Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
Effectiveness of Secretome from Human Umbilical Cord Mesenchymal Stem Cells in Gel (10% SM-hUCMSC Gel) for Chronic Wounds (Diabetic and Trophic Ulcer) – Phase 2 Clinical Trial
Authors Tan ST , Aisyah PB, Firmansyah Y, Nathasia N , Budi E, Hendrawan S
Received 27 February 2023
Accepted for publication 30 May 2023
Published 23 June 2023 Volume 2023:16 Pages 1763—1777
DOI https://doi.org/10.2147/JMDH.S408162
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Sukmawati Tansil Tan,1 Putri Bennya Aisyah,2 Yohanes Firmansyah,2 Nathasia Nathasia,2 Erwin Budi,2 Siufui Hendrawan3,4
1Department of Dermatology and Venereology, Faculty of Medicine, Tarumanagara University, Jakarta, Indonesia; 2Faculty of Medicine, Tarumanagara University, Jakarta, Indonesia; 3Department of Biochemistry and Molecular Biology, Faculty of Medicine, Tarumanagara University, Jakarta, Indonesia; 4Tarumanagara Human Cell Technology Laboratory, Tarumanagara University, Jakarta, Indonesia
Correspondence: Siufui Hendrawan, Email [email protected]
Background: Chronic wounds carry financial burdens and increase morbidity and mortality, especially in diabetic ulcers and Hansen’s Morbus. More than 50% of chronic ulcers are difficult to heal with regular treatment and require new types of therapy such as the use of secretome of human umbilical cord mesenchymal stem cells (SM-hUCMSC).
Methods: This experimental study was carried out to see the effectiveness of using SM-hUCMSC in diabetic ulcers and Hansen’s Morbus in four medical facilities (multicentre). The level of active secretion has been measured by default in 10% SM-hUCMSC gel, used as a treatment intervention. The primary outcome is wound healing in terms of the length, width, and extent of the wound. The secondary is the side effects of treatment 2 weeks after administration. Follow-up visits will be scheduled at 1 and 2 weeks post-treatment.
Results: Forty-one chronic ulcers successfully followed the study until the end. In patients with chronic ulcers, the mean ulcer length, width, and area were 1.60 (0,50– 13,0), 1.3 (0,5– 6,0), and 2.21 (0,25– 78) cm square, respectively, before interventions and 1 (0– 12), 0,8 (0– 6,0), and 1 (0– 72) square cm after interventions at the second follow-up. The change between the beginning and end of the intervention was significant (p-value < 0.05).
Conclusion: The use of 10% SM-hUCMSC gel topically has been proven effective in accelerating the process of wound healing, especially chronic ulcers with side effects that are not present in this study.
Keywords: chronic wound healing, secretome of human umbilical cord-derived MSCs gel, diabetic chronic ulcers, leprosy
Two Letters to the Editor have been received and published for this article
Introduction
Chronic wound is defined as a wound that is difficult to heal according to the natural healing process with longer healing time to produce functional and anatomical integrity, usually more than 3 months, or a wound that has undergone a healing process but is imperfect in terms of anatomy and functional.1 The terminology of chronic wounds is often referred to as ulcer wounds that are difficult to heal and usually persist in 4 weeks or more.2 Chronic wounds are divided into four main groups based on the etiology of the cause, venous ulcers, diabetic ulcers, namely arterial insufficiency ulcers, and pressure ulcers.3 The prevalence of chronic wounds in developed countries is 1% to 2% of the total population.4 The prevalence of chronic wounds will often increase with increasing age and the presence of comorbidities that inhibit the wound healing process.5 The most severe complication of chronic ulcers is the occurrence of persistent infections, such as gangrene, infective venous eczema, cellulitis, bleeding, and amputation of the lower limb.6
A clinician has to work hard to take care of and handle patients with long-term injuries. Clinicians have to deal with the fact that there are not many wound care techniques that have been proven to work and that chronic ulcers do not heal very often. A special study from Germany says that each year, more than 4.5 million people are treated for long-term injuries that cost up to 5 billion Euros to care for.7 The extremely low rate of wound healing in chronic wounds6 has a substantial impact on the health and quality of life of patients and their family. Social isolation, shame, anxiety, difficulties, sadness, loss of function and movement, pain, the financial burden of hospitalization, complications of sickness, and even death are some of the consequences.8 Previous research has demonstrated that persistent ulcers are a considerable burden and frequently result in a very low quality of life for their victims. In order to improve the quality of life of persons with chronic ulcers, it is vital to provide wound care and ulcer management that are both efficient and cost-effective.9
The two most common types of chronic wounds encountered in developing areas are diabetic ulcers and trophic ulcers. The most common chronic ulcer is ulcers due to diabetes mellitus or often referred to as diabetic foot ulcers, with an annual rate of 2% to 6% and pre-incident from 2% to 10%.7 This generated a substantial $ 8659 per patient burden on the healthcare system.10 In the US, the total medical expense for treating diabetic foot ulcers is from $ 9 to $ 13 billion.11 The International Diabetes Foundation has raised public awareness of the risks and expenses associated with diabetic foot ulcers.12 The majority (85%) of diabetes patients who had limb amputations did so due to gangrene or severe diabetic foot ulcers.13
According to the World Health Organization (WHO), in 2016, there were a total of 214,783 new cases found in 143 countries. The World Health Organization declared 22 states as “global priority countries” for leprosy because they accounted for 94–96% of the disease burden and 92.3% of cases with level II disability.14–16 The Southeast Asia region has several new case discoveries and the number of cases of leprosy with the highest grade II disability compared to Africa, America, the Mediterranean, the western Pacific, and Europe. Some Southeast Asian countries that contributed the most were Indonesia, Myanmar, and the Philippines.14–16 Indonesia accounted for 16,826 of 214,783 new cases in the world detected in 2016, 1363 patients were accompanied by grade II disabilities, with 62 of them children, and 229 chances of relapse were found. Currently, Indonesia cannot be declared free from leprosy.17
Along with technological advancements and the fact that 50% of chronic wounds still do not heal,18 as well as the fact that current therapies for chronic wounds do not work in some circumstances and are expensive and time-consuming, novel wound care strategies are required. Following the trend of wound healing mechanisms, stem cell treatment is a novel alternative to traditional ways of wound healing. Researchers therefore rely on stem cell therapy, which has enormous promise due to its growth factors.19
Wound care using stem cells is a new treatment option with promising results.20 Stem cells have the potential to differentiate into many cell types.21 Stem cell therapy has shown great potential in the treatment of various conditions, such as orthopedic disorders, inflammatory diseases, liver failure, and autoimmune disorders.22 Until this report was made, no severe side effects have been found from the use of stem cell therapy. Therefore, stem cell treatment is currently considered safe.23 Until now, there has been an increase in the use of mesenchymal stem cells (MSC) in various types of disorders due to the multipotent characteristics of MSC. Several studies have shown that stem cells derived from bone marrow (BM-SC) can act as precursors that develop from the germinal lining pathway into various cell types such as adipose, chondrocytes, and solid osteocytes.24 Recent research also reveals MSC can also function as a therapeutic cell that modulates the microenvironment and immunological competence, accelerates wound repair, and reduces fibrosis or scar formation or both due to height.25 Stem cells contain Fibroblast Growth Factor (FGF) or Vascular Endothelial Growth Factor (VEGF) needed for wound healing.26 VEGF is an essential component for maintaining hypoxic tissue.18 A case report from Sarasua et al states that wound closure occurred after 18 days after BM-SC injection in pressure ulcers stage IV.27
The human umbilical cord’s secretome could be used to treat diseases in a number of medical fields, such as skin, surgery, and internal medicine. Dermatologists have looked at the secretome of the human umbilical cord to see if it can help the skin grow back and heal wounds. It has been shown to have growth factors and cytokines that can speed up the mending process and make skin cells grow more quickly. This makes it a hopeful way to treat long-lasting wounds, like diabetic foot ulcers, as well as conditions that stop the skin from healing itself, like aging and skin damage from the environment.28–30
In surgery, the secretome of the human umbilical cord has been looked at to see if it can help heal and grow back damaged tissue. It has been shown to have growth factors and cytokines that can help make new blood vessels and encourage the growth of cells that help mend tissue. Because of this, it could be a good way to treat diseases like burns and ischemic injuries that damage tissue.31–33
The secretome of the human umbilical cord has been studied in the field of internal medicine to see if it can help control the immune system and lower inflammation. It has been shown to have factors that can stop the production of cytokines that cause inflammation and boost the production of cytokines that fight inflammation. Inflammatory diseases like rheumatoid arthritis, inflammatory gut disease, and multiple sclerosis could be helped by this.34–36
Overall, the human umbilical cord’s secretome could be used to treat a wide range of diseases, and ongoing study in this area could lead to the development of new treatments for a number of different illnesses. The primary mechanism underlying the beneficial effects of secretomes on tissue regeneration is their ability to generate a variety of bioactive trophic factors that stimulate surrounding parenchymal cells to initiate tissue repair. These bioactive factors modulate the local immune system, promote angiogenesis, prevent cell apoptosis, and stimulate specific cell survival, proliferation, and differentiation. MSCs secrete trophic factors that promote cell regeneration and repair, such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and epidermal growth factor (EGF). When grafted to injured tissue sites, MSCs differentiate into connective tissue elements, promote vasculogenesis, and secrete healing-promoting cytokines and growth factors. Previous research has demonstrated that Wharton Jelly Mesenchymal Stem Cells (WJ-MSCs) serve as a transplantable source of juvenile, non-tumorigenic, and immunomodulatory cells that regenerate liver, heart, bone, cartilage, fat, pancreas, nerves, blood vessels, and skin components. WJ-MSCs release proangiogenic and wound healing factors, including TGF, VEGF, platelet-derived growth factor, insulin-like growth factor, and interleukin (IL)-6.37–41
This trial is a preliminary Phase II study in which we will evaluate the safety, feasibility, and potential efficacy of topical administration of secretome of human umbilical cord mesenchymal stem cells (SM-hUCMSC) as an alternative treatment for chronic ulcers caused by both diabetic ulcers and leprosy-related tropic ulcers. If the results of this study confirm the safety, feasibility, and potential beneficial effects of interventions related to clinical parameters, then mini RCTs and possibly large multicenter RCTs with longer follow-up will be initiated with the goal of accumulating more clinical evidence for the topical use of SM-hUCMSC in the treatment of chronic ulcers.
Materials and Methods
Research Design and Subject
This research is a phase II clinical trial using the method of “non-randomized controlled trial” and “open trial.” The minimum sample size used in this study was planned for 20 respondents (preliminary study). The inclusion criteria in this study were all subjects with chronic ulcers, patients aged 18–80 years, not recovering with routine therapy for at least 1 month, chronic ulcers degrees 2 and 3, are willing to take part in the study, as well as with the respondent’s good health to follow this study. In this study there were no minimum wound area criteria for participation in the study. Exclusion criteria in this study included anemia and pregnancy.
This research was conducted at Sukma Clinic, Indra Clinic, Mayapada Hospital, and Sitanala Hospital. Before the patient received the intervention, the patient was first screened through a thorough history, briefed on the knowledge about this study, physical examination, and laboratory examination to ensure patient safety and to find out any possible side effects during the completion of the intervention. Physical examination is done thoroughly, starting from the examination of vital signs and examination of the head to foot. Laboratory tests used in this study included an examination of fasting blood sugar levels.
Secretome Intervention and Preparation
The interventions given in this study were secretome of human umbilical cord mesenchymal stem cells (SM-hUCMSC). Mesenchymal Stem Cell that has been isolated from the umbilical cord was cultured until passage 6 (P6) in a T175 Flask (Corning). The culture medium was deprived and replaced with a basal medium with no supplement addition after 70–80% confluence reached then incubated at 37°C, 5% CO2 incubator with hypoxic (5% O2) conditions. After 72 hr of incubation, the conditioned medium was collected and stored at a deep freezer (−80°C) for long-term storage. SM-hUCMSC was modified to become 10% gel by Sukma. This SM-hUCMSC is given topically to the wound area at a sufficient dose according to the size of the wound. Giving SM-hUCMSC is offered twice a day for 2 weeks.
Due to their distinct MSC characteristics, we utilized passage six cells for CM production. At 70–80% confluence, the MSC cells were cultured without a growth supplement in MEM-Alpha. To allow the secretion of the secretome, the MSC cells were incubated for additional 72 hr. MSCs were exposed to hypoxic [5% O2] environments to determine the optimal conditions for secretome secretion. The CM was placed in a deep freezer to preserve its protein content for long-term storage. Pro-collagen 1 levels were 655,100.00 pg/mL, vascular endothelial growth factor (VEGF) levels were 21.42 pg/mL, and basic fibroblast growth factor (bFGF) levels were 34.64 pg/mL, as determined by ELISA.
Preparation of 10% gel was carried out by mixing 1 mL of WJMSC-CM (100%) containing VEGF, bFGF, ProCollagen, KGF, and several growth factors, paracrine and cytokines in 9 g of 80% Aloe vera gel, 3% Propolis, Vitamin 1% C, 3% olive oil, 5% Natrosol, and 0.5% Katon. Product stability tests have been carried out periodically to ensure the stability of the growth factor content. Sterility, bacteria, and endotoxin tests on gel preparations have also been carried out in accordance with the standards of Good Laboratory Practice (GLP).
Study Protocol and Outcome
The variables in this study were divided into two, namely, the independent variable was the use of 10% gel SM-hUCMSC were administered topically, and the dependent variables were wound healing and side effects caused by the interventions given. Wound healing or repair in this study was assessed from several variables, namely the presence of granulation tissue growth, reduced edema, reduced erythema, and improvement in wound size both in terms of length, width, and area of wound measured using a standard ruler and digital photo.
The study lasted for two weeks and one month, with the measurement of dependent variables carried out three times, namely before the intervention was carried out, two first weeks after the administration of the intervention (first follow-up), and 1 month after the intervention (second follow-up). The researcher also gave a contact number to the patient for a personal consultation if side effects occur during the intervention period.
Data will be analyzed based on the “intention to treat” method. Data on research variables will be presented with univariate and bivariate tables. Population characteristics will be analyzed using descriptive statistics, and changes in wound characteristics will be presented using analytic statistical tables. The statistical analysis used in this study is to use generalized linear methods if the data distribution is normal and the Wilcoxon Signed Ranks Test if the data distribution is not normal for numerical data.
Side effects of treatment at 2 weeks of 10% gel SM-hUCMSC administration, defined as1 local side effects, including signs of local inflammation (heat, rubor, dolor, tumor, and function Lesa), worsening of wounds, or appearance of new wounds or hematomas after 10% gel SM-hUCMSC administration and2 other side effects, which are considered as side effects according to “Common Terminology Criteria for Adverse Events version 4.0.” The secondary safety outcome is the presence of severe side effects at first week after the intervention, up to 2 weeks after the intervention, which is defined as an event that requires the respondent to undergo hospitalization, persistent or significant death or disability.
Wound healing will be monitored both clinically and through digital photographs in sequence. The picture will be taken in standard mode. Manual and single ulcer measurements have proven to be unreliable; therefore, the use of digital images with reference scales to measure ulcers is reported as a valid and reliable method for objectively describing ulcers.
Results
This study had 38 participants that met the inclusion criteria and were willing to participate in the investigation. From 38 respondents, 6 (15.8%) withdrew out or did not receive sufficient care during the study, including three respondents with leprosy and three with diabetes mellitus due to relocation. This study concluded with 32 participants, numerous participants suffering from multiple injuries, and 41 chronic ulcers being treated.
The majority of the 32 participants in the study were female (43.8%), and they had an average age of 57.03 (9.32) years, height of 161.34 (8.60) cm, and body weight of 59.59 (6.80) kg. The etiology of chronic ulcers in 32 participants of the study was 27 participants with leprosy and 5 participants with diabetes mellitus. The concomitant and patient characteristics are listed in detail in Table 1 and Table 2.
 |
Table 1 Demographic Characteristics of Research Respondents |
 |
Table 2 Wound Size Characteristics |
According to descriptive test results, the mean wound length at baseline was 1.60 (0.50–13.0) cm, at the first follow-up it was 1.2 (0–13) cm, and at the second follow-up it was 1 (0–12) cm. The mean wound width at baseline was 1.3 (0.5–6.0) cm, the wound width at the first follow-up was 1.0 (0.0–6.0) cm, and the wound width at the second follow-up was 0.8 (0.0–6.0) cm. The mean wound area was 2.21 (0.25–78) cm2 at baseline, 1.2 (0–78) cm2 at the first follow-up, and 1 (0–72) cm2 at the second follow-up, according to descriptive test results.
The Non-Parametric Wilcoxon Signed Ranks Test is applied to determine statistical significance. Length, width, and area of the wound changed significantly between the baseline and the first follow-up (p-value 0.001), the baseline and the second follow-up (p-value 0.001), and the first follow-up and the second follow-up (p-value 0.001) (Table 3 and Figures 1–3).
 |
Table 3 Changes in Chronic Wound Length, Width, and Area from the Beginning. The First Week and the Second Week |
 |
Figure 1 Wound length from the beginning, first week, and second week for all chronic wounds. |
 |
Figure 2 Wound width from the beginning, first week, and second week for all chronic wounds. |
 |
Figure 3 Area changes for all chronic wounds beginning, first week, and second week. |
After one month of intervention, the trial concluded. During the intervention period and up to 2 months post-intervention, SM-hUCMSC 0.1% applied topically caused no adverse effects (Figure 4).
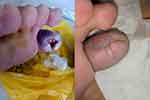 |
Figure 4 Example of diabetic ulcer repair – before (left) and after intervention (right). |
Discussion
Stem cell therapy, with the main focus on the use of secretome, is a therapy that focuses on the physiology of injured tissue that will release various growth factors and cytokines derived from stem cells. In vitro, the secretome from these stem cells can be harvested in a medium called the conditioned medium. This therapeutic principle has been widely used in the field of regenerative medicine, especially chronic wounds.42
Several previous studies have shown that MSC has the potential to increase wound healing rates by accelerating wound closure time.43 In our previous study, we use secretomes from human umbilical cord mesenchymal stem cells (SM-hUCMSC) with a hypoxic condition. We analyzed the levels of b-FGF and VEGF expression of MSC markers detected by Flow Cytometry and Pro-Collagen 1 using ELISA; CCK-8 evaluated fibroblast cell viability. The potential of MSC in vivo shows the process of basic fibroblast growth factor (b-FGF), vascular endothelial growth factor, and chemokines in tissue repair models in murine hind limb ischemia. MSCs, through paracrine mechanisms, enable interactions with various cells: immune cells, fibroblasts, endothelial cells, and other cells to modulate wound healing.44 Further, in vitro studies have identified paracrine effects between MSC, local tissue cells, and various immune cells that are regulated by secretion factors produced by MSC.45 In vivo administration of MSC also shows the migration of endothelial cells, macrophages, and reduction of effector T cells, which triggers angiogenic and regenerative processes by MSC.46 After the first week of treatment, we saw in all of our cases that the color of the wound bed changed to pink, proving the validity of the preceding statement. We hypothesized that the first action of this treatment is VEGF, which induces neovascularisation and builds angiogenesis, allowing nutrition to be supplied again and facilitating the diapedesis of inflammation cells, macrophages, and neutrophils, which remained impeded in the wound’s surrounding area. Macrophages and neutrophils begin their role as bacteria-killing cells and decontaminate the injury site. Macrophages serve a vital role as phagocytic cells in wound healing. Enzymes like cytokines, tumor necrosis factor (TNF), interleukins, and collagenase generated during phagocytosis have a role in removing foreign material and promoting fibroblasts and angiogenesis. Secreted by macrophages, vascular endothelial growth factor (VEGF), and Platelet-derived growth factor (PDGF) move granulation tissue from the proliferation phase to the tissue regeneration phase.;47 In acute wound healing, this may initiate a new phase of inflammation, however in chronic wound healing, loss of the first phase of inflammation is one of the causes limiting wound healing. After the initial phase has begun, the following phase of proliferation is more accessible (Figures 5 and 6).
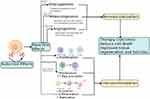 |
Figure 5 Mechanisms of angiogenesis and vasculogenesis in chronic ulcer treatment.48 |
 |
Figure 6 Schematic of secretome from stem cell derivatives in inducing wound healing.49 |
MSC-CM contains many neurotrophic factors, which have various beneficial factors in modulating and inducing vascularization and regeneration in the wound area, nerve repair, muscle, nerve, and skin regeneration by means of accelerating cell count increase, decreasing the incidence of post-inflammatory fibrosis and increasing fiber organization.42 The pus in the wounds was decreasing after the second weeks of treatment in our samples may show the activities of Neutrophil factors in this study.
Previous studies have revealed that MSCs can affect the activation and proliferation of immune cells. In simple terms, MSC regulates various1 anti-inflammatory factors such as IL1 receptor antagonist (IL1RA), IL27, IL17E, IL12p70, IL10, neurotrophin 3 (NT-3), ciliary neurotrophic factor (CNTF), IL18 binding protein (IL18-BP), interleukin (IL) 13 and all growth factors such as tumor growth factor-1 (TGF-1);2 pro-inflammatory such as IL9, IL8, IL6, and IL 1b. MSCs have an important role to play in balancing levels of anti-inflammatory and pro-inflammatory cytokines. However, it is also remarkable that MSC inhibits proinflammatory cytokines, such as interferon (IFN) and tumor necrosis factor (TNF), while increasing the release of anti-inflammatory IL10.42 Very interesting in this study, it seems anti-inflammation effects in all of the patients showed significant rate decrease in erythema, edema, and pain after 2 weeks of treatment, of course, this will impact their quality of life and suffering.
MSC also demonstrated the ability to enhance the healing process through extracellular matrix manipulation.50 Conditioned media (CM) of human cord blood MSC has been shown to inhibit the expression of matrix metalloproteinase (MMP) −1, which functions to reduce the degradation of the collagen matrix and plays a role in fibroblast regeneration.43 In the same study, this media played a role in wound healing through increased production of collagen and elastin by fibroblasts. MSC derived from human adipose has been shown to improve wound healing rates in vitro, and Lee et al showed that treatment with MSC significantly increased the proliferation of immortalized human keratinocyte (HaCaT) cells and skin fibroblasts, which resulted in increased wound healing rates.51 In our study, we also found that the width, length is decreased, and also improving bed of wounds, means fibroblast regeneration and epithelization were active in those cases. Assume that while VEGF maintains angiogenesis, bFGF in our gel attracts the fibroblast to work on the wound bed to crochet a granulation net. The rich pro-collagen content in our CM gel additionally promotes granulation formation’s strength. Granulation production marks the end of the proliferative phase of wound healing and the beginning of the maturation phase. After 2 weeks of process and progression toward the maturity phase, granulation is now robust enough to sustain the wound’s closure. Because of this, we discontinued the therapy, and now patients are taught how to manage their wounds on their own while under our supervision. We are also happy to communicate with each of them if they still require our guidance, and we can also monitor the final results. Stem cells have been utilized extensively, both externally and inside. Park et al discovered that ASC that secretes VEGF, b-FGF, TGF-1, HGF, KGF, PDF, and type 1 collagen, when injected intradermally twice weekly, reduces face wrinkles and skin thickness in 2 months.52 Kim et al tried to provide subcutaneous ASC injection for wrinkles induced by UB-B radiation in mice and were found to release antiapoptotic factors, collagen synthesis, and fibroblasts, and were shown to increase skin thickness and collagen bundles.53
The main problem with the topical administration of stem cells is the mechanism by which cells can be integrated into the wound.25 Stoff et al conducted a study by injecting concentrated human MSC (hMSC) into incisional wounds in rabbit skin animal models. This study showed HMSC migrated from the injection site and crossed the dermal-epidermal junction area of the wound on day 14 and had reached the intersection between the wound bed and the underlying fascia on day 21. The results of this study prove that MSC can migrate directly through connective tissue. This study also shows that the administration of MSC makes collagen fiber deposition more effective, more organized, and less likely to cause scarring.54 We also support this finding that topical use of gel was significant, and wounds heal better after 2 weeks than before treatment.
In this study, we modified 10% SM-hUCMSC gel as one of the solutions to this problem; it is important to pay attention to the concentration of active substances, the quality of SM-hUCMSC, the base of products, stabilization products, and the method and amount of application, as these variables can affect the efficacy of topical wound treatment.
We expect that this treatment will serve as an additional growth factor with therapeutic potential for persistent skin ulcers. By applying gel containing 10% SM-hUCMSC to the wound twice a day for 2 weeks, as stated in the aforementioned studies, we hypothesized that all active components are absorbed well, and all of the necessary base ornament factors in wound healing steps are formed in 2 weeks; patients then continue treatment on their own. This novel solution is designed to be simpler to administer, portable, inexpensive, effective, efficient, and beneficial. This study provides positive data for the use of topical MSC in the treatment of persistent ulcers caused by diabetes or leprosy. It is anticipated that this study will be continued into a Phase 3 clinical trial with improved research methods in order to increase clinical evidence about the treatment of chronic ulcers.
Conclusions and Recommendations
It has been shown that a topical administration of secretome of human umbilical cord mesenchymal stem cells (SC-hUCMSC) 10% can effectively promote wound healing, notably for chronic ulcers caused by diabetes and leprosy. This is demonstrated by the significant reduction of the wound’s length, width, and area that occurred between the time of the SM-hUCMSC intervention and the present. In phase 2 clinical research, the utilization of this technique was also assessed for its level of safety; however, there was no evidence of either local or systemic adverse effects. We plan to do more clinical testing (Phase 3) with larger samples and the use of control media, such as antibiotics or a placebo, in order to expand the data foundation for using SM-hUCMSC topically to treat chronic ulcers. This will be done in order to treat chronic ulcers.
Abbreviation
CM-hUCMSC, Conditioned Medium of Human Umbilical Cord Mesenchymal Stem Cells; ASC, Adipose-Derived Stromal Cell; b-FGF, Basic Fibroblast Growth Factor; BM-SC, Bone Marrow Stem Cell; CCK-8, Cell Counting Kit-8; CM, Conditioned Media; cm, Centimeter; CNTF, Ciliary Neurotrophic Factor; CTGF, Connective Tissue Growth Factor; ECM, Extracellular Matrix; EGF, Epidermal Growth Factor; ELISA, The Enzyme-Linked Immunosorbent Assay; FGF, Fibroblast Growth Factor; GM-CSF, Granulocyte-Macrophage Colony-Stimulating Factor; HaCaT, Human Keratinocyte; HGF, Hepatocyte Growth Factor; hMSC, Human Mesenchymal Stem Cells; HTS, Hypertrophic Scars; hUC-MSC, Human Umbilical Cord Mesenchymal Stem Cells; IFN, Interferon; IGF, Insulin-Like Growth Factor; IGFBP, Insulin-Like Growth Factor Binding Protein; IL, Interleukin; IL18-BP, IL18 binding protein; IL1RA, IL1 receptor antagonist; Kg, Kilogram; KGF, Keratinocyte Growth Factor; MMP, Matrix Metalloproteinase; MSC, Mesenchymal Stem Cells; NT-3, Neurotrophin 3; PDGF, Platelet-Derived Growth Factor; PGF, Placental Growth Factor; PT SST, Perseroan Terbatas Sukma Skin Treatment; RCT, Randomized Controlled Trial; SC-hUCMSC, Secretome of Human Umbilical Cord Mesenchymal Stem Cells; SC-PWJSC, Secretome From Placental Wharton Jelly Stem Cell; TGF-1, Tumor Growth Factor-1; TNF, Tumor Necrosis Factor; UTHREC, Universitas Tarumanagara Human Research Ethics Committee; VEGF, Vascular Endothelial Growth Factor; WHO, World Health Organization; α-SMA, Alpha-Smooth Muscle.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethical Clearance
Ethical Clearance for this study was obtained from Universitas Tarumanagara Human Research Ethics Committee Institute of Research and Community Engagement (Register Number: PPZ20192072 and Number of Letter: 1007-Int-KLPPM/Untar/VI/2020). This study has been registered at ClinicalTrials.gov with ID number: NCT04134676.
Inform Consent
Informed consent was obtained from all individual participants included in this study. The purpose and procedures of the study were explained to each participant, including any potential risks or benefits. Participants were informed of their right to withdraw from the study at any time without penalty. Confidentiality of participants’ data was ensured, and all data were anonymized and securely stored. The study protocol was approved by the relevant ethical review board/institutional review board, and the research was conducted in accordance with the principles of the Declaration of Helsinki”.
Acknowledgments
This study has been presented in the form of a grant fund accountability presentation. The authors are grateful to the following individuals for their generous help: Department Research. PT. Sukma Skin Treatment (PT. SST) to help us solve several problems in statistics. Department of Management of PT. Sukma Skin Treatment (PT. SST) for administration and Universitas Tarumanagara Human Research Ethics Committee Institute of Research and Community Engagement for ethics. Thank you to all the Mayapada Hospital, Sitanala Hospital, Indra Clinic, and Sukma Clinic for their permission to carry out this research. Do not forget we also thank all nurses, doctors, and research assistants who have worked together so that this research can be completed properly. We also thank Yuyus Kusnadi, Christine Ayu Lagonda, and Dilafitria Fauza for preparing the human umbilical cord mesenchymal stem cell secretome.
Funding
This project was supported by a grant from Institute of Research and Community Engagement Universitas Tarumanagara (Grant/Award Number: 012 Int-DIR.PPKM/ 3). As a kind of internal accountability, the complete methodology and outcomes of this research have been posted to the Tarumanagara University repository.
Disclosure
The authors declared that they have no conflicts of interest.
References
1. Werdin F, Tennenhaus M, Schaller H-E, Rennekampff H-O. Evidence-based management strategies for treatment of chronic wounds. Eplasty. 2009;9:25.
2. Cazander G, Pritchard DI, Nigam Y, Jung W, Nibbering PH. Multiple actions of Lucilia sericata larvae in hard-to-heal wounds. BioEssays. 2013;35(12):1083–1092. doi:10.1002/bies.201300071
3. Robson MC, Barbul A. Guidelines for the best care of chronic wounds. Wound Repair Regen. 2006;14(6):647–648. doi:10.1111/j.1524-475X.2006.00173.x
4. Gottrup F. A specialized wound-healing center concept: importance of a multidisciplinary department structure and surgical treatment facilities in the treatment of chronic wounds. Am J Surg. 2004;187(5):S38–S43. doi:10.1016/S0002-9610(03)00303-9
5. Wicke C, Bachinger A, Coerper S, Beckert S, Witte MB, Königsrainer A. Aging influences wound healing in patients with chronic lower extremity wounds treated in a specialized wound care center. Wound Repair Regen. 2009;17(1):25–33. doi:10.1111/j.1524-475X.2008.00438.x
6. Järbrink K, Ni G, Sönnergren H, et al. Prevalence and incidence of chronic wounds and related complications: a protocol for a systematic review. Syst Rev. 2016;5(1). doi:10.1186/s13643-016-0329-y.
7. Jöster M, Kröger K. Prevalence of chronic wounds in different modalities of care in Germany. Eur WOUND Manag Assoc J. 2018;2018:75.
8. Macdonald J. Global Initiative for Wound and Lymphoedema Care (GIWLC). Wounds UK. 2009;4:92–95.
9. Denny K, Lawand C, Perry SD. Compromised wounds in Canada. Health Q. 2014;17(1):7–10. doi:10.12927/hcq.2014.23787
10. Ragnarson Tennvall G, Apelqvist J. Health-economic consequences of diabetic foot lesions. Clin Infect Dis. 2004;39(Supplement_2):S132–S139. doi:10.1086/383275
11. Rice JB, Desai U, Cummings AKG, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care. 2014;37(3):651–658. doi:10.2337/dc13-2176
12. Jeffcoate W, Bakker K. World diabetes day: footing the bill. Lancet. 2005;365(9470):1527. doi:10.1016/S0140-6736(05)66437-9
13. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med. 2017;49(2):106–116. doi:10.1080/07853890.2016.1231932
14. UNAIDS. Global AIDS Update 2016. World Health Organization; 2016.
15. World Health Organization. Global Leprosy Strategy 2016–2020. World Health Organization; 2016.
16. World Health Organization. Global leprosy update, 2016: accelerating reduction of disease burden. Relev Epidemiol Hebd. 2017;92(35):501–519.
17. Kemenkes RI. Data dan Informasi profil Kesehatan Indonesia 2018 [Ministry of Health of the Republic of Indonesia]. Data dan Inf Profil Kesehat Indones; 2018.
18. Yolanda MM. Adult stem cell therapy in chronic wound healing. J Stem Cell Res Ther. 2014;04(01). doi:10.4172/2157-7633.1000162
19. Bluestein D, Javaheri A. Pressure ulcers: prevention, evaluation, and management. Am Fam Physician. 2008;78(10):1186–1194.
20. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi:10.1080/14653240600855905
21. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi:10.1091/mbc.e02-02-0105
22. Qomi RT, Sheykhhasan M. Adipose-derived stromal cell in regenerative medicine: a review. World J Stem Cells. 2017;9(8):107. doi:10.4252/wjsc.v9.i8.107
23. Zollino I, Zuolo M, Gianesini S, et al. Autologous adipose-derived stem cells: basic science, technique, and rationale for application in ulcer and wound healing. Phlebology. 2017;32(3):160–171. doi:10.1177/0268355516641546
24. Singh R, Singh R, Rohilla RK, Siwach R, Verma V, Kaur K. Surgery for pressure ulcers improves general health and quality of life in patients with spinal cord injury. J Spinal Cord Med. 2010;33(4):396–400. doi:10.1080/10790268.2010.11689718
25. Sorrell JM, Caplan AI. Topical delivery of mesenchymal stem cells and their function in wounds. Stem Cell Res Ther. 2010;1(4). doi:10.1186/scrt30
26. Utomo W, Dewi YI, Abdurrasyid T. Efektifitas nigella sativa oil untuk mencegah terjadinya ulkus dekubitus pada pasien tirah baring lama [Effectiveness of nigella sativa oil to prevent decubitus ulcers in long bed rest patients]. J Ners Indones. 2014;2(2):151–157.
27. González Sarasúa J, Pérez López S, Viejo M Á, et al. Treatment of pressure ulcers with autologous bone marrow nuclear cells in patients with spinal cord injury. J Spinal Cord Med. 2011;34:301.
28. Damayanti RH, Rusdiana T, Wathoni N. Mesenchymal stem cell secretome for dermatology application: a review. Clin Cosmet Investig Dermatol. 2021;14:1401–1412. doi:10.2147/CCID.S331044
29. Silveira BM, Ribeiro TO, Freitas RS, et al. Secretome from human adipose-derived mesenchymal stem cells promotes blood vessel formation and pericyte coverage in experimental skin repair. PLoS One. 2022;17(12):e0277863. doi:10.1371/journal.pone.0277863
30. Park S-R, Kim J-W, Jun H-S, Roh JY, Lee H-Y, Hong I-S. Stem cell secretome and its effect on cellular mechanisms relevant to wound healing. Mol Ther. 2018;26(2):606–617. doi:10.1016/j.ymthe.2017.09.023
31. Cunningham CJ, Redondo-Castro E, Allan SM. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J Cereb Blood Flow Metab. 2018;38(8):1276–1292. doi:10.1177/0271678X18776802
32. Ahangar P, Mills SJ, Cowin AJ. Mesenchymal stem cell secretome as an emerging cell-free alternative for improving wound repair. Int J Mol Sci. 2020;21(19):7038. doi:10.3390/ijms21197038
33. Wang M, Xu X, Lei X, Tan J, Xie H. Mesenchymal stem cell-based therapy for burn wound healing. Burn Trauma. 2021;9. doi:10.1093/burnst/tkab002/6261432:
34. Zriek F, Di Battista JA, Alaaeddine N. Mesenchymal stromal cell secretome: immunomodulation, tissue repair and effects on neurodegenerative conditions. Curr Stem Cell Res Ther. 2021;16(6):656–669. doi:10.2174/1574888X16666210202145639.
35. De Kock J, Rodrigues RM, Branson S, et al. Inflammation alters the secretome and immunomodulatory properties of human skin-derived precursor. Cells. 2020;9(4):914. doi:10.3390/cells9040914
36. Colombini A, Libonati F, Lopa S, et al. Immunomodulatory potential of secretome from cartilage cells and mesenchymal stromal cells in an arthritic context: from predictive fiction toward reality. Front Med. 2022;9. doi:10.3389/fmed.2022.992386.
37. Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7(1):1–13. doi:10.1186/s13287-016-0363-7
38. English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol. 2013;91(1):19–26. doi:10.1038/icb.2012.56
39. Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23(9):1045–1059. doi:10.3727/096368913X667709
40. Patel DM, Shah J, Srivastava AS. Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells Int. 2013;2013:1–15. doi:10.1155/2013/496218
41. Fu Y, Karbaat L, Wu L, Leijten J, Both SK, Karperien M. Trophic effects of mesenchymal stem cells in tissue regeneration. Tissue Eng Part B Rev. 2017;23(6):515–528. doi:10.1089/ten.teb.2016.0365
42. Vizoso F, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18(9):1852. doi:10.3390/ijms18091852
43. Jeon YK, Jang YH, Yoo DR, Kim SN, Lee SK, Nam MJ. Mesenchymal stem cells’ interaction with skin: wound-healing effect on fibroblast cells and skin tissue. Wound Repair Regen. 2010;18(6):655–661. doi:10.1111/j.1524-475X.2010.00636.x
44. Bronckaers A, Hilkens P, Martens W, et al. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143(2):181–196. doi:10.1016/j.pharmthera.2014.02.013
45. Elman JS, Murray RM, Wang F, et al. Pharmacokinetics of natural and engineered secreted factors delivered by mesenchymal stromal cells. PLoS One. 2014;9(2):e89882. doi:10.1371/journal.pone.0089882
46. van Rhijn-Brouwer FCC, Gremmels H, Fledderus JO, et al. A randomised placebo-controlled double-blind trial to assess the safety of intramuscular administration of allogeneic mesenchymal stromal cells for digital ulcers in systemic sclerosis: the MANUS Trial protocol. BMJ Open. 2018;8(8):e020479. doi:10.1136/bmjopen-2017-020479
47. Sinno H, Prakash S. Complements and the Wound Healing Cascade: an Updated Review. Plast Surg Int. 2013;2013:1–7. doi:10.1155/2013/146764
48. Samakova A, Gazova A, Sabova N, Valaskova S, Jurikova M, Kyselovic J. The pi3k/Akt pathway is associated with angiogenesis, oxidative stress and survival of mesenchymal stem cells in pathophysiologic condition in ischemia. Physiol Res. 2019;S131–S138. doi:10.33549/physiolres.934345
49. Cerqueira MT, Pirraco RP, Marques AP. Stem cells in skin wound healing: are we there yet? Adv Wound Care. 2016;5(4):164–175. doi:10.1089/wound.2014.0607
50. Ojeh NO, Navsaria HA. An in vitro skin model to study the effect of mesenchymal stem cells in wound healing and epidermal regeneration. J Biomed Mater Res Part A. 2014;102(8):2785–2792. doi:10.1002/jbm.a.34950
51. Lee SH, Jin SY, Song JS, Seo KK, Cho KH. Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann Dermatol. 2012;24(2):136. doi:10.5021/ad.2012.24.2.136
52. Park BS, Jang KA, Sung JH, et al. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatologic Surg. 2008;34:1323.
53. Jin HJ, Bae YK, Kim M, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14(9):17986–18001. doi:10.3390/ijms140917986
54. Stoff A, Rivera AA, Banerjee NS, et al. Promotion of incisional wound repair by human mesenchymal stem cell transplantation. Exp Dermatol. 2009;18(4):362–369. doi:10.1111/j.1600-0625.2008.00792.x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
