Back to Journals » Nutrition and Dietary Supplements » Volume 15
Effectiveness of Quadruple Fortified Salt Compared to Double and Single Fortified Salts in Improving Haemoglobin Levels Among Moderately Anemic Women Aged 18–49 Years in Rural Low Resource Setting: Randomized Clinical Trial
Authors Mdoe P, Mannar V, Justine M, Guga G, Gadiye R, Assey V, Kimathi C, Abdallah F, Paschal J, Mduma E , Diosady L
Received 4 April 2023
Accepted for publication 11 August 2023
Published 8 September 2023 Volume 2023:15 Pages 77—89
DOI https://doi.org/10.2147/NDS.S412893
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Paschal Mdoe,1 Venkatesh Mannar,2 Museveni Justine,1 Godfrey Guga,1 Rose Gadiye,1 Vincent Assey,3 Caroline Kimathi,1 Fatma Abdallah,3 John Paschal,1 Estomih Mduma,1 Levente Diosady2
1Department of Obstetrics and Gynecology, Haydom Lutheran Hospital, Manyara, Tanzania; 2Department of Food Engineering, University of Toronto, Toronto, ON, Canada; 3Department of Food and Nutrition, Tanzania Food and Nutrition Center, Dar Es Salaam, Tanzania
Correspondence: Paschal Mdoe, P.O. Box 9000, Manyara, Tanzania, Tel +255754429346, Email [email protected]
Background: Micronutrients (iron, iodine, vitamin B12 and folate) deficiency is prevalent globally affecting more than two billion people majority being from low- and middle-income countries. Women of reproductive age are in an increased risk of iron deficiency. About 29.4% of women aged 15– 49 years worldwide are estimated to be affected by iron deficiency. Food fortification with micronutrients is important in addressing micronutrients deficiency.
Aim: To evaluate if the quadruple fortified with iodine, iron, vitamin B12, and folic acid (QFS), will be more effective in improving the hemoglobin level of women aged 18 to 49 years compared to the double fortified with iodine and iron (DFS) and iodized salt in rural Tanzania.
Methods: A double-blinded three-arm randomized controlled trial was conducted between July 2020 and December 2021 at the Haydom Lutheran Hospital catchment area. We randomized women aged 18– 49 years with haemoglobin between 8 and 12 g/dl who were neither pregnant nor lactating into three groups 55 Iodized salts (IS), 57 Double fortified salt (DFS), and 57 quadruple fortified salt (QFS). The participants used study salt for 10 months.
Results: Over the ten months of use of study salts, the overall mean haemoglobin level of women was significantly higher in QFS by 0.43g/dl compared to IS. The ferritin levels were significantly higher in QFS and DFS by 9.60ng/mL and 9.09ng/mL, respectively, compared to IS. Vitamin B12 was insignificantly higher in QFS by 52.19pg/mL compared to DFS, and folate concentration were insignificantly higher in QFS by 7.57nmoL/L and 4.51nmoL/L compared to DFS and IS groups, respectively.
Conclusion: Salt fortification with iron, iodine, folate, and Vitamin B12 is feasible and has the potential to increase the serum ferritin, Vitamin B12 and folate levels with subsequent improvement of haemoglobin levels of individuals with relatively low haemoglobin.
ClinicalTrial.org Number: NCT04404751.
Keywords: iron-deficiency anemia, iodine, iron, vitamin B12, folate
Introduction
The global prevalence of anemia is estimated to be about 25% between 1993 and 20051,2 of which in low-income countries is 46.5%.3 The risk of anemia differs by age where women aged 15–49 years are at increased risk, the biggest problem seen among pregnant women by 42%.4 The highest prevalence of anemia was reported in Mozambique at 53.9%, whereas in Tanzania was around 45%.5
There are several causes of anemia, namely, micronutrients deficiency (inadequate diet or inadequate absorption of nutrients), infections (e.g., malaria, parasitic infestations, tuberculosis, HIV), inflammations, chronic diseases, inherited red blood disorders, and obstetrics and gynecological conditions. Micronutrient deficiency is the commonest cause of anemia globally accounting for more than 115,000 maternal and 591,000 perinatal global deaths annually, most of them being from Africa and Asia.6–8 Iron, folic acid, and Vitamin B12 deficiencies have been recorded as the most important causes of anemia globally.9 Between 1995 and 2011 the global mean haemoglobin level shows slight improvement, from 43% to 38% among pregnant women.10 Iron, folic acid, and Vitamin B12 deficiencies are estimated to affect more than two billion people worldwide mainly pregnant women, lactating women, and young children due to increased metabolic demand for growth.11–13 There are reports of suboptimal levels of folate and vitamin B12 in many low- and middle-income countries which are associated with increased morbidity and mortality among pregnant women.14,15 Furthermore, low intake of iron, iodine, folic acid, and Vitamin B12, affects a woman’s health, and pregnancy outcomes.16 It is, therefore, imperative that action is taken to reduce the rates of anemia in the population through community-level measures. This could be achieved by routine supplementation or fortification strategies.
Nutritional education, dietary modifications, and food fortification are among the documented ways which have been used to improve micronutrient levels among women and children globally.15 Large-scale food fortification has been shown to increase blood serum micronutrient concentrations in several populations. The adequate serum level of these micronutrients has reduced the incidences of anemia, goiter, and neural tube defect.17,18
Salt, which is universally consumed, has been used for the fortification of iodine and more recently in India as double-fortified salt (DFS) with iodine and iron.19 However, in Tanzania iron, folic acid, and vitamin B12-fortified salts have not been attempted. We conducted a randomized controlled clinical trial to evaluate the effectiveness of salt quadruple fortified with iodine, iron, vitamin B12, and folic acid (QFS) and compared it to the effect of salt double fortified with iodine and iron (DFS) and iodized salt on improving the haemoglobin level of women aged 18 to 49 years in rural Tanzania.
Methodology
Study Setting
This study was conducted at the community level at Haydom which is situated in the north-central part of Tanzania, approximately 300km from Arusha, the nearest urban center. Haydom is a rural area characterized by poor road infrastructure in a high-altitude setting. The population is approximately 20,000 inhabitants who are primarily dependent on subsistence agriculture, with some market sales of food products among inhabitants.20,21
Haydom Lutheran Hospital (HLH) is the central medical services hub for Haydom. The HLH catchment area consists of four administrative divisions in three districts and two regions. These are the Dongobesh and Haydom divisions in Mbulu District, the Basotu division in Hanang district and the Nduguti division in Mkalama (Singida region).
Geographically, Haydom is diverse; villages are distributed between two branches of the Great Rift Valley and are located at various elevations on the highland plateau where the hospital is located. The Tanzania demographic and health survey of 2010 showed that iron deficiency anemia was 35% among women of reproductive age (15–49 years), respectively.22
Study Design
A double-blinded randomized controlled trial with three arms was conducted to compare the effectiveness of quadruple-fortified salt versus double-fortified salt versus iodized salt (control) in improving haemoglobin concentrations of women aged 18 to 49 years at Haydom Lutheran hospital catchment area. The double blinded RCT was selected due to its novel capacity of assessing the causal effect.23
Inclusion Criteria
(1) moderately low Hb (Hb 8 to 12g/dl) (2) residence at the respective study site for 12 months and no plans of moving away during the study period; (3) Women aged 18–49 years at the time of enrolment; (4) general good health, no known history of major medical illnesses, thyroid dysfunction gastrointestinal or metabolic disorders and not on any long-term medication.
Exclusion Criteria
(1) Severe anemia (Hb <8 g/dl) (2) refusal to consent to participate, (3) sickle cell disease, (4) planning to become pregnant or to relocate out of the study area within the study period, (5) pregnant and lactating women (6) participating in another clinical trial with a nutritional supplement.
Recruitment, Study Population, and Randomization
We conducted a census of all the households in the HLH catchment area to identify those with potential women to join the study. Potential women were screened and those found eligible in their households were recruited and randomized to one of the study arms. Households with recruited study participants (whose households consented) in the catchment were supplied with fortified salt (study salt) for their normal daily use (cooking common dishes). A total of 174 women aged 18 to 49 years in their households were randomly allocated into either of the 3 arms. From each household, one woman aged 18 to 49 years who had relatively low haemoglobin (Hb >8 and ≤12g/dl) at the beginning of the study, who had no chronic illness, was not pregnant and who voluntarily agreed to participate was recruited into the study through randomization. Households with participants were randomly assigned to either Iodised salt (control) or double fortified salt or quadruple fortified salt group with a 1:1:1 allocation as per a computer-generated random sequence. Each household was supplied with salt based on their assigned study arm.
Study Procedure
For a family size of <5 household members 0.5 kg of salt was distributed weekly for the first month thereafter up to 5kg of salt was distributed every two months depending on the household usage. For household sizes above this 0.5 kg of salt was added for each extra household member. Compliance with the usage of study salt provided was monitored rigorously once every week with research staff visiting the houses and checking on the usage by weighing the salt container provided to them and completing a compliance questionnaire monthly. The questionnaire was used to collect comprehensive information related to illness, use of medication, use of supplements, use of study salts, and use of other salts. This enables the research assistants to adjust the amount of salt provided to the household periodically. Stool samples were collected from each participant and tested for intestinal worms, and those found with worms were treated accordingly. Additionally, all participants were dewormed (given a single dose of Mebendazole which treats a wide spectrum of worms) at the beginning of the study irrespective of their infestation status. Capillary blood was tested in the field using HemoCue Hb 201 and hemoglobin (Hb) recorded for screening purposes and study testing. Those found with Hb >8 and <12g/dl, and met other eligibility were enrolled. After enrolment 8 mL of venous blood was collected (by research staff who are well-trained in phlebotomy) at the beginning (0 month) of the study, at 3, 8 months, and during study exit at month 10. Blood was tested for: hemoglobin, iron status (Serum Ferritin), soluble transferrin receptor and zinc protoporphyrin, red cell folate and vitamin B12 status, and C-reactive protein. Urine was collected for iodine testing. The urine samples were analysed using the improved protocol of ammonium persulfate digestion method based on Sandell-Kolthoff reaction.24 The aliquoted serum blood was thawed and prepared to be tested for Vitamin B12 and Ferritin. These blood biomarkers are quantitatively measured by using Immuno Enzymometric Assay from TOSOH AIA System Analyzer. Commercially available controls were used for quality control procedure which are standardize on WHO 1 st IS 80/602 (1983) Vitamin B12. The highest measurable concentration of ferritin in undiluted serum is 1000ng/mL, while the lowest measurable concentration is 3.0ng/mL. The highest measurable concentration of Vitamin B12 in undiluted serum is 2000pg/mL, while the lowest measurable concentration is 50pg/mL.
All laboratory tests were done within Tanzania using international standards.25
Procedures for Salt Fortification, Technology Breakthrough
The process of multiple salt fortification consists of producing an extruded premix containing ferrous fumarate, folic acid, and vitamin B12, which, when added to iodized salt, produces a stable, white QFS. The retained levels will be 30–35% of Recommended Dietary Intake (RDI) for iron and folic acid and 100% RDI for iodine and vitamin B12. QFS is indistinguishable in taste, colour, and smell from regular salt.
Power Calculation and Sample Size Estimation
Considering the study done by Andersson et al 200818 and the findings of a systematic review and Meta-analysis report by Ramirez-Luzuriaga et al 2018,26 the sample size was calculated by using G*power to provide sufficient power for testing the effective difference between the groups, to achieve the power of 80%, precision of 5%, and medium effect size of 0.25 to a minimum of 53 women aged 18 to 49 years with moderately low haemoglobin was needed in each arm. Therefore, accounting for dropout, loss to follow-up, and missing samples, we added 10% to the total sample size, and assigned 58 each to three arms, resulting in a total of 174 participants.
Data Collection
Structured interviews were conducted during the baseline visit to collect information on socio-demographic characteristics including age, education level, marital status, literacy, income, number of children and adults living in the household and medical history. At baseline dietary intake was assessed with a food type and intake frequency questionnaire and a 24-hour dietary recall. A research assistant (RA) visited each household monthly to assess and record the salt usage. Furthermore, the RA measured the weight (at 100g precision by using SECA Digital Scale for adults) and height (at a precision of 0.1 cm by using SHORR three pieces height boards) of the participants. Venous blood samples for estimation of haemoglobin and ferritin levels were collected (by a nurse trained in phlebotomy) at recruitment, and after 3, 6, 8, and 10 months of intervention. The Hb was tested at the field and another blood sample was immediately placed in a controlled temperature cool box with ice and sent to the research laboratory for further analysis. Serum samples were aliquoted and stored at minus 80 C until analysis. The stool was collected from each participant at recruitment to assess for helminth infestation.
To monitor the smooth implementation of the study, the RA visited every household weekly the first month of the study to monitor the salt consumption and supply of salt. Thereafter, they visited households at monthly intervals. They collected information on any adverse effects that may be associated with salt consumption, eg, vomiting, diarrhea, allergic reaction, etc., as reported by consumers. The available local healthcare logistics and systems were used to refer the study participant who needed medical attention to the hospital.
Data Management
Quality control of the data was performed by two data editors before data entry. The data entry was done by two independent data clerks to capture entry inconsistencies and correct them, and final quality assurance was done by the data manager. Data were served into data servers at two different sites in a secure server. Data were analyzed using R version 4.1.0 (Foundation for Statistical Computing), the descriptive analysis was used to describe the data where mean, standard deviation, median, and quartiles were used to present data. We used line charts with 95% confidence interval error bars to assess the outcome variables (haemoglobin, ferritin, folic, and Vitamin B12) over time during the intervention by the salt group. Since we have two independent factors (within-subject factors), that is intervention groups (salt group) and we measure each subject repeated at a different time point, and the outcome variables were measured at a continuous level. We used the Analysis of variance two-way repeated measures to assess if the salt groups (interventions) and time points (target months) have effects on the outcomes (haemoglobin, ferritin, folic, and Vitamin B12 levels) at 0.05 level of significance. The post hoc test Turkey multiple comparisons of means with 95% family-wise confidence level was used for pairwise comparisons to check for main and interaction effects at different levels.
Results
A total of 689 households were visited between August 2019 and February 2020. Out of these 362 women of reproductive age were assessed for eligibility. Out of 362 assessed, 178 (49.2%) were eligible. Out of 178 eligible, 4 (2.2%) refused to participate, 174 were randomized into three arms 59 received Iodized Salt (IS), 58 received double fortified salt (DFS), and 57 received Quadruple Fortified Salt (QFS). Of the randomized participants, 55 (93.2%), 57 (98.3%), and 56 (98.2%) completed follow-up in the IS, DFS, and QFS arms, respectively, as set out in Figure 1.
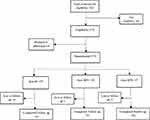 |
Figure 1 Consortium flow chart. |
All participants were women with a median age of 38.5 years and an average of 4 children. Each household had an average of 3 adult people living together. Almost three-quarters of the participants in each arm were married, and more than two-thirds had spent at least 7 years in school (primary education). Two-thirds of the participants were farmers, and more than half had normal body mass index (BMI) (Table 1).
 |
Table 1 Demographic Characteristics of the Randomized Participants |
The haemoglobin level of women of reproductive age increased in all study groups over the duration of the study. However, QFS and DFS groups had higher levels of haemoglobin levels at all time points from 3 months onwards as compared to IS groups (Figure 2). Overall mean haemoglobin levels in months 3, 8, and 10 were significantly higher than the baseline by 1.23g/dl, 1.66g/dl and 1.74g/dl, respectively. The QFS group had a significantly higher haemoglobin level by 0.43g/dl as compared to IS group. At month 10, the haemoglobin level on DFS, QFS and IS were significantly higher than the baseline by 2.07g/dl, 1.87g/dl, and 1.28g/dl, respectively. At month 10, the level of haemoglobin on DFS and QFS was higher compared to IS by 0.58g/dl and 0.54g/dl, respectively, however, their differences were not significant.
 |
Figure 2 The graph showing the trends of haemoglobin level over period during study by salt groups (quadruple fortified salt (QFS), double fortified salt (DFS) and iodised salt (IS)). |
The ferritin level increased over time within 8 months for the DFS and QFS, while in the IS the ferritin level decreased over time. The QFS and DFS groups had a significantly higher mean ferritin level (9.09ng/mL and 9.60ng/mL, respectively) compared to IS group. For the pairwise comparison of interaction effects, at month 8, the ferritin levels were higher on QFS and DFS compared to IS by 14.61ng/mL and 16.40ng/mL, respectively, however, their differences were not significant (Figure 3).
 |
Figure 3 The trends of serum ferritin levels (ng/mL) of the women over the 8 months by salt groups (quadruple fortified salt (QFS), double fortified salt (DFS) and iodised salt (IS)) with error bars. |
The Vitamin B12 levels decrease over time for all salt groups, however a steep decline was observed in DFS and IS, while the QFS had a gentle slope in decline. The QFS group had significantly higher levels of Vitamin B12 by 52.19pg/mL compared to DFS. At month 8, the levels of vitamin B12 were higher on QFS compared to DFS and IS by 87.58pg/mL and 28.10pg/mL respectively, however, their differences were not significant (Figure 4).
 |
Figure 4 The trends of Vitamin B12 levels (pg/mL) of the women over the 8 months by salt group (quadruple fortified salt (QFS), double fortified salt (DFS) and iodised salt (IS)) with error bars. |
The folic levels increase over time for all salt groups within the first three months however folic level in QFS group was above other groups. At 8th month, the QFS group had a significantly higher level of folic concentration by 7.57nmoL/L and 4.51nmoL/L compared to DFS and IS, respectively (Figure 5).
 |
Figure 5 The trends of folic levels (nmoL/L) of the women over the 8 months by salt groups (quadruple fortified Salt (QFS), double fortified salt (DFS) and iodised salt (IS)) with error bars. |
The two-way RM-ANOVA outputs showed that both time and salt groups had significant impact on the levels of haemoglobin, vitamin B12 and Folic acid. The ferritin levels seem to be significantly affected by salt groups. The salt groups and time have not shown any interaction effect on the outcome levels (Table 2).
 |
Table 2 Analysis of Variance (Two-Way) Repeated Measures Outputs for Haemoglobin, Ferritin Vitamin B12 and Folic Acid Levels by Salt Group and Target Months |
Discussion
Deficiencies of iron, iodine, Vitamin B12, and folic acid) are widespread in low- and middle-income countries. The deficiencies compromise not only the physical and cognitive capacity of millions of people, but also significantly contribute to the anaemia related morbidity and mortalities. Food fortification is a cost-effective strategy for combating these deficiency diseases with demonstrated health, economic, and social benefits. We report on a clinical trial done in the Haydom Lutheran Hospital catchment area involving salt fortification with iodine, iron, vitamin B12, and folate. In this trial, the amount of salt supplied in the households (0.1–2.5 Kg/person/week) was almost six times the amount recommended by WHO (<0.035Kg/person/week).27 The reason for oversupply was to ensure that the trial participant consumes what she usually consumes even if the salt was to be shared with others in this community with low-social economic status, though sharing study salt was discouraged. Moreover, this study did not consider the absorption capacity and hence was not set precisely on the amount of micronutrients supplementation.
This clinical trial found that both quadruple fortified salt (QFS) and double fortified salt (DFS) use, significantly increased both haemoglobin and ferritin levels for women of reproductive age with relatively low initial haemoglobin levels over ten months of use as compared to iodized salt (IS) use. Vitamin B12 levels were higher in QFS at 8 months of use as compared to DFS and IS groups, indicating that the Vitamin B12 added to salt was absorbed.
Our study showed a significant rise in haemoglobin levels of women with relatively low haemoglobin after ten months of QFS or DFS use as compared to the control group (IS) use. The rise of haemoglobin was sharp at three and eight months, thereafter the rise was more moderate. Other studies have previously shown a similar increase in haemoglobin over time of the use of iron-fortified salt and other iron-fortified foods.16 The impact of iron fortification on haemoglobin level has been found in both healthy and iron-deficient populations.16 In our study, we recruited women with relatively low haemoglobin levels, however, we did not explore the cause of their low haemoglobin levels. Participants in our study had relatively low ferritin levels at recruitment which was likely related to their low haemoglobin. Furthermore, all participants were blindly dewormed irrespective of their infestation status. This might have contributed to the improved haemoglobin levels of participants in both groups, however, only 2 participants tested positive for microscopic worm tests, and this reduces the likeliness that deworming contributed to the observed haemoglobin increase. Importantly, the observed low haemoglobin in the IS group, compared to the significantly higher haemoglobin levels in the QFS and DFS groups from three months onwards clearly indicates that the added micronutrients in the DFS and QFS groups were responsible for the observed increases in haemoglobin.
Serum ferritin level is an important marker for the body’s iron store levels.28 An average adult person loses about 1 mg of iron daily through sloughing cells from the skin and mucosal lining.29 Additionally, premenopausal women lose about 2 mg of iron daily during menstruation.30 The iron requirement is maximized in growing individuals namely neonates and children.31 Lack of iron supplementation through iron-rich food or fortified food leads to iron deficiency which is the commonest cause of low haemoglobin and subsequently anemia32–35 In our study, we found an exponential increase in haemoglobin levels following the use of QFS and DFS over ten months of use. Compared to iodized salt use which showed stable serum ferritin levels over the study period, both QFS and DFS had significantly higher levels of serum ferritin levels at 3 and 8 of use. Similarly, Gondolf et al found that serum ferritin levels of infants were significantly increased following the use of iron supplementation.36 A systematic review and a meta-analysis of the effect of iron-fortified flour on the iron status of populations worldwide showed that iron-fortified flour significantly increased the mean haemoglobin and serum ferritin levels.37 The findings from this study confirm that iron fortification using salt potentially increases the serum ferritin level of individuals.
In our study, Vitamin B12 showed decreasing trends in DFS and IS study groups, but in QFS the B12 levels were almost similar at zero and at eight months period. The vitamin B12 in the QFS seems to have been absorbed into the serum thus maintaining the level of serum Vitamin B12, despite increased demand for Vitamin B12 is proportional to increase haemoglobin production. Folate, Vitamin B12, and iron are crucial elements in erythropoiesis. The production of erythrocytes requires Folic acid and vitamin B12 which are key during DNA synthesis. The deficiency of these micronutrients affects erythropoiesis.38 Koury et al 2004, reported that the combination of vitamin B12, folate, erythropoietin, and iron was more effective in stimulating erythropoiesis among premature infants.39 The findings concur with our findings as QFS had a slightly higher mean haemoglobin level at eight months compared to DFS, however, the difference was not significant. These findings (our study and Haiden et al 2006) imply the importance of the combined effect of iron, Vitamin B12, and folate in raising haemoglobin levels. Furthermore, folate has other health benefits including prevention of birth anomalies (neural tube defect), improved cognitive functions, healthy embryo development, and reduction of risk of cancer.40,41
The finding from this study clearly shows the impact of QFS and DFS on raising the serum ferritin levels with subsequent increase in haemoglobin levels. Furthermore, the QFS may have additional health benefits derived from the combination of Vitamin B12 and folate.
Strength and Limitation
This clinical trial was conducted in a rural community among relatively healthy women with moderately low haemoglobin levels over a period of ten months. Being used in routine food preparation on daily household uses ensured the intake of daily required salt with less chance of unhealthy salt use. Furthermore, the study design and random assignment of study arms increased the credibility of the findings.
The study limitations include the inability to ascertain the effect of infection (eg, subclinical urinary tract infection) on ferritin levels, and not considering initial ferritin levels at recruitment. The collection of subjective information on daily eating practice, salt use (study salt or other salts), illness, and medication lacks precision and can mislead the assessment of the association between exposure and the outcome measures, measured objectively. We did not have a formal system to collect potential side effects in the HH as it was possible for another member of the HH to consume the salt, but we presumed that in normal domestic consumption, it was unlikely. The number of participants and the study being in one setting, compromise the generalization of the findings.
Conclusion
This study present that is feasible for the population in LMIC with moderate anaemia to consume salt fortified with multiple micronutrients. The consumption of salt fortified with iron, iodine, folate, and Vitamin B12 (QFS) was associated with a significant increase in hemoglobin compared to the use of double fortified salt (DFS) and Iodized salt (IS) in non-pregnant, non-lactating women aged between 18 and 49 years with moderate anaemia. The study also found the potential increase of serum ferritin, Vitamin B12, and folate levels micronutrients in the QFS group compared to the other two groups, ie, DFS and IS.
Data Sharing Statement
The data that support the findings of this study are available from the Haydom Lutheran Hospital through [email protected].
Ethical Approval and Consent to Participate
The clinical trial was approved by the Tanzania National Institute for Medical Research ethical review committee (NIMR), the Ministry of Health, community development, gender elderly and children (NIMR/HQ/R.8a/Vol. IX/2719), and the Tanzania Medicine and Medical Devices Authority (TMDA) (TZ19CT0009). These (NIMR and TMDA) are the only legal authorities in Tanzania with the mandate of reviewing, approve and oversee the conduct of clinical trials involving human subjects. The permission to conduct the research was also obtained from the local authority in the region and districts where the trial was conducted. The written informed consent was obtained from the head of the household and from the participating woman. The trial was registered on 27/05/2020 on ClinicalTrial.org (NCT04404751). All trial methods were performed in accordance with the relevant international and national guidelines and regulations including the declaration of Helsinki.
Acknowledgment
We acknowledge all women who took part in this study and the Neelkanth salt Limited for mixing the salt with extracts.
Author Contributions
All authors contributed to data analysis, drafting, or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This research was funded by the University of Toronto, Canada. The funders had no role in the design of the study, data collection, and analysis, interpretation of the findings, or writing the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. 2009;12(4):444–454. doi:10.1017/S1368980008002401
2. Balarajan Y, Ramakrishnan U, Ozaltin E, Shankar AH, Subramanian SV. Anaemia in low-income and middle-income countries. Lancet. 2011;378(9809):2123–2135. doi:10.1016/S0140-6736(10)62304-5
3. Ali SA, Khan U, Feroz A. Prevalence and determinants of anemia among women of reproductive age in developing countries. J Coll Physicians Surg Pak. 2020;30(2):177–186. doi:10.29271/jcpsp.2020.02.177
4. Kinyoki D, Osgood-Zimmerman AE, Bhattacharjee NV, Kassebaum NJ, Hay SI, Hay SI, Local Burden of Disease Anaemia Collaborators. Anemia prevalence in women of reproductive age in low- and middle-income countries between 2000 and 2018. Nat Med. 2021;27(10):1761–1782. doi:10.1038/s41591-021-01498-0
5. Teshale AB, Tesema GA, Worku MG, Yeshaw Y, Tessema ZT, Spradley FT. Anemia and its associated factors among women of reproductive age in Eastern Africa: a multilevel mixed-effects generalized linear model. PLoS One. 2020;15(9):e0238957. doi:10.1371/journal.pone.0238957
6. Georgieff MK. Iron deficiency in pregnancy. Am J Obstet Gynecol. 2020;(20):30328. doi:10.1016/j.ajog.2020.03.006
7. Juul SE, Derman RJ, Auerbach M. Perinatal iron deficiency: implications for mothers and infants. Neonatology. 2019;115(3):269–274. doi:10.1159/000495978
8. World Health Organization. Global Anaemia Reduction Efforts Among Women of Reproductive Age: Impact, Achievement of Targets and the Way Forward for Optimizing Efforts. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO.
9. Chaparro CM, Suchdev PS. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann N Y Acad Sci. 2019;1450(1):15–31. doi:10.1111/nyas.14092
10. Stevens GA, Finucane MM, De-Regil LM, et al.; Nutrition Impact Model Study Group (Anaemia). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1(1):e16–e25. PMID: 25103581; PMCID: PMC4547326.
11. Muthayya S, Rah JH, Sugimoto JD, Roos FF, Kraemer K, Black RE. The global hidden hunger indices and maps: an advocacy tool for action. PLoS One. 2013;8(6):e67860. doi:10.1371/journal.pone.0067860
12. Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–260. doi:10.1016/S0140-6736(07)61690-0
13. McLean E, De Benoist B, Allen LH. Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr Bull. 2008;29(Supplement 1):38–51. doi:10.1177/15648265080292S107
14. Bhutta ZA. Micronutrient needs of malnourished children. Curr Opin Clin Nutr Metab Care. 2008;11(3):309. doi:10.1097/MCO.0b013e3282fbf5a0
15. Best C, Neufingerl N, Del Rosso JM, Transler C, van den Briel T, Osendarp S. Can multi micronutrient food fortification improve the micronutrient status, growth, health, and cognition of schoolchildren? A systematic review. Nutr Rev. 2011;69(4):186–204. doi:10.1111/j.1753-4887.2011.00378.x
16. Huffman SL, Baker J, Shumann J, Zehner ER. The case for promoting multiple vitamin and mineral supplements for women of reproductive age in developing countries. Food Nutr Bull. 1999;20(4):379–394. doi:10.1177/156482659902000401
17. Olson R, Gavin-Smith B, Ferraboschi C, Kraemer K. Food fortification: the advantages, disadvantages and lessons from sight and life programs. Nutrients. 2021;13(4):1118. doi:10.3390/nu13041118
18. Andersson M, Thankachan P, Muthayya S, et al. Dual fortification of salt with iodine and iron: a randomized, double-blind, controlled trial of micronized ferric pyrophosphate and encapsulated ferrous fumarate in southern India. Am J Clin Nutr. 2008;88(5):1378–1387. doi:10.3945/ajcn.2008.26149
19. Evjen-Olsen B, Olsen ØE, Kvåle G. Achieving progress in maternal and neonatal health through integrated and comprehensive healthcare services – experiences from a programme in northern Tanzania. Int J Equity Health. 2009;8(8):27. doi:10.1186/1475-9276-8-27
20. Rekdal OB. Money, milk and sorghum beer: change and continuity among the Iraqw of Tanzania. Africa. 1996;66(3):367–385. doi:10.2307/1160958
21. Mduma ER, Gratz J, Patil C, et al. The etiology, risk factors, and interactions of enteric infections and malnutrition and the consequences for Child Health and Development Study (MAL-ED): description of the Tanzanian site. Clin Infect Dis. 2014;59(suppl–4):S325–30. doi:10.1093/cid/ciu439
22. National Bureau of Statistics (NBS). Micronutrients: results of the 2010 Tanzania demographic and health survey; 2011. Available from: www.nbs.go.tz.
23. Hariton E, Locascio JJ. Randomised controlled trials - the gold standard for effectiveness research: study design: randomised controlled trials. BJOG. 2018;125(13):1716. PMID: 29916205; PMCID: PMC6235704. doi:10.1111/1471-0528.15199
24. Sandell EB, Kolthoff IM. Micro determination of iodine by a catalytic method. Mikrochim Acta. 1937;1(1):9–25. doi:10.1007/BF01476194
25. World Health Organization. Serum and red blood cell folate concentrations for assessing folate status in populations. Vitamin and Mineral Nutrition Information System; 2015:1–7.
26. Ramírez-Luzuriaga MJ, Larson LM, Mannar V, Martorell R. Impact of double-fortified salt with iron and iodine on haemoglobin, anemia, and iron deficiency anemia: a systematic review and meta-analysis. Adv Nutr. 2018;9(3):207–218. doi:10.1093/advances/nmy008
27. World Health Organization. Guideline: Sodium Intake for Adults and Children. Geneva: World Health Organization; 2012. PMID: 23658998.
28. Orino K, Lehman L, Tsuji Y, et al. Ferritin and the response to oxidative stress. Biochem J. 2001;357(1):241–247. doi:10.1042/bj3570241
29. Cook JD, Skikne BS, Lynch SR, Reusser ME. Estimates of iron sufficiency in the US population. Blood. 1986;68(3):726–731. doi:10.1182/blood.V68.3.726.726
30. Bothwell TH, Charlton RW. A general approach of the problems of iron deficiency and iron overload in the population at large. Semin Hematol. 1982;19(1):54–67.
31. Gibson RS, MacDonald AC, Smit-Vanderkooy PD. Serum ferritin and dietary iron parameters in a sample of Canadian preschool children. J Can Dietetic Assoc. 1988;49:23–28.
32. Silla LM, Zelmanowicz A, Mito I, et al. High prevalence of anemia in children and adult women in an urban population in southern Brazil. PLoS One. 2013;8(7):e68805. doi:10.1371/journal.pone.0068805
33. De Benoist B, McLean E, Egli I, Cogswell M. WHO/CDC. Library cataloguing-in-publication data. In: Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia. Geneva: WHO Press, World Health Organization; 2008:40.
34. Duque X, Martinez H, Vilchis-Gil J, et al. Effect of supplementation with ferrous sulfate or iron bis-glycinate chelate on ferritin concentration in Mexican schoolchildren: a randomized controlled trial. Nutr J. 2014;13(1):71. doi:10.1186/1475-2891-13-71
35. Leonard AJ, Chalmers KA, Collins CE, et al. The effect of nutrition knowledge and dietary iron intake on iron status in young women. Appetite. 2014;81:225–231. doi:10.1016/j.appet.2014.06.021
36. De-Regil LM, Jefferds ME, Sylvetsky AC, et al. Intermittent iron supplementation for improving nutrition and development in children under 12 years of age. Cochrane Database Syst Rev. 2011;2011(12):CD009085. doi:10.1002/14651858.CD009085.pub2
37. Gondolf UH, Tetens I, Michaelsen KF, Trolle E. Iron supplementation is positively associated with increased serum ferritin levels in 9-month-old Danish infants. Br J Nutr. 2013;109(1):103–110. PMID: 22443990. doi:10.1017/S000711451200058X
38. Sadighi J, Nedjat S, Rostami R. Systematic review and meta-analysis of the effect of iron-fortified flour and iron status of the populations worldwide. Public Health Nutr. 2019;22(180):3465–3484. doi:10.1017/S1368980019002179
39. Koury MJ, Ponka P. New insights into erythropoiesiS: the roles of folate, vitamin B12, and iron. Annu Rev Nutr. 2004;24(1):105–131. PMID: 15189115. doi:10.1146/annurev.nutr.24.012003.132306
40. Haiden N, Klebermass K, Cardona F, et al. A randomized, controlled trial of the effects of adding vitamin B12 and folate to erythropoietin for the treatment of anemia of prematurity. Pediatrics. 2006;118(1):180–188. PMID: 16818564. doi:10.1542/peds.2005-2475
41. Imbard A, Benoist JF, Blom HJ. Neural tube defects, folic acid and methylation. Int J Environ Res Public Health. 2013;10(9):4352–4389. doi:10.3390/ijerph10094352
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
