plan consisting of recommended exercises and education articles that were accessible through the app.
The app provided the plan as “sessions”. Each session included exercises that were specific to acute back, knee, shoulder, hip, neck/upper back, elbow/wrist/hand, or ankle/foot pain. Sessions consisted of stretching, strengthening, and balance and mobility activities, based on the participant’s functional limitations and goals, and presented various repetitions of each exercise (depending on the difficulty and type of exercise). As participants progressed through the acute program, a PT adjusted their exercises through changes in variation, number of repetitions, hold time, and/or use of resistance bands, to gradually advance them toward their goals. Once exercises for each session were completed, the app presented educational resources and articles about acute and subacute MSK pain-related topics. As a wholly virtual program, participants had the flexibility of choosing when and where to meet with PTs via video and complete sessions.
Participants
Each week between July and October 2021, study participants who met inclusion and exclusion criteria based on information provided in the application were identified. Inclusion criteria were age 18 years or older; back, knee, shoulder, hip, or neck pain; visual analog scale (VAS) pain score > 0; pain for less than 12 weeks; and covered by employer’s health plan. Exclusion criteria were signs of fracture, joint instability, infection, cancer, and cauda equina syndrome. Individuals who did not provide informed research consent or who had pain for more than 12 weeks were also excluded from the study.
The digital intervention group received an initial video visit with a PT and a published care plan. Nonparticipants applied for the acute program but were declined because their employers did not yet offer the acute program as a benefit. The nonparticipant sample was stratified on pain region (ie back, knee, shoulder, hip, neck). A propensity score match was conducted based on baseline pain and function. Participants were invited to complete an emailed survey 3 weeks after registration (nonparticipants) or video visit (intervention). Surveys were also sent at 6 weeks and 12 weeks after registration (nonparticipants) or video visit (intervention) to individuals who had completed the 3 week survey and agreed to be recontacted.
Ethical Considerations
The study was conducted in accordance with the Declaration of Helsinki and approved by WIRB-Copernicus Group Institutional Review Board (OHRP/FDA IRB registration number IRB00000533) at WIRB-Copernicus Group. Study participants acknowledged and provided research consent.
Variables
The following section describes outcomes, exposures, and covariates.
Outcomes
The primary outcome was chronic pain prevention, a dichotomous outcome defined as sustained pain relief by week 12. Those with pain relief met all of the following criteria: minimally clinically important difference (MCID) in pain from baseline, no more than mild pain, MCID in functional improvement, and no indication of depression or anxiety. Pain relief was measured at 3, 6, and 12 weeks, and those with fluctuating relief (eg met the above criteria at 3 and 12 weeks, but not at 6 weeks) were considered to have not achieved chronic pain prevention.
The following scales were used to define pain, function, and mental health. Pain was based on the question: “Over the past 24 hours, how bad was your [back/knee/shoulder/hip/neck] pain?” from 0 (none) to 100 (worst pain imaginable). A person achieved a MCID in pain improvement if they showed at least a 20 point decrease or a 30% improvement.19 Participants who reported pain scores ≤34 were categorized as having mild pain.20
Function outcomes were dependent on pain location. We collected responses to the 11-item Roland Morris Disability Questionnaire (RMDQ-11, back only), Knee injury and Osteoarthritis Outcome Score Physical Function Short form (KOOS-PS, knee only), Hip disability and Osteoarthritis Outcome Score Physical Function Short form (HOOS-PS, hip only), Shoulder Pain and Disability Index (SPADI, shoulder only), and Neck Pain and Disability Scale short form (sf-NPAD, neck only). Next, we calculated the change from baseline to 12-week follow up. The dichotomous variable for MCID in functional improvement is defined to be as at least 30% improvement on the RMDQ-11;21,22 or 8 point improvement on the KOOS-PS;23–25 or 9.3 point improvement on the HOOS-PS;26,27 or 13 point improvement on the SPADI;28–30 or 12 point improvement on the sf-NPAD;31,32 or no limitations at follow up.
Depression and anxiety scores were collected based on responses to the Patient Health Questionnaire 2-item (PHQ-2) and Generalized Anxiety Disorder 2-item (GAD-2) scales, respectively.
Confounders
Model covariates included registration month (July, August, September, October), age at baseline, pain region (back, knee, shoulder, hip, neck), weeks of pain, and use of healthcare services at 12 weeks (no/yes). The healthcare services were conservative care (eg, office visit with a doctor or a physical therapist), over the counter medications, prescription pain medications, and invasive procedures (eg, emergency department or urgent care center visit, overnight stay in a hospital, injections, or surgery).
Data Sources
Baseline data was acquired through the online application at program registration. Follow-up surveys and up to two reminders were emailed at 3, 6, and 12 weeks after registration (nonparticipants) or first PT video visit (intervention). Time until chronic pain relief was calculated by taking the difference in days between the completed survey date and the date of registration (nonparticipants) or first PT video visit (intervention). Respondents received gift cards for $20 at 3 weeks, $25 at 6 weeks, and $35 at 12 weeks, for their participation.
Study Size
Sample size was based on detecting non-inferiority of the intervention versus nonparticipants at 6 weeks after registering or PT video visit. For VAS pain, a non-inferiority margin of 10 points was used because it is less than the 20 point reduction for MCID in pain improvement.15 Assuming standard deviations (SD) of 21.4 for pain,33 80% power, and a one-sided 2.5% significance level, the required sample size was 57 people per arm (n=114).
Statistical Analysis
Participants who completed the survey 3, 6, and 12 weeks after registration (nonparticipants) or video visit (intervention) were included in the study sample. Summary statistics were estimated for baseline characteristics of age, pain region, registration month and baseline pain. To evaluate differences between groups at baseline, t-tests and chi-square tests were conducted for continuous and categorical variables, respectively.
Unadjusted and adjusted logistic regression models were conducted to examine the association between group and chronic pain prevention. Covariates included baseline age, pain region, registration month, number of weeks of pain experienced, and healthcare service use at 12 weeks. Odds ratios (OR) and 95% confidence intervals (CIs) were estimated. Estimated predicted probabilities and marginal effects are presented. Time to event analysis was used to evaluate the time to pain relief between groups. Kaplan–Meier curves were used to show probabilities of developing chronic pain over time, and a Log rank test was used to compare treatment groups. A proportional hazards model was conducted to compare the association between time to pain relief between treatment groups. The primary analysis employed complete case analysis, ie, excluded cases with any missing values. All analyses were performed in R version 4.0.5 (R Core Team, Vienna, Austria).
Results
Sample Characteristics
Table 1 shows baseline characteristics for nonparticipant and intervention groups. Overall, there were 75 participants in the intervention group and 96 nonparticipants. Other than age (intervention participants were older than nonparticipants, p=0.010), no significant differences were found between the two groups at baseline. The mean (SD) age of the total sample was 44.2 (11.8) years. At registration, the mean VAS pain was 43.3 (SD 22.9), out of 100. The largest percent of the sample registered for back pain (31.0%) and the smallest registered for hip pain (10.5%).
 |
Table 1 Baseline Characteristics (n=171) |
Descriptive Results
12.5% (12 out of 96 participants) of the comparison group achieved chronic pain prevention compared to 38.9% (29 out of 75 participants) of the intervention group.
Main Results
Table 2 shows results from unadjusted and adjusted logistic regression models for chronic pain prevention. The odds of achieving chronic pain prevention by 12 weeks was higher for the intervention (OR: 4.41, 95% CI: [2.10, 9.76]) versus the nonparticipant group. After adjusting for age, pain region (back, knee, shoulder, hip, neck), weeks of pain, and use of healthcare services at 12 weeks, the estimated percentage who achieved chronic pain prevention was 15.0% higher in the intervention group than the nonparticipant group (Table 3).
 |
Table 2 Logistic Regression Results Examining Chronic Pain Prevention by 12 Weeks in Unadjusted and Adjusted Models Comparing the Intervention Group to the Nonparticipant Group |
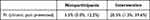 |
Table 3 Adjusted Predicted Proportion of Groups Achieving Chronic Pain Prevention by 12 Weeks |
In time-to-event analysis, results show that the intervention group had significantly shorter time to pain relief than the control group (Figure 1) (p<0.001; Log rank test). Probabilities of having unresolved chronic pain in nonparticipants were 95.8%, 87.5%, and 77.7% at 28, 49, and 91 days, respectively, compared to 85.3%, 61.3%, and 46.5% in intervention participants. An adjusted analysis using proportional hazards regression found that intervention participants progressed faster towards pain relief than nonparticipants (Table 4) (HR: 2.83, 95% CI: [1.56, 5.13]).
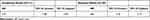 |
Table 4 Proportional Hazards Regression Results Examining Time to Chronic Pain Prevention in Unadjusted and Adjusted Models Comparing the Intervention Group to the Nonparticipant Group |
 |
Figure 1 Kaplan–Meier curves for chronic pain prevention by 12 weeks. |
Discussion
MSK pain has a significant impact on patients’ quality of life by limiting mobility and dexterity, interfering with sleep quality, and affecting mood and mental health.1,34 It is recommended that chronic MSK pain be treated through a multidisciplinary management approach, consisting of lifestyle modification, education, behavioral therapy, physical exercise, and other rehabilitative approaches.35 The studied digital MSK acute intervention program provides its users with a similar multidisciplinary approach and aims to prevent acute pain from worsening into chronic pain.
This study aimed to evaluate whether a digital MSK acute intervention program prevented acute pain from developing into chronic pain against a nonparticipant comparison group. Because of the complex nature of pain, MSK pain can be affected by both physical and psychological factors that can make it difficult to accurately define. As a result, several factors, including pain, function, depression, and anxiety, were taken into consideration in determining the prevention of chronic pain. Additionally, the study also evaluated the timeline in which chronic pain relief was achieved between the groups.
Our study showed that acute program participants were more likely to achieve prevention in chronic pain than nonparticipants. These results are consistent with research showing that early treatment of acute pain through physical activity may help prevent chronic pain. One study showed that those with LBP who were advised to stay active had half the rate of recurrence of LBP at one year compared to participants who were advised to rest.12 A separate study found that those with first time MSK pain in an early active intervention group had 8 times lower chance of developing chronic pain at 12 months, compared to those who experienced treatment as usual (rest for 2–3 weeks and sick leave).36 Results of another cohort study emphasized the importance of active exercise treatments in the prevention of LBP, finding that patients who had less than 1.45 active treatment sessions per week increased the one-year risk of recurrence by 82%.37
Our results found that after adjusting for multiple variables, 3.7 times more intervention participants achieved chronic pain prevention compared to nonparticipants (20.5% of intervention participants versus 5.5% of nonparticipants). While these numbers seem low compared to the literature reporting 26–32% of patients with acute LBP transition to chronic LBP,10,11 our study not only considered improvements in pain, function, and mental health outcomes as having pain relief, but also deemed those with sustained pain relief by week 12 as individuals who did not transition to chronic pain. Considering a variety of clinical outcomes in our definition of chronic pain may have resulted in more conservative estimates than studies who only considered pain-related outcomes.
Results from this study also indicate that those who participate in a digital MSK acute intervention achieve pain relief sooner than nonparticipants. The Kaplan–Meier curves show a statistically significant gap between the intervention and nonparticipant group in the 3 to 6 week time frame, emphasizing the effectiveness of the digital intervention. This is consistent with recent meta-analyses reporting that exercise is an effective treatment for both acute and subacute low back pain in the immediate term.38
To our knowledge, this is the first study to evaluate the effects of a digital intervention on preventing acute pain from transitioning into chronic pain. Additional study strengths include use of data from two prospective cohorts who were matched on similar age, pain, and pain region factors at baseline. Furthermore, this study included a comparison group, which is imperative considering the natural history and fluctuation of acute and subacute MSK conditions. Lastly, a variety of outcomes (pain, function, depression, anxiety) were considered when defining chronic pain prevention, and the program was evaluated in real world settings. Findings of this study are generalizable to a population with acute and subacute MSK pain with expressed interest in a digital acute MSK program.
This study also presents its limitations. As an observational study, we cannot establish causality of the intervention’s effect on chronic pain prevention. In addition, the intervention and nonparticipant groups differed in age at baseline. To address this, we controlled for measured variables in adjusted models. We observed significant associations between the digital intervention group and decreased chronic pain development, however, we could not account for unmeasured confounders, such as motivation. Intervention group participants may have been more motivated to manage pain and report pain improvement, which may bias results upwards. Furthermore, this study proposes a new, multidimensional approach for defining chronic pain prevention that is yet to be validated. But, our approach is consistent with recommendations in pain prevention literature that emphasize the complexity of pain and how it requires a more multifaceted, patient-tailored solution.3,14,39 By incorporating function and psychosocial factors, such as anxiety and depression, in our pain measurement strategy, this study expands past the one-dimensional definition of pain and is able to better capture its complexity.
Future research is needed on evaluating the effects of a digital MSK program on chronic pain prevention. To build on current findings, we recommend a larger study that incorporates more variables, such as sleep quality, motivation, education, and overall health, to construct an even more comprehensive definition of chronic pain. Future studies should also consist of a longer follow-up period where LBP recurrence is examined, as results after one year would be more meaningful in evaluating chronic pain resolution. Additional studies can also evaluate outcomes for each pain region (ie, back, knee, shoulder, hip, neck) separately.
Conclusion
The results of this study provide clinicians with encouraging evidence that a digital MSK program can help with patient needs and can serve as a potential intervention to their acute or subacute pain. Effective early interventions for prevention of chronic pain is imperative in maintaining or improving health, quality of life, and function. Additionally, such interventions could also be cost-effective by reducing the necessity of invasive and potentially ineffective solutions for chronic back pain. As a result, a digital MSK acute program can be used as an early intervention for prevention of chronic pain, as this study’s results suggest that not only are program participants more likely to prevent chronic pain by 12 weeks, but they also achieve pain relief sooner than nonparticipants.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
Hinge Health, Inc., funded the study and provided the digital MSK program to participants.
Disclosure
MH and MT are employed at Hinge Health and received salary and nominal equity compensation. The authors report no other conflicts of interest in this work.
References
1. World Health Organization (WHO). Musculoskeletal Conditions; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions.
2. Institute for Health Metrics and Evaluation (IHME). GBD compare. Seattle, WA: IHME, University of Washington; 2015. Available from: http://vizhub.healthdata.org/gbd-compare.
3. Hruschak V, Cochran G. Psychosocial predictors in the transition from acute to chronic pain: a systematic review. Psychol Health Med. 2018;23(10):1151–1167. doi:10.1080/13548506.2018.1446097
4. Meyer C, Denis CM, Berquin AD. Secondary prevention of chronic musculoskeletal pain: a systematic review of clinical trials. Ann Phys Rehabil Med. 2018;61(5):323–338. doi:10.1016/j.rehab.2018.03.002
5. Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222.
6. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715–724. doi:10.1016/j.jpain.2012.03.009
7. U.S. Bureau of Labor Statistics. Occupational injuries and illnesses resulting in Musculoskeletal Disorders (MSDs); 2020. Available from: https://www.bls.gov/iif/oshwc/case/msds.htm.
8. Centers for Disease Control and Prevention (CDC). Acute low back pain; 2022. Available from: https://www.cdc.gov/acute-pain/low-back-pain/index.html.
9. Gatchel RJ, Reuben DB, Dagenais S, et al. Research agenda for the prevention of pain and its impact: report of the work group on the prevention of acute and chronic pain of the federal pain research strategy. J Pain. 2018;19(8):837–851. doi:10.1016/j.jpain.2018.02.015
10. Chou R. Will this patient develop persistent disabling low back pain? JAMA. 2010;303(13):1295. doi:10.1001/jama.2010.344
11. Stevans JM, Delitto A, Khoja SS, et al. Risk factors associated with transition from acute to chronic low back pain in US patients seeking primary care. JAMA Netw Open. 2021;4(2):e2037371. doi:10.1001/jamanetworkopen.2020.37371
12. Matsudaira K, Hara N, Arisaka M, Isomura T. Comparison of physician’s advice for non-specific acute low back pain in Japanese workers: advice to rest versus advice to stay active. Ind Health. 2011;49(2):203–208. doi:10.2486/indhealth.MS1193
13. Holley AL, Wilson AC, Palermo TM. Predictors of the transition from acute to persistent musculoskeletal pain in children and adolescents: a prospective study. Pain. 2017;158(5):794–801. doi:10.1097/j.pain.0000000000000817
14. Tighe P, Buckenmaier CC, Boezaart AP, et al. Acute pain medicine in the United States: a status report. Pain Med. 2015;16(9):1806–1826. doi:10.1111/pme.12760
15. Hewitt S, Sephton R, Yeowell G. The effectiveness of digital health interventions in the management of musculoskeletal conditions: systematic literature review. J Med Internet Res. 2020;22(6):e15617. doi:10.2196/15617
16. Wang G, Yang M, Hong M, Krauss J, Bailey JF. Clinical outcomes after a digital musculoskeletal (MSK) program for acute and subacute pain: an observational, longitudinal study with comparison group. J Med Internet Res Rehabil Assist Technol. 2022;2022:34.
17. Younger J, McCue R, Mackey S. Pain outcomes: a brief review of instruments and techniques. Curr Pain Headache Rep. 2009;13(1):39–43. doi:10.1007/s11916-009-0009-x
18. Gordon DB, Polomano RC, Pellino TA, et al. Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172–1186. doi:10.1016/j.jpain.2010.02.012
19. Olsen MF, Bjerre E, Hansen MD, Tendal B, Hilden J, Hróbjartsson A. Minimum clinically important differences in chronic pain vary considerably by baseline pain and methodological factors: systematic review of empirical studies. J Clin Epidemiol. 2018;101(87):87–106.e2. doi:10.1016/j.jclinepi.2018.05.007
20. Boonstra AM, Schiphorst Preuper HR, Balk GA, Stewart RE. Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. Pain. 2014;155(12):2545–2550. doi:10.1016/j.pain.2014.09.014
21. Stroud MW, McKnight PE, Jensen MP. Assessment of self-reported physical activity in patients with chronic pain: development of an abbreviated Roland-Morris disability scale. J Pain. 2004;5(5):257–263. doi:10.1016/j.jpain.2004.04.002
22. Jordan K, Dunn KM, Lewis M, Croft P. A minimal clinically important difference was derived for the Roland-Morris disability questionnaire for low back pain. J Clin Epidemiol. 2006;59(1):45–52. doi:10.1016/j.jclinepi.2005.03.018
23. Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: international Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Ou. Arthritis Care Res. 2011;63(S11):S208–S228. doi:10.1002/acr.20632
24. White DK, Master H. Patient-reported measures of physical function in knee osteoarthritis. Rheum Dis Clin N Am. 2016;42(2):239–252. doi:10.1016/j.rdc.2016.01.005
25. Beletsky A, Gowd AK, Liu JN, et al. Time to achievement of clinically significant outcomes after isolated arthroscopic partial meniscectomy: a multivariate analysis. Arthrosc Sports Med Rehabil. 2020;2(6):e723–e733. doi:10.1016/j.asmr.2020.06.002
26. Davis AM, Perruccio AV, Canizares M, et al. The development of a short measure of physical function for Hip OA HOOS-Physical Function Shortform (HOOS-PS): an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16(5):551–559. doi:10.1016/j.joca.2007.12.016
27. Ehrich EW, Davies GM, Watson DJ, Bolognese JA, Seidenberg BC, Bellamy N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27(11):2635–2641.
28. Angst F, Schwyzer HK, Aeschlimann A, Simmen BR, Goldhahn J. Measures of adult shoulder function: disabilities of the Arm, Shoulder, and Hand Questionnaire (DASH) and Its Short Version (QuickDASH), Shoulder Pain and Disability Index (SPADI), American Shoulder and Elbow Surgeons (ASES) Society Standardized Shoulder. Arthritis Care Res. 2011;63(S11):S174–S188. doi:10.1002/acr.20630
29. Schmitt JS, Di Fabio RP. Reliable change and minimum important difference (MID) proportions facilitated group responsiveness comparisons using individual threshold criteria. J Clin Epidemiol. 2004;57(10):1008–1018. doi:10.1016/j.jclinepi.2004.02.007
30. Roy JS, MacDermid JC, Woodhouse LJ. Measuring shoulder function: a systematic review of four questionnaires. Arthritis Rheum. 2009;61(5):623–632. doi:10.1002/art.24396
31. Jorritsma W, Dijkstra PU, de Vries GE, Geertzen JHB, Reneman MF. Detecting relevant changes and responsiveness of neck pain and disability scale and neck disability index. Eur Spine J. 2012;21(12):2550–2557. doi:10.1007/s00586-012-2407-8
32. Blozik E, Kochen MM, Herrmann-Lingen C, Scherer M. Development of a short version of the neck pain and disability scale. Eur J Pain. 2010;14(8):864.e1–864.e7. doi:10.1016/j.ejpain.2009.12.006
33. Shebib R, Bailey JF, Smittenaar P, Perez DA, Mecklenburg G, Hunter S. Randomized controlled trial of a 12-week digital care program in improving low back pain. Npj Digit Med. 2019;2(1):1. doi:10.1038/s41746-018-0076-7
34. Tang NKY, McBeth J, Jordan KP, Blagojevic-Bucknall M, Croft P, Wilkie R. Impact of musculoskeletal pain on insomnia onset: a prospective cohort study. Rheumatology. 2015;54(2):248–256. doi:10.1093/rheumatology/keu283
35. Oliveira CB, Maher CG, Pinto RZ, et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J. 2018;27(11):2791–2803. doi:10.1007/s00586-018-5673-2
36. Linton S. Psychological interventions for patients with chronic back pain. World Health Organization (WHO); 1993. Available from: https://apps.who.int/iris/handle/10665/58224.
37. Krause F, Niederer D, Banzer W, Vogt L. Medical exercise and physiotherapy modes and frequency as predictors for a recurrence of chronic non-specific low back pain. J Back Musculoskelet Rehabil. 2021;34(4):665–670. doi:10.3233/BMR-200149
38. Gianola S, Bargeri S, Del Castillo G, et al. Effectiveness of treatments for acute and subacute mechanical non-specific low back pain: a systematic review with network meta-analysis. Br J Sports Med. 2022;56(1):41–50. doi:10.1136/bjsports-2020-103596
39. Platts-Mills TF, Dayaa JA. Musculoskeletal injures in older adults: preventing the transition to chronic pain and disability. N C Med J. 2017;78(5):318–321. doi:10.18043/ncm.78.5.318
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
