Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
Effectiveness of the Stress Process Model-Based Program in Dementia Caregiving (DeCare-SPM) for Family Caregivers: A Study Protocol for a Randomized Controlled Trial
Authors Wang J , Chen H, Yang L, Yu X, Zhang D, Zhao Q, Xiao M
Received 25 September 2023
Accepted for publication 1 November 2023
Published 16 November 2023 Volume 2023:16 Pages 3507—3519
DOI https://doi.org/10.2147/JMDH.S438342
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jun Wang,1,* Hongmei Chen,2,* Lin Yang,2 Xiuli Yu,3 Dandan Zhang,3 Qinghua Zhao,1 Mingzhao Xiao4
1Department of Nursing, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 2Department of Gynecology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 3Qinggang Senior Care Center, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 4Department of Urology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mingzhao Xiao, Department of Urology, The First Affiliated Hospital of Chongqing Medical University, 1 Youyi Road, Chongqing, 400016, People’s Republic of China, Tel +8613608399433, Fax +86-23-89012206, Email [email protected]
Abstract: This paper aims to describe a randomized controlled trial protocol evaluating the effectiveness, cost, and process of a stress process model-based program in dementia caregiving (DeCare-SPM) for family caregivers. Family caregivers of individuals with dementia will be recruited from memory clinics and community settings and randomly assigned to either DeCare-SPM or usual care. DeCare-SPM comprises three face-to-face sessions (ie, problem-based coping, emotion-based coping, meaning-based coping), and a fourth session (ie, social support) including weekly telephone-based consultation for four weeks and then monthly face-to-face boosters. Outcomes will be measured at baseline (T0), and at one (T1), three (T2), and six months (T3). The primary outcome is positive aspects of caregiving and secondary outcomes are caregiving (ie, sense of competence, caregiver burden, social support, anxiety, depression, and quality of life), dementia-related (ie, care dependency, neuropsychiatric symptoms, and quality of life), and stress-related biomarkers of blood and saliva. In addition, process and economic evaluations will be performed. Mixed-effects models will be used to assess intervention effects. Content analysis will be performed on the qualitative data. This paper described the protocol for comprehensive evaluation of the effectiveness, cost, and process of the theory-driven DeCare-SPM to inform how and why interventions work. It highlights the need to reduce challenges and enhance the positive aspects of dementia care. The DeCare-SPM will provide evidence-based insights into how to support and empower family caregivers in their important roles, thereby, leading to improved dementia care.
Keywords: Alzheimer’s disease, stress coping, informal caregivers, community, psychosocial
Introduction
Dementia is a progressive neurodegenerative disorder that affects millions of individuals worldwide, causing significant cognitive decline and functional impairment and placing a considerable burden on individuals, families, healthcare systems, and society.1 Dementia takes a heavy toll on the global economy, with approximately 50% of its cost attributed to informal care (care provided by family members and close friends).1 Family caregivers are the main sources of informal care and play a vital role in the positive outcomes of individuals with dementia (IWD). Nevertheless, family caregivers of IWD, referred to as “invisible second patients”, suffer from high levels of psychological distress, social isolation, and physical strain, which can cause adverse health outcomes and decrease quality of life.2–4 In China, 95% of IWD are cared for by family caregivers in the community or at home because of the traditional Chinese culture (eg, getting home, Confucianism) and limited formal caregivers and support resources.5,6 China has the largest population of IWD globally. Consequently, it is essential to develop and evaluate effective interventions that address the unique needs of family caregivers to enhance their quality of life and reduce the likelihood of burnout.
Previous studies have identified factors contributing to caregiver burden, including the duration and intensity of caregiving, care recipients’ level of dependency, and caregivers’ personal resources and coping strategies.7,8 Subsequently, numerous interventions focusing on risk and resilience factors have emerged as promising approaches to address the multifaceted needs of the family caregivers of IWD. These approaches mainly focus on on-site or Internet-based psychoeducation, consultation, skill training, support groups, and psychosocial programs comprising multi-component interventions such as skills training, cognitive therapy, and social support.9,10 Most of these interventions have primarily addressed the deficit of knowledge, skills, and/or support from the perspective of caregivers’ negative emotions and adverse experiences, and ignored how caregivers adopt meaning-based coping with stress. While these efforts are necessary and beneficial in mitigating the negative effects associated with caregiving, such as caregiver burden, anxiety, and depression,9,11 there is increasing recognition of the need to shift the paradigm to not only mitigate the negatives but also to enhance the positive aspects of caregiving.
An integrative review conceptualized four main themes of positive aspects of caregiving: a sense of personal accomplishment and gratification, feelings of mutuality in a dyadic relationship, an increase in family cohesion and functionality, and a sense of personal growth and purpose in life.12 In recent years, the positive aspects of caregiving for IWD have become important coping resources.13,14 These aspects are not new; the experience of caregiving can be rewarding and fulfilling, offering opportunities for personal growth, increased sense of purpose, and making new friends, and participating in meaningful activities.15,16 The positive psychology perspective, which emphasizes the cultivation and enhancement of these positives, holds substantial promise for promoting caregiver well-being.17 A published program on benefit-finding interventions developed by Cheng et al in Hong Kong expanded a series of studies focusing on their short- and long-term effects and used a qualitative approach to assess the positive aspects of caregiving.18–20 In addition, interventions such as evidence-based bibliotherapy,21 iSupport,22 gratitude intervention,23 positive diaries,24 and online positive emotion regulation skills25 have been conducted, which indicate a significant effect on the positive aspects of caregiving in family caregivers of IWD. Conversely, a systematic review showed that psychoeducational interventions, which combine education, skill-building, and coping strategies, do not yield a positive impact on perceived benefits.26 Positive psychology perspective to understand caregiving in dementia has been emphasized.
There is inadequate evidence regarding the development and evaluation of positive psychology-based interventions based on a theoretical framework and context, and their impact on the caregiver and dementia-related outcomes. Additionally, these studies have mainly focused on the effectiveness of interventions, that is, outcome measures.13,14 Few have explored how and why interventions work, as well as their acceptability and cost-effectiveness. Thus, there is considerable demand and urgency to implement feasible, acceptable, and cost-effective interventions in the real world to support family caregivers of IWD and improve their quality of life.
Theoretical Framework
The Stress Process Model (SPM) is a well-established theoretical framework that delineates the relationships between stressors, resources, and psychological well-being in caregiving.27 It posits that individuals deal with stressors through two processes: cognitive appraisal and coping. Cognitive appraisal consists of primary and secondary appraisal. Primary appraisal evaluates the beneficial and detrimental perceptions of stressors, while secondary appraisal assesses whether an individual can do anything to overcome or prevent harm and change the prospects of benefits. Through cognitive appraisal, individuals evaluate whether an environmental encounter is related to their happiness. Coping has two functions: regulating stress emotions (emotion-based coping) and changing troubled environmental relationships that cause distress (problem-based coping).
The revised theory of the stress process adopted by Folkman (2008) introduced meaning-based and positive emotional coping.28 This model assumes that if solutions fail, meaning-based coping is triggered. It can help individuals generate positive emotions and stimulate re-appraisal. These emotions and evaluations can influence the stress process by collecting coping resources and providing the necessary motivation, sustaining problem-based coping in the long term. Collecting coping resources is the process of receiving social support during stress coping.
The SPM provides a basis for the development of targeted interventions to mitigate family caregiver stress in dementia care and enhance the positive aspects of caregiving by identifying key stressors and coping resources. The theoretical framework is illustrated in Figure 1. The present study protocol outlines (a) the design and methodology of a novel intervention called the SPM-based Program in Dementia Caregiving (DeCare-SPM), which integrates the principles of the SPM, and (b) the evaluation of outcome, process, and cost-effectiveness of the DeCare-SPM.
Materials and Methods
Study Design
The study protocol is for a pragmatic, randomized controlled trial of family caregivers of community-dwelling and/or home-based IWD in Chongqing, China. In addition to the outcome measures, a process evaluation with a mixed-methods design and economic evaluation will be conducted. The intervention group will undergo the DeCare-SPM and the control group will receive the usual care. The study protocol is registered with the Chinese Clinical Trial Registry (registration number: ChiCTR2300072697). The study protocol follows the SPIRIT checklists.29 The design is illustrated in Figure 2.
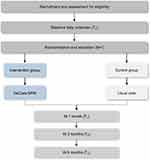 |
Figure 2 Flow Diagram protocol of study. |
Sample Selection
Participants will be recruited via e-mail, WeChat, or telephone by the primary researchers with the assistance of healthcare providers for dementia, or in person at memory clinics or communities. At the memory clinic, two independent investigators will review the patients weekly to identify eligible IWD and contact their family caregivers. Those who meet the inclusion criteria will be requested to provide informed consent. In addition, the recruitment profiles and poster links will be sent to potential participants through their healthcare providers such as family doctors and staff in community service centers via WeChat or e-mails. Family caregivers of IWD who are willing to participate can contact the investigators who will explain the study and evaluate their eligibility via telephone or face-to-face interviews. The inclusion criteria are: (a) care recipients are diagnosed with dementia by medical institutions, including Alzheimer’s disease and vascular dementia; (b) primary family caregivers aged 18 years or above and spend an average of at least four hours per day in caregiving; (c) community-based or home-based care provided for at least three months and no plans to move to long-term care facilities within six months; and (d) volunteered to participate in the DeCare-SPM. The exclusion criteria are: (a) caregivers with mental illness, (b) involvement in other trials, and (c) inability to attend the training because of physical distance.
Sample Size
The sample size was computed by calculating the difference between two independent means (two groups) using G* Power 3.1. A sample size of 31 in each group was calculated, considering an effect size of 0.8, an alpha error of 0.05, a power of 0.80, a 1:1 allocation, and an attrition rate of 20%. Participants will be randomized 1:1 to the control, or the DeCare-SPM. We aim to recruit 80 family caregivers.
Randomization and Allocation Concealment
First, all eligible participants will be classified as spouse or adult-child caregivers. They will be caring for IWD, which is categorized into mild, moderate, or severe stages. Family caregivers enrolled in the study will be randomly allocated in a 1:1 ratio to either the control group or the DeCare-SPM. Randomization ensures an equivalent distribution among the groups. An independent statistician will assign participants to groups using a computer-generated random sequence, and the process will be blinded to other investigators. The statistician will be blinded to the participants and will not participate in statistical analysis. Owing to the nature of the intervention study, we are unable to blind the participants.
Intervention
The DeCare-SPM was developed based on needs analysis (interviews with family caregivers), practice analysis (interviews with the interdisciplinary dementia care team), and a systematic review of positive psychology interventions. In addition, iSupport for dementia and a training and support manual for caregivers of IWD were important inputs for developing the intervention.30 The underlying theory of intervention was the revised SPM that explained the caregiving process of family caregivers of IWD. The DeCare-SPM comprises four sessions. The details of the intervention components are presented in Table 1.
 |
Table 1 Intervention Components |
Session 1: Problem-based coping (being a caregiver). Daily care and behavioral symptom management are the main stressors for family caregivers and are the areas where they need the maximum support. The goal of this session is to improve knowledge and skills of dementia-related issues and foster a sense of competence. The content will employ teaching approaches, such as lectures, case teaching, and video learning, about the introduction of dementia, daily care, and strategies for dealing with behavioral and psychological symptoms. In addition, caregivers will be encouraged to share their beneficial experiences in the process of caring for IWD.
Session 2: Emotion-based coping (caring for me). In the transition to the role of caregivers of IWD, family caregivers face primary stressors that can cause role strain, stress, and burden, which are regarded as secondary stressors affecting their mental health. Therefore, stress management is crucial to assist family caregivers. The goal of this session is to reduce negative feelings. The content will employ lectures and skills training on stress management techniques, such as relaxation, mindfulness exercises, sleep, and pleasant events. Home practice, including stress techniques three to four times per week, is recommended.
Session 3: Meaning-based coping (finding the meaning of caregiving). A sense of meaning is an individual’s understanding, pursuit, and realization of the purpose and goal of their life, and consequently, a sense of achievement. Coping styles related to maintaining a sense of meaning in life are of particular value in family caregivers’ caregiving process. This session aims to enhance the positive feelings. Self-health management, source of meaning, and the power of role models, positive mind development, and goal setting will be discussed with family caregivers. They will be then taught to reset goals for their life and dementia care.
Session 4: Seeking social support (supporting for me). Social support refers to the instrumental and emotional support provided to an individual by family, friends, and others. This session aims to promote social support and maintain intervention efficacy. Caregiver support groups will be established to expand the support networks of family caregivers and provide support. Telephone-based support in this session is designed to guide family caregivers to seek social support and solve individual care-related issues. Additionally, boosters are included to reinforce knowledge and skills acquired by family caregivers.
The first three sessions will be conducted in three consecutive half-days through group-delivered face-to-face conferences. Each session will be delivered over a half-day period, last for 90–120 minutes, and will include 20–30 caregivers in each group. Session 4 will provide a weekly one-on-one telephone-based consultation for four weeks, then, boosters will be performed by group-delivered, 30–40 minutes, face-to-face conferences once a month until the end of the follow-up time. Trained clinical professionals and psychologists will administer all interventions. Table 2 presents the study timeline.
 |
Table 2 Study Timelines |
 |
Table 3 Data Sources and Data Collection Approach |
The participants allocated to the control group will not partake in the DeCare-SPM during the intervention phase. They will continue with the usual care services available during this period without any restrictions, such as medication prescriptions and other support from medical staff. To our knowledge, few family caregivers in the community have access to psychosocial support from healthcare providers within the region of the study. Hence, we anticipate that this factor will not significantly impact the study outcome. In instances where a caregiver from the control group seeks assistance from the researcher, the researcher will provide the requisite knowledge to cater to the caregiver’s needs.
Outcomes Measures
Demographic characteristics of family caregivers and care recipients will be collected at baseline, including sociodemographics (eg, age, sex, and education), clinical characteristics (eg, stage and type of dementia), time spent on caregiving, and relationships with care recipients. The primary and secondary outcomes are described below, and the assessment schedules are listed in Table 2. Family caregivers will complete questionnaires at baseline (T0), one month (T1), three months (T2), and six months (T3). The questionnaires chosen in the protocol have demonstrated good reliability and validity and have been used locally.
The primary outcome is the positive aspect of caregiving evaluated using the Chinese version 9-item Positive Aspects of Caregiving Scale (PACS-9).31 The PACS contains two dimensions, self-affirmation (six items) and life outlook (three items), rated on a 5-point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree); higher scores indicate a higher PAC.
Secondary outcomes are related to the health of family caregivers (ie, sense of competence, caregiver burden, social support, anxiety and depression, and quality of life), outcomes for IWD (i.e, care dependency, neuropsychiatric symptoms [NPS], and quality of life), and stress-related biomarkers (ie, cortisol, interleukin [IL]-1β, IL-6, and IL-10 of saliva).
The sense of competence will be evaluated using the Short Sense of Competence Questionnaire (SSCQ), a 7-item version of the 27-item Sense of Competence Questionnaire (SCQ) that assesses feelings of being capable of caring for IWD.32 It has been used in the Chinese family caregivers of IWD with good reliability.33 A 5-point Likert scoring method is adopted ranging from 1 (strongly agree) to 5 (strongly disagree); higher scores indicate a higher sense of competence. Caregiver burden will be measured using the 6-item Zarit Burden Interview (ZBI-6) developed by Higginson et al, which has been used in our previous study.34,35 It uses a 5-point Likert scale from 0 (never) to 4 (always); higher scores indicate a heavier caregiver burden. Social support is assessed using the 6-item Interpersonal Support Evaluation List (ISEL-6), a short version of the original 40-item ISEL.36 The scale contains three dimensions (appraisal, belonging, and tangible support) rated from 1 (definitely false) to 4 (definitely true), with a higher score indicating a higher perception of social support. Anxiety and depression will be evaluated using the Hospital Anxiety and Depression Scale (HADS) validated among Chinese family caregivers, which consists of anxiety (seven items) and depression (seven items).37 The scores range from 0 (not a problem) to 3 (a high level of problem), with each subscale score ranging from 0 to 21. A subscale score of 7 indicates an increased risk of anxiety or depressive disorder, and a total score >14 indicates psychological distress. Quality of life for family caregivers will be measured using the Chinese version European Quality of Life-5 Dimensions (EQ-5D), a widely used instrument for evaluating an individual’s health-related quality of life.38 Responses are classified as “no problem”, “moderate problem”, and “extreme problem”. Additionally, the instrument includes a Visual Analog Scale (VAS) to report the overall health status from 0 (worst possible health status) to 100 (best possible health status).
Care dependency for IWD will be evaluated using Katz’s index of independence in activities of daily living (ADL), a three-level rating (dependent/partially independent/independent) scale comprising six functions.39 It is categorized as mild (dependence for 1–2 functions), moderate (dependence for 3–4 functions), and severe disability (dependence for 5–6 functions). The Neuropsychiatric Inventory (NPI-Q)-Chinese version will be used to assess the severity of NPS.40 Items are rated on a 4-point scale (0–4) and a 3-point severity scale (0–3). The total score for each symptom is computed by multiplying the frequency and severity scores, ranging from 0 to 12 points. The NPI scores range from 0 to 144 points, with a higher score indicating a more severe NPS. Quality of life for IWD will be measured using the Chinese version Quality of Life-Alzheimer’s Disease (AD-QOL) proxy version with 13 items.41 Responses are scored on a 4-point Likert scale ranging from 1 (poor) to 4 (excellent), with higher scores indicating better QOL.
Stress-related biomarkers are cortisol, IL-1β, IL-6, and IL-10, which are measured in saliva using enzyme-linked immunosorbent assay (ELISA). We will collect 1–2 mL of unstimulated saliva, participants will lean forward above a sterile bottle placed on ice to allow saliva to flow naturally, then transfer the saliva to a frozen tube, place it in an icebox, and store at −80 C for cryopreservation. Saliva will be collected at T0, T1, T2, and T3.
Economic Evaluation
The EQ-5D is used for family caregivers and the AD-QOL for IWD, both collecting data on quality-adjusted life years (QALYs). We will collect both the direct and indirect costs of the intervention program. Direct costs are for the research and development, implementation, and maintenance of the intervention, data for which will be gathered from financial records and reports from the project team. Indirect costs refer to the time cost of caregivers, which will be collected by surveying the total hours spent providing care for IWD in the past week.
Process Evaluation
For the generalizability of the results and to support future implementation of the intervention, a comprehensive assessment of the process measures is indispensable.42 Implementation and process evaluation will be performed based on The Medical Research Council (MRC) process evaluation framework42 and the Reach, Effectiveness, Adoption, Implementation, Maintenance (RE-AIM) framework,43 as presented in Figure 3. The MRC framework helps us understand how and why interventions work and the context in which they are the most effective. In addition, it helps explore the unexpected pathways and consequences that have not been previously considered. The RE-AIM framework helps us structure the different implementation factors, namely reach, adoption, implementation, and maintenance. Effectiveness was not part of the process evaluation because it is evaluated in the outcome measures section. Table 3 presents the data sources and collection approaches for process evaluation. Contextual factors will be assessed from baseline information, including individual characteristics, interpersonal relationships, community services, and the healthcare system. Reach refers to the recruitment, retention, and representativeness of individuals willing to participate in the interventions, which will be generated from screening and follow-up records and baseline and follow-up surveys. Adoption will be assessed by the acceptability and perception of intervention components immediately after the implementation of interventions and at six months. The Client Satisfaction Questionnaire (CSQ‐8) will be used to evaluate the satisfaction with the program.44 Qualitatively, focus group interviews with family caregivers will be conducted to explore their experiences and perceptions of the interventions. Each focus group will consist of 6–8 participants to ensure diverse perspectives while fostering an environment conducive to open dialogue. The sessions will be facilitated by trained moderators using a semi-structured interview guide, which will be pilot-tested for clarity and relevance. Implementation measures the extent, consistency, and quality of the delivered intervention and the engagement of the participants. Data will be collected by monitoring the implementation checklist and a self-designed engagement questionnaire comprising attention, engagement, interest, interaction, and performance. Maintenance refers to the extent to which intervention components become part of caregiving and is evaluated by questions on how frequently family caregivers use the intervention components they have been trained to care for IWD. Additionally, we will identify facilitators and barriers that may influence each RE-AIM dimension through focus group interviews with participants and semi-structured interviews with project staff at the end of follow-up.
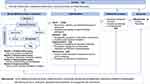 |
Figure 3 Process evaluation framework. Abbreviations: DeCare-SPM, Stress Process Model-based Program in Dementia Caregiving; PAC, Positive Aspects of Caregiving. |
Data Analysis
Descriptive statistics such as means, standard deviations (SD), and frequencies will be calculated. Differences in sociodemographic between the control and intervention groups at baseline will be evaluated using t-tests or chi-squared tests for continuous and categorical variables, respectively. Mixed-effects models will be used to analyze temporal changes (T0 to T3) between experimental conditions and to account for within-subject correlations. If time-by-group interaction effects are significant, post hoc pairwise comparisons will be conducted to identify specific differences between the groups at each time point. Moreover, we will calculate effect sizes (ie, Cohen’s d) to evaluate the magnitude and clinical significance of the intervention effects. The intention-to-treat principle will be applied.
Cost-effectiveness analyses (CEA) and cost-utility analyses (CUA) will be performed. First, we will estimate the incremental cost-effectiveness ratio (ICER) by dividing the difference in total costs of the intervention program by the difference in incremental effects. The total benefit is determined by converting improvements in quality of life to QALYs and then monetizing it using a certain exchange rate (ie, per capita gross domestic product [GDP]). Non-parametric bootstrap resampling will be employed to address the uncertainty of the ICER estimation and a sensitivity analysis will be conducted on the costs and benefits to understand the impact of uncertainties on the results.
Qualitative data generated during the process evaluation will be analyzed using content analysis.45 It involves transcribing focus group data, generating initial codes, and refining them through consensus. The codes are grouped into themes, which are then reviewed and refined. Transcription of records and text encoding of the qualitative research data will be performed using NVivo 12.0.
Ethical Considerations
The study protocol was approved by the Ethics Committee of the first affiliated hospital of Chongqing Medical University (Approval No. 2022-016). This trial will be conducted in accordance with the Declaration of Helsinki. The DeCare-SPM is voluntary and unpaid, and the caregivers will be informed of their right to participate or withdraw freely from the study at any time. The withdrawal reasons will be recorded. The principal investigator will be responsible for obtaining written informed consent from the participants. They will undergo training to ensure that they provide comprehensive information about the study and address any questions or concerns the participants may have. Each participant will be assigned a unique code to protect their privacy, and only the investigators will have access to the code and the participants’ identities.
Study Monitoring and Auditing
Two trained investigators will participate in the on-site face-to-face conferences throughout, and record the process evaluation. Reflective records concerning the protocol implementation after each conference will be discussed. In addition, we have developed training manuals and evaluation methods for facilitators and research assistants to standardize interventions and data collection. Quantitative data will be double-entered for validation and analyzed based on an intentionality analysis framework. For the qualitative data, two authors will review the transcribed content and perform the coding independently to ensure accuracy; a meeting will be held to reach a consensus. When amendments to the protocol are necessitated due to unforeseeable factors such as manpower, financial resources, or time constraints, we will organize meetings to discuss the potential impacts on the outcomes. Concurrently, we will seek approval from the Ethics Committee for the proposed amendments.
Discussion
We developed the DeCare-SPM, based on the SPM, that aims to enhance the positive aspects of caregiving among family caregivers of IWD. The program comprises four sessions (problem-based coping, emotion-based coping, meaning-based coping, and social support). This protocol will comprehensively evaluate its effectiveness, cost, and process. The program design acknowledges that while caregiving can be stressful, it brings positive experiences such as personal growth and an increased sense of purpose.12 These findings can guide the development of future interventions, highlighting the need to balance the challenges and rewards of dementia care. The DeCare-SPM is expected to provide evidence-based insights on how to support and empower family caregivers in their important roles, ultimately leading to improved dementia care.
Support for dementia care has been a research priority for reducing the global burden of dementia. In China, there is an urgent need to develop and implement feasible and cost-effective programs to satisfy the needs of family caregivers due to the large population of IWD. However, there is a lack of structured and comprehensive dementia care systems and limited dementia care facilities.46 Therefore, the primary responsibility of dementia care often falls on the family, deeply ingrained in the Confucian principle of “filial piety”. Family caregivers face numerous challenges, including a lack of knowledge about dementia, limited access to professional help, societal stigma associated with mental health conditions, and the physical and emotional toll of caregiving,2 causing isolation, stress, and depression, further exacerbating the situation and leading to poor care outcomes for IWD. To mitigate these challenges and improve family caregivers’ well-being, it is crucial to establish a comprehensive care support program.
We conducted a needs analysis, practice analysis, and synthesis of empirical evidence to develop the DeCare-SPM, which contributes to the clinical applicability of the program to better satisfy the needs of family caregivers of IWD. Compared with studies that focused on meaning-centered psychotherapy,47,48 this program not only offers problem-solving for the challenging aspects of dementia care but also provides stress regulation strategies, which are probably more acceptable to caregivers as they primarily require knowledge and care skills. The findings of the need and practice analysis were consistent with the principles of DeCare-SPM development and in accordance with a previous study.49 Thus, this multidimensional psychosocial program, which includes problem-based coping, emotion-based coping, meaning-based coping, and social support, caters to caregivers’ holistic needs and enhances their capabilities and well-being. In addition, intervention components are theory-based, which expands our understanding of how interventions based on the SPM can promote positive aspects of caregiving, potentially improving the well-being of family caregivers.
We aim to conduct a randomized controlled trial that compares the DeCare-SPM with usual care, expecting a clinically significant increase in the positive aspects of caregiving. Multiple health-related outcomes will be evaluated and objective biomarkers related to stress will be examined to compensate for the limitations of subjectively reported outcome measures. Due to the accessibility and convenience of the intervention for family caregivers and limited resources for dementia care support, group-based interventions have been proposed to attain better effectiveness and utility. Thus, an economic evaluation will be performed to explore the most cost-efficiency. To the best of our knowledge, cost evaluations of psychosocial interventions have rarely been studied. Because of the rapidly increasing population of IWD, cost-efficient psychosocial care is important from an economic point of view to ensure the feasibility of implementation in community- and home-based dementia care.
The process evaluation of the DeCare-SPM in our study protocol is a critical component that provides insight into the program’s implementation and factors that influence its effectiveness.42 By examining the program’s implementation process, we expect to understand not only whether the program works but also how and why it works. Process evaluation is complex requiring various methods. Considering the limited time, resources, and focus of the program, we focused on implementation, including recruitment, reach, fidelity, dose, and satisfaction, based on the MRC and RE-AIM frameworks.42,43 Furthermore, process evaluation captures the variability in program implementation across different settings and populations. This will provide valuable information on the program’s generalizability and its potential for wider dissemination and implementation in various contexts.42 Upon completing our study, we are poised to adopt an integrated strategy for the dissemination and amplification of our findings. Our primary outcomes will be submitted to esteemed peer-reviewed journals, ensuring that our insights are accessible to the academic sphere at large. In tandem with this effort, an exhaustive implementation manual will be formulated to offer guidance to practitioners and researchers interested in replicating or adapting the scope of our intervention. Moreover, to expedite the wide-scale uptake and comprehension of the DeCare-SPM, we will envisage organizing dedicated training modules and workshops. Such endeavors are not merely to disseminate our findings but also to pave the way for the program’s extensive application and resonance across diverse caregiving settings.
Strengths and Limitations of the Study
Although the effectiveness of psychosocial interventions on the health-related outcomes of family caregivers of IWD has been explored, there is a paucity of studies examining the effects of a complex intervention on the positive aspects of caregiving. In particular, few studies are on the basis of a robust theoretical model of psychology and an interdisciplinary approach to dementia research.50 This study protocol focuses on the positive aspects of caregiving, a relatively underexplored area in dementia care research. Additionally, the SPM provides a comprehensive framework for understanding the complex dynamics of caregiving experiences. The rigorous study design, including process evaluation, strengthens its methodological robustness. This allows for an in-depth understanding of not only the program’s outcomes but also the processes and factors influencing its effectiveness.
The protocol has several limitations. First, the self-report bias of outcomes may influence the findings; for instance, family caregivers may feel compelled to emphasize the positive aspects of their experiences. The measurement of caregiver burden, quality of life, and stress-related biomarkers in this protocol can provide a more balanced perspective. In addition, the inability to mask participants owing to the nature of the intervention can cause an unavoidable study bias. Furthermore, potential cultural and individual differences in caregiving experiences may limit the generalizability of the findings. Caregivers from different cultural backgrounds or those with different relationships with IWD may perceive and respond to caregiving stressors differently. Finally, while process evaluation provides valuable insights into the implementation process, it may not capture all the nuances of real-world implementation, limiting the comprehensive understanding of the program’s effectiveness.
Conclusion
This study protocol, based on the SPM, was developed as a novel approach to enhance the positive aspects of caregiving for family caregivers of IWD. The protocol outlines a comprehensive evaluation of the program’s effectiveness on the positive aspects of caregiving and its positive impacts on the mental health and well-being of family caregivers and IWD, incorporating a process evaluation to assess contextual and implementation factors and mechanisms of the impact. This study protocol underscores the need for healthcare systems to recognize and bolster the positive aspects of caregiving while managing the associated stress. Furthermore, the outcomes of this protocol will provide a reference for the design of future interventions from interdisciplinary insights aimed at promoting caregiver well-being, thus, influencing dementia care policies.
Acknowledgments
We would like to thank all study participants for their considerable support and assistance.
Funding
The study protocol was supported by the National Social Science Foundation of China (Grant: 21BRK013).
Disclosure
The authors report no competing interests in this work.
References
1. World Health Organization (WHO). Dementia. 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia.
2. Larson EB, Stroud C. Meeting the challenge of caring for persons living with dementia and their care partners and caregivers: a report from the National Academies of Sciences, Engineering, and Medicine. JAMA. 2021;325(18):1831–1832. doi:10.1001/jama.2021.4928
3. The Lancet Neurology. Dementia care: beyond a diagnosis. Lancet Neurol. 2022;21(11):947. doi:10.1016/S1474-4422(22)00402-1
4. Seidel D, Thyrian JR. Burden of caring for people with dementia - comparing family caregivers and professional caregivers. A descriptive study. J Multidiscip Healthc. 2019;12:655–663. doi:10.2147/JMDH.S209106
5. Yiu HC, Zang Y, Chew JHS, Chau JPC. The influence of Confucianism on the perceptions and process of caring among family caregivers of persons with dementia: a qualitative study. J Transcult Nurs. 2021;32(2):153–160. doi:10.1177/1043659620905891
6. Yiu HC, Zang Y, Chau JPC. Barriers and facilitators in the use of formal dementia care for dementia sufferers: a qualitative study with Chinese family caregivers in Hong Kong. J Geriatr Nurs. 2020;41(6):885–890. doi:10.1016/j.gerinurse.2020.06.018
7. Liu Z, Sun W, Chen H, et al. Caregiver burden and its associated factors among family caregivers of persons with dementia in Shanghai, China: a cross-sectional study. BMJ open. 2022;12(5):e057817. doi:10.1136/bmjopen-2021-057817
8. van den Kieboom R, Snaphaan L, Mark R, Bongers I. The trajectory of caregiver burden and risk factors in dementia progression: a systematic review. J Alzheimers Dis. 2020;77(3):1107–1115. doi:10.3233/JAD-200647
9. Sun Y, Ji M, Leng M, Li X, Zhang X, Wang Z. Comparative efficacy of 11 non-pharmacological interventions on depression, anxiety, quality of life, and caregiver burden for informal caregivers of people with dementia: a systematic review and network meta-analysis. Int J Nurs Stud. 2022;129:104204. doi:10.1016/j.ijnurstu.2022.104204
10. Aravena JM, Gajardo J, Saguez R, Hinton L, Gitlin LN. Nonpharmacologic interventions for family caregivers of people living with dementia in Latin-America: a scoping review. Am J Geriatr Psychiatry. 2022;30(8):859–877. doi:10.1016/j.jagp.2021.10.013
11. Dickinson C, Dow J, Gibson G, Hayes L, Robalino S, Robinson L. Psychosocial intervention for carers of people with dementia: what components are most effective and when? A systematic review of systematic reviews. Int Psychogeriatr. 2017;29(1):31–43. doi:10.1017/S1041610216001447
12. Yu DSF, Cheng ST, Wang J. Unravelling positive aspects of caregiving in dementia: an integrative review of research literature. Int J Nurs Stud. 2018;79:1–26. doi:10.1016/j.ijnurstu.2017.10.008
13. Cheng ST, Mak EPM, Kwok T, Fung H, Lam LCW. Benefit-finding intervention delivered individually to Alzheimer family caregivers: longer-term outcomes of a randomized double-blind controlled trial. J Gerontol B Psychol Sci Soc Sci. 2020;75(9):1884–1893. doi:10.1093/geronb/gbz118
14. Wang J, Li X, Liu W, et al. The positive aspects of caregiving in dementia: a scoping review and bibliometric analysis. Front Public Health. 2022;10:985391. doi:10.3389/fpubh.2022.985391
15. Moe A, Alnes RE, Nordtug B, Blindheim K, Steinsheim G, Malmedal W. Coping with everyday life for home-dwelling persons with dementia: a qualitative study. J Multidiscip Healthc. 2021;14:909–918. doi:10.2147/JMDH.S300676
16. Yuan Q, Zhang Y, Samari E, et al. Positive aspects of caregiving among informal caregivers of persons with dementia in the Asian context: a qualitative study. BMC Geriatr. 2023;23(1):51. doi:10.1186/s12877-023-03767-8
17. Quinn C, Toms G. Influence of positive aspects of dementia caregiving on caregivers’ well-being: a systematic review. Gerontologist. 2019;59(5):e584–e596. doi:10.1093/geront/gny168
18. Cheng ST, Lau RWL, Mak EPM, et al. A benefit-finding intervention for family caregivers of persons with Alzheimer disease: study protocol of a randomized controlled trial. Trials. 2012;13(1):10. doi:10.1186/1745-6215-13-98
19. Cheng ST, Fung HH, Chan WC, Lam LC. Short-term effects of a gain-focused reappraisal intervention for dementia caregivers: a double-blind cluster-randomized controlled trial. Am J Geriatr Psychiatry. 2016;24(9):740–750. doi:10.1016/j.jagp.2016.04.012
20. Cheng ST, Chan WC, Lam LCW. Long-term outcomes of the benefit-finding group intervention for Alzheimer family caregivers: a cluster-randomized double-blind controlled trial. Am J Geriatr Psychiatry. 2019;27(9):984–994. doi:10.1016/j.jagp.2019.03.013
21. Wang S, Cheung DSK, Leung AYM, Davidson PM. Bibliotherapy for improving caregiving appraisal of informal caregivers of people with dementia: a pilot randomized controlled trial. Res Nurs Health. 2021;44(4):692–703. doi:10.1002/nur.22143
22. Teles S, Ferreira A, Paúl C. Feasibility of an online training and support program for dementia carers: results from a mixed-methods pilot randomized controlled trial. BMC Geriatr. 2022;22(1):1–17. doi:10.1186/s12877-022-02831-z
23. DeGregory C. The Effects of Multiple Gratitude Interventions Among Informal Caregivers of Persons with Dementia and Alzheimer’s Disease. University of South Carolina; 2014.
24. Fuju T, Yamagami T, Yamaguchi H, Yamazaki T. A randomized controlled trial of the “positive diary” intervention for family caregivers of people with dementia. Perspect Psychiatr Care. 2021;58(4):1949–1958. doi:10.1111/ppc.13013
25. Moskowitz JT, Cheung EO, Snowberg KE, et al. Randomized controlled trial of a facilitated online positive emotion regulation intervention for dementia caregivers. Health Psychol. 2019;38(5):391–402. doi:10.1037/hea0000680
26. Han A. Interventions for attitudes and empathy toward people with dementia and positive aspects of caregiving: a systematic review and meta-analysis. Res Aging. 2020;42(2):72–82. doi:10.1177/0164027519884766
27. Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: an overview of concepts and their measures. Gerontologist. 1990;30(5):583–594. doi:10.1093/geront/30.5.583
28. Folkman S. The case for positive emotions in the stress process. Anxiety Stress Coping. 2008;21(1):3–14. doi:10.1080/10615800701740457
29. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–207. doi:10.7326/0003-4819-158-3-201302050-00583
30. Pot AM, Gallagher-Thompson D, Xiao LD, et al. iSupport: a WHO global online intervention for informal caregivers of people with dementia. World Psychiatry. 2019;18(3):365–366. doi:10.1002/wps.20684
31. Zhang R, Li Z. Reliability and validity of Chinese version of positive aspects of caregiving. Chin J Nurs. 2007; 12:1068–1071. Chinese.
32. Vernooij-Dassen MJ, Felling AJ, Brummelkamp E, Dauzenberg MG, van den Bos GA, Grol R. Assessment of caregiver’s competence in dealing with the burden of caregiving for a dementia patient: a Short Sense of Competence Questionnaire (SSCQ) suitable for clinical practice. J Am Geriatr Soc. 1999;47(2):256–257. doi:10.1111/j.1532-5415.1999.tb04588.x
33. Lian Y. Study on timely diagnosis of dementia and support for caregivers of people with dementia in community care settings [doctoral dissertation], Army Medical University; 2020. Chinese.
34. Higginson IJ, Gao W, Jackson D, Murray J, Harding R. Short-form Zarit caregiver burden interviews were valid in advanced conditions. J Clin Epidemiol. 2010;63(5):535–542. doi:10.1016/j.jclinepi.2009.06.014
35. Wang J, Liu W, Yu S, et al. Social networks effects on spouse and adult-child dementia caregivers’ experiences: a cross-sectional study. J Am Med Dir Assoc. 2023;24(9):1374–1380.e1371. doi:10.1016/j.jamda.2023.04.006
36. Cohen S, Mermelstein R, Kamarck T, Hoberman H. Measuring the functional components of social support. In: Sarason IG, editor. Social Support: Theory, Research, and Application. The Hague, Holland: Martinus Nijhoff; 1985:73–94.
37. Li Q, Lin Y, Hu C, et al. The Chinese version of hospital anxiety and depression scale: psychometric properties in Chinese cancer patients and their family caregivers. Eur J Oncol Nurs. 2016;25:16–23. doi:10.1016/j.ejon.2016.09.004
38. Yang Z, Busschbach J, Liu G, Luo N. EQ-5D-5L norms for the urban Chinese population in China. Health Qual Life Outcomes. 2018;16(1):210. doi:10.1186/s12955-018-1036-2
39. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185(12):914–919. doi:10.1001/jama.1963.03060120024016
40. Leung VP, Lam LC, Chiu HF, Cummings JL, Chen QL. Validation study of the Chinese version of the neuropsychiatric inventory (CNPI). Int J Geriatr Psychiatry. 2001;16(8):789–793. doi:10.1002/gps.427
41. Wan LP, He RL, Ai YM, et al. Item function analysis on the Quality of Life-Alzheimer’s Disease (QOL-AD) Chinese version, based on the Item Response Theory (IRT). Chin J Epidemiol. 2013;34(7):728–731. Chinese.
42. Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions: medical research council guidance. BMJ. 2015;350:h1258. doi:10.1136/bmj.h1258
43. Oosterveld-Vlug M, Onwuteaka-Philipsen B, Ten Koppel M, et al. Evaluating the implementation of the PACE steps to success programme in long-term care facilities in seven countries according to the RE-AIM framework. Implement Sci. 2019;14(1):107. doi:10.1186/s13012-019-0953-8
44. Liu H, Peng H, Song X, Xu C, Zhang M. Using AI chatbots to provide self-help depression interventions for university students: a randomized trial of effectiveness. Internet Interv. 2022;27:100495. doi:10.1016/j.invent.2022.100495
45. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288. doi:10.1177/1049732305276687
46. Jia L, Quan M, Fu Y, et al. Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol. 2020;19(1):81–92. doi:10.1016/S1474-4422(19)30290-X
47. van der Spek N, Vos J, van Uden-Kraan CF, et al. Efficacy of meaning-centered group psychotherapy for cancer survivors: a randomized controlled trial. Psychol Med. 2017;47(11):1990–2001. doi:10.1017/S0033291717000447
48. Winger JG, Ramos K, Kelleher SA, et al. Meaning-centered pain coping skills training: a pilot feasibility trial of a psychosocial pain management intervention for patients with advanced cancer. J Palliat Med. 2022;25(1):60–69. doi:10.1089/jpm.2021.0081
49. Cheng ST. The principles and techniques of benefit-finding for dementia caregivers: reply to Gersdorf. Am J Geriatr Psychiatry. 2018;26(3):405–406. doi:10.1016/j.jagp.2017.11.003
50. Ibsen TL, Eriksen S. Interdisciplinary research: an important contribution to dementia care. J Multidiscip Healthc. 2022;15:317–321. doi:10.2147/JMDH.S350132
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.