Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
Effectiveness of Medication Reconciliation in a Chinese Hospital: A Pilot Randomized Controlled Trial
Authors Chai D , Liu Z , Wang L, Duan H, Zhao C, Xu C, Zhang D, Zhao Q, Ma P
Received 22 August 2023
Accepted for publication 2 November 2023
Published 24 November 2023 Volume 2023:16 Pages 3641—3650
DOI https://doi.org/10.2147/JMDH.S432522
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Dongyan Chai,1,2,* Zhihui Liu,3,* Liuyi Wang,3 Hongyan Duan,2,3 Chenglong Zhao,1 Chengyang Xu,2 Dongyan Zhang,1 Qiongrui Zhao,4 Peizhi Ma1
1Department of Pharmacy, Henan Provincial People’s Hospital, Zhengzhou University People’s Hospital, Henan University People’s Hospital, Zhengzhou, Henan People’s Republic of China; 2International Medical Center of Henan Province, Henan Provincial People’s Hospital, Zhengzhou, Henan, People’s Republic of China; 3Department of General Practice, Henan Provincial People’s Hospital, Zhengzhou, Henan, People’s Republic of China; 4Department of Clinical Research Service Center, Henan Provincial People’s Hospital, Zhengzhou, Henan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Peizhi Ma, Department of Pharmacy, Henan Provincial People’s Hospital, Zhengzhou University People’s Hospital, Henan University People’s Hospital, Weiwu Road 7, Zhengzhou, Henan, People’s Republic of China, Tel +86 18538298188, Fax +86 037165580803, Email [email protected]
Background: Implementing medication reconciliation (MR) was complex and challenging because of the variability in the guidance provided for conducting. The processes of MR adopted in China were different from that recommended by the World Health Organization. A pilot study to inform the design of a future randomized controlled trial to determine the effectiveness of these two workflows was undertaken.
Methods: Patients taking at least one home/regular medication for hypertension, diabetes, or coronary heart disease were recruited at admission, and then were randomized using a computer-generated random number in a closed envelope. In the study group, the pharmacist reviewed electronic medical record systems before communication with patients. In the control group, pharmacists communicated with patients at patient’s admission. The time investment of pharmacists for MR process, the number of unintended medication discrepancies, and physician acceptance were tested as outcome measures.
Results: One hundred and forty adult patients were randomized, of which 66 patients in the intervention received MR within 24 hours, while 58 patients in control received MR at some point during admission. The most common condition in the study group was hypertension (coronary heart disease in the control group). The workflow of the study group can save an average 7 minutes per patient compared with the WHO recommended process [17.5 minutes (IQR 14.00, 28.25) vs 24.5 minutes (IQR17.75, 35.25), p = 0.004]. The number of unintended discrepancies was 42 in the study group and 34 in the control group (p = 0.33). Physicians’ acceptance in the study and control groups were 87.5% and 92.3%, respectively (p = 0.87).
Conclusion: The results suggest that changes in outcome measures were in the appropriate direction and that the time limit for implementing MR can be set within 48 hours. A future multi-centre RCT study to determine the effectiveness of MR is feasible and warranted.
Keywords: medication reconciliation, workflow, prospective studies, pharmacist, randomized controlled trial
Introduction
Medication discrepancies are a common phenomenon in healthcare, which can adversely impact quality of care, patient safety, and resource utilization at transitions of care.1 As a result of poor communication between healthcare professionals or between healthcare professionals and patients/carers, medication discrepancies often occur at transitions of care, where patients always receive new medications or have changes made to their existing medications. Thus, improved methods of ensuring an accurate medication history at admission are needed.2 Medication reconciliation (MR) is a method that can reduce medication-list discrepancies.3–5 MR is a process of creating the most accurate list possible of all medications a patient is taking and comparing that list against the physician’s admission, transfer, and/or discharge. However, differences in the definition of MR used by different organizations lead to variability in MR practices.6,7 Inconsistent workflows have been recognized as a major challenge in expanding MR.8,9 At present, there are two models of MR process, namely, the proactive process and the retroactive process.10 In the proactive process, the interview with patients is performed upon patient arrival, whereas medical records are reviewed prior to communication with patients during the retrospective process. Several leading organizations worldwide, such as the World Health Organization (WHO) and the Joint Commission, and the Institute for Healthcare Improvement (IHI), give priority to the proactive process.11–14 This standard operating protocol (SOP) of WHO, named High 5s SOP,15 comprises four steps: (1) obtaining the best possible medication history (BPMH), which involves a systematic approach of acquiring a thorough list of all medications and verification of this information with at least 1 other reliable source of information; (2) confirming the accuracy of the patient’s history; (3) reconciling the BPMH with prescribed medication(s); and (4) supplying accurate medical information.
It is widely recognized that a process that works effectively in one area may not produce the same effect in other areas. Therefore, to set up a successful workflow, every step of a process must be adapted to the local context.16 Because of this, the WHO encourages all its member countries to implement respective SOP of MR in their healthcare institutions.
Performing MR is a time-consuming process.17,18 The patient interview of obtaining the BPMH as the crucial step takes 12 to 46 minutes per patient.19,20 In the electronic medical record system (EMRs), all medical staff can review health records (eg, medication history, laboratory results, simple text reports of microbiology and pathology, or radiographic images) and receive medical assistance from prescription-audit systems, and clinical pharmacists can review lists of medications procured from ambulatory prescription, information stored in local EMRs, and computerized orders. Merandi et al thought the EMRs is one of the first areas of focus that can improve MR and a workflow of guiding the clinician to reconcile all medications should be created to standardize the MR process.21 In recent years, MR is still in an experimental stage in China.22 For the BPMH to be obtained, at least two data sources are required, which may include a patient/family/caregiver interview, an EMR medication list, a patient prescription list/pill bottles, and a discharge summary.11 However, there is a lack of information on which sources should be prioritized. Due to the limited number of pharmacists in China and inability to communicate with patients in a timely manner, the pharmacist always reviews EMRs as a source of BPMH before communication with patients. In short, the retroactive process is more common with the promotion of EMRs in China.
A study showed that EMR usability can have an impact on clinical workflow, safety, quality, communication, and collaboration.23 In a study, researchers integrated EMRs into the clinical workflow to improve the MR process.24 Moreover, studies show that the use of EMRs can bring numerous benefits to the MR process; for example, it can improve healthcare efficiency and patient safety and reduce costs by reducing medication discrepancies, potential adverse drug events, and drug–drug interactions.25 Rungvivatjarus et al24 also suggested that the effective integration of EMRs into the workflow is related to successful MR performance.
Most of the evidence regarding the standardization of MR process has been from western healthcare systems.13,26,27 A review of the English literature suggests no previous studies describing the perfect time to review EMRs in MR progress. Thus, high-quality evidence is necessary, which demonstrates effectiveness of workflow to ensure optimized resources (EMRs). The most suitable approach to set up an effective workflow of a complex intervention is to perform a multi-center, RCT study, which collates data from different environments (ie, patients, wards, and pharmacists) and outcomes that are more reliable.
Aims
The aim of this study was therefore to pilot an RCT to inform the design of a future definitive study to determine an effect estimate of the MR workflow. The objectives of the pilot study were to:
- Identify the most suitable outcome measure for a future definitive trial with respect to proximity and response to the intervention and quality of data obtained;
- The effect estimate would provide information into sample size calculations for the planning of future, confirmative randomized controlled trials.
- We aimed to gain experience with respect to the feasibility of such studies, including aspects such as recruitment rate, the time limit for implementing MR and the physician’s acceptability of the intervention.
Methods
Setting
This was a single-center, prospective, pilot randomized controlled trial, to which patients were allocated (1:1) to the intervention or the control group. The trial was registered in Chinese Clinical Trial Registry (ChiCTR2100044652). Patients were considered for inclusion from Monday to Friday during daytime shifts and were recruited between June and December 2021. The study was conducted to compare the effectiveness of the current MR process adopted in China (ie, retroactive) and the WHO-recommended MR process (ie, proactive). Ethics approval was obtained from the medical ethics committees of Henan Provincial People’s Hospital (2021No.34).
Clinical pharmaceutical care services on the study ward were delivered between 08:00 and 17:30, Monday to Friday. The clinical pharmaceutical care model is ward-aligned, and pharmacist routinely attend ward rounds. Weekend clinical pharmaceutical care services were limited and excluded from this study. As part of their usual weekday activities, ward pharmacists were expected to obtain BPMH by a face-to-face interview and review all prescriptions, to ensure safety and appropriateness.
Participants and Enrolment
Patients hospitalized were required to complete a basic survey upon admission to the hospital, which contained items related to whether they had taken preadmission/home oral medicine and their medical history. The survey form with preadmission/home oral medicine was given to the research nurse for assessing during the study period to identify potential participants. Patients were recruited based on the following inclusion and exclusion criteria: Adult (≥18 years old); at least one home/regular medication; can speak Mandarin; and medical history of hypertension, diabetes, or coronary heart disease. Exclusion criteria were an inability to provide complete medical information owing to language, hearing, or other disabilities; an inability to provide a full medication list; or being severely ill.
Severely ill was defined as organ-level pathophysiology (for example, shock and respiratory failure), and intensive care services were delivered on maintaining organ-level homeostasis (for example, assisted breathing and circulatory support).
Each eligible patient was given a closed envelope containing random numbers, which was required to transfer to the intern pharmacist. After confirmation, the pharmacist will open the envelope to obtain grouping information.
Interventions
The MR process was conducted within 24 hours of hospitalization or transition of care by a pharmacist-managed team, like in previous studies28,29 and the WHO High5s implementation guide.15 The study group’s workflow proceeded in the order of (1) review of EMRs after the physician had completed admission notes (within 8 hours of the patient’s admission) and write all medicine in EMRs in a fixed MR form (Supplementary Table 1) as one source of BPMH, (2) a face-to-face interview with the patient or caregiver to create the BPMH, (3) reconciliation of the medication list in light of the patient’s current condition, and (4) discussion of unintended discrepancies with the physician. The control group’s workflow was as follows: (1) a face-to-face interview with the patient or caregiver to create BPMH when they were admitted to the ward, (2) review EMRs to confirm the accuracy of the history. Subsequent steps repeated Steps 3 and 4 in the study group (Figure 1).
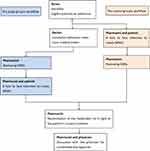 |
Figure 1 Flow chart showing the different workflows of the pilot study. Abbreviations: EMRs, electronic medical record system; BPMH, Best Possible Medication History. |
Outcome Measurements
Although undertaken as a pilot study with study aims to identify the most suitable outcome measure, the time investment of pharmacists for MR process was nominally selected as the primary outcome measure for this pilot trial. Accordingly, the authors collected data on the time taken for completion of each step in the MR process, which was divided into four parts: reviewing EMRs, confirming BPMH with patient, reconciling BPMH and admission orders, and discussion with physicians.
Secondary outcome measures were the number of unintended medication discrepancies and physician acceptance. Unintended medication discrepancy was defined as a difference between a patient’s current prescription and previous prescription across different institutions (excluding deliberate changes owing to medical conditions) which contribute substantially to adverse drug events.30
Researchers have often used medication discrepancies as an evaluation index for accuracy in the comparative analyses of an incremented MR process and a standard one (eg, by integrating new technologies or adjusted workflows).31 The meaning of MR may sometimes be misunderstood, entailing a low acceptance of MR recommendations.32 Thus, the number of unintended medication discrepancies and physician acceptance were reported.
Sample Size
There are no published data available on the time taken to complete different MR processes in China. The guidance on sample sizes for pilot studies varies, with 30–50 patients per arm thought to be sufficient.33 We aimed to recruit at least 50 patients for each of the control and intervention groups. Considering the expected dropout rate 20% and beneficial to patients, we expanded sample size to 140 for total groups.
Randomization
Patients were randomized in simple by random numbers, which were automatically generated by statisticians. Author QR Zhao, who did not recruit and allocate patients, prepared opaque, sealed, and sequentially numbered envelopes containing the randomization numbers. Each enrolled patient was given a closed envelope containing a number by nurse on admission. Subsequently, this envelope was handed to the intern pharmacist: those with odd numbers were assigned to the study group, otherwise, were assigned to the control group. Only pharmacists were aware of the grouping.
Data Collection
The pharmacist team included a certified clinical pharmacist with more than 10 years of experience and an intern pharmacist. The clinical pharmacist who had participated in professional training on diabetes, CHD, and hypertension was primarily responsible for obtaining BPMH and reconciling the medication list. Meanwhile, the intern pharmacist was responsible for recording the time taken for completing the MR process. Information regarding demographic and clinical characteristics and hospitalization records were all collected from EMRs by the intern pharmacist. Each patient’s BPMH was obtained by the clinical pharmacist in a standardized form, containing name, dose, frequency, and route of prescription, over‐the‐counter medications, dietary supplements, herbal medicines, results of MR.
Polypharmacy is defined as the routine use of five or more medications. This includes over-the-counter, prescription and/or traditional and complementary medicines used by a patient.34
Statistical Analysis
Statistical analysis was carried out using SPSS, version 25.0. Continuous data were presented as means ± standard deviation, with categorical data as percentages. Further, for continuous data with a normal distribution, Student’s t-tests were used; otherwise, Mann–Whitney U-test were used and presented as median and interquartile range (IQR); for categorical data, chi-square tests were used. Univariate and multivariate logistic regression analyses were used to identify factors associated with unintended medication discrepancies. A two-sided p < 0.05 was considered as statistically significant. The logistic regression model was used to determine the associations between patient’s clinical characteristics and unintentional medication discrepancies in univariate and multivariable logistic regression analyses. The following variables were tested by univariate logistic regression analysis: age, sex, group (study vs control), hypertension, CHD, diabetes, and polypharmacy. Variables with a significant relationship (P<0.30) by univariate analysis were imported into the multivariable model.
Results
One hundred and forty-six patients were assessed by research nurse at admission of which 6 were assessed for not eligibility (4 were not met inclusion criteria and 2 declined to participate), resulting in a recruitment rate of 95.89%. One hundred and forty patients were randomly assigned, and then 5 patients were excluded. The main reason of those patients not received MR was exceeding the 24-hour window for intervention. Among the 124 participants, 66 were assigned to the study group and 58 to the control group (Figure 2). All enrolled patients were of Han ethnicity. Most patients in both groups were male, and 36.36% in the study group and 34.48% in the control group were aged >65 years. In both groups, the most common reason for taking medication was hypertension (81.8% in the study group; 57.1% in the control group). Participants’ occupations were divided by whether they performed manual labor or not and being unemployed; the proportion of manual workers, non-manual workers, and unemployed participants was 9.1%, 75.8%, 15.2% in the study group, respectively, and 10.3%, 74.1%, 15.5% in the control group, respectively. Regarding education, 47.0% of participants had a college degree in the study group and 48.3% in the control group. The proportion of patients with Polypharmacy in both groups exceeded 40%. Additional characteristics are shown in Table 1.
 |
Table 1 Demographic and Clinical Characteristics |
 |
Figure 2 CONSORT Flow Diagram. |
Time Investment for MR
The data of time investment did not have a normal distribution, so Mann–Whitney U-test was used and presented as median and IQR. The average time taken to complete the MR process for each patient in the study group [17.5 (14.00, 28.25) minutes] was shorter than that in the control group [24.5 (17.75, 35.25) minutes]. Regarding each step of the MR process, the average time taken to confirm BPMH for each patient was 7.0 (5.00, 11.50) minutes in the study group, which was significantly shorter than the 11.5 (8.00, 17.25) minutes in the control group (Table 2).
 |
Table 2 Unintended Discrepancies and Time Consumption |
Unintended Medication Discrepancies
There were no significant differences between the study group and control group in the number of unintended discrepancies (42 vs 34) or the number of patients with unintended discrepancies (32 vs 26). In the study group, among 66 BPMHs obtained, there were 42 unintended medication discrepancies across 32 cases (48.5%). In the control group, among 58 BPMHs obtained, there were 34 unintended medication discrepancies across 26 cases (44.8%) (Table 2).
Multivariate logistic regression showed that diabetes (odds ratio [OR], 8.664; 95% confidence interval [CI], 3.664–20.488) and polypharmacy (OR, 1.414; 95% CI, 1.178–1.966) were statistically associated with unintended medication discrepancies (Table 3).
 |
Table 3 Univariate and Multivariate Analyses of Possible Risk Factors of Unintentional Medication Discrepancies |
Physician Acceptance
The proportion of physicians who accepted unintended medication discrepancies was higher in the control group than in the study group, but there were no significant differences (87.5% vs 92.3%, Table 2).
Discussion
This is a single center pilot study. The results from this study, which was performed to inform the design of a future RCT, suggest that such evaluation indicators are feasible and outcome data can be effectively obtained. In the future, a multi-centre RCT study can be conducted to recruit patients with various medical histories, not limited to hypertension, diabetes, or coronary heart disease to verify the effectiveness of workflow.
We consider that the time investment of pharmacists for the MR process would be the most appropriate primary outcome measure for such a trial. Healthcare systems in China are facing constant pressure to improve work efficiency, so we hope that results from this study will help improve efficiency of MR. If our future study elicits positive results, MR could be disseminated to more healthcare institutions across China.
Physician acceptance of these discrepancies should be selected as the secondary endpoints. In the clinical setting, the meaning of MR may sometimes be misunderstood, leading to a focus on filling out formularies, which entails a low acceptance of the recommendations for MR. Although the rates for physician acceptance of unintended medication discrepancy in the two groups were not significantly different. The proportion of physicians who accepted unintended medication discrepancies was higher in the control group (87.5% vs 92.3%, respectively; Table 2). This result can be explained. One of the most obvious differences between the proactive and retroactive MR processes is the timing when pharmacists interview patients. In the control group, the pharmacist was required to create BPMH as early as possible. Once pharmacists found problems, they would contact physician. That means the time of communication between pharmacist and physician would be much earlier than the intervention group, which could partly increase the trust and acceptance of physicians. Therefore, more sample size were required. We cannot exclude the possibility of different results obtained.
With a requirement that all patients receive MR within 24 hours of admission, it is unsurprising that some patients were excluded because clinical pharmacists cannot complete MR in a timely manner. Clinical pharmacists in China are responsible for consultations, meetings, and pharmaceutical affairs management in addition to the affairs in the ward, and only one certified clinical pharmacist and an intern pharmacist in this study. This may explain why some patients were excluded for failing to contact with the pharmacist, especially in the control group. In order to solve this problem, there are two solutions: first, refer to other literature,35–37 set the time for implementing MR within 48 hours; Second, a shift pharmacist is necessary to ensure patient recruitment and MR implementation.
In the study group, the time investment for MR process was significantly shorter than that in the control group, especially for polypharmacy patients. In line with previous research, documenting the medication list was the most time-consuming and challenging aspect of MR.38,39 It took less time to obtain the BPMH for patients in the study group than for those in the control group [7.0 (5.00, 11.50) vs 11.5 (8.00, 17.25) minutes, respectively; p <0.001]. There are two possible reasons. First, regarding definition, EMRs refer to systematic electronic collections of digital patient records and electronic prescribing systems that serve to accurately capture patient status at all times,40,41 allowing healthcare workers to monitor patients more effectively and facilitating the making of quick and well-informed decisions.42 Second, previous medication history in EMRs can be one of the two sources of BPMH.
Limitations
First, this study was conducted in a single center in central China, limiting the generalizability of its findings and discussions. Moreover, severely ill people were excluded to avoid research bias, who will be always polypharmacy patients, which did not deny the necessity of MR for critically ill patients. Additionally, improvements in patients’ clinical indicators were not analyzed. Hence, future multicenter research should analyze whether the MR approach proposed in this study is applicable to patients with severe conditions, and examine patients’ clinical situation and improvements in clinical indicators.
Strengths
In this pilot randomized controlled trial, the domestic MR process in China, which prioritizes the review of EMRs, can save on average 7.0 minutes per patient, compared with the WHO-recommended MR process; meanwhile, the accuracy and physician acceptance were comparable. Although prior studies have noted EMRs are one of the primary means of improving MR effectiveness, there is a lack of a standard process for the use of EMR.20,21 To better apply EMRs in the process of MR, this study developed a workflow to review EMRs before communicating with the patient in a study group. In the healthcare context, although the value of information is widely recognized, managers are inclined to explore effective ways to use current electronic tools due to economic pressure. Without capital investment, the findings in the current study showed that prioritizing EMRs to interview can improve MR efficiency and reduce capital and labor costs. Furthermore, multi-center RCT study will contribute to the making of standardized workflow of MR in China with the assistance of EMRs.
Data Sharing Statement
In accordance with the ethical approval of this study, only the members of the research group who are involved in the study will have access to data. This means that we are not allowed to share the raw data from this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Almanasreh E, Moles R, Chen TF. The medication discrepancy taxonomy (MedTax): the development and validation of a classification system for medication discrepancies identified through medication reconciliation. Res Soc Admin Pharm. 2019;16(2):142–148. doi:10.1016/j.sapharm.2019.04.005
2. Cornish PL, Knowles SR, Marchesano R, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165(4):424–429. doi:10.1001/archinte.165.4.424
3. Shenoy AM, Bennett A, Segal AZ. Tips and resources for medication reconciliation. Continuum Minneap Minn. 2019;25:543–549. doi:10.1212/con.0000000000000706
4. Pronovost P, Weast B, Schwarz M, et al. Medication reconciliation: a practical tool to reduce the risk of medication errors. J Crit Care. 2003;18(4):201–205. doi:10.1016/j.jcrc.2003.10.001
5. Bemt PMVD, Broek SVD, Nunen AKV, et al. Medication reconciliation performed by pharmacy technicians at the time of preoperative screening. Ann Pharmacother. 2009;43:868–874. doi:10.1345/aph.1l579
6. Almanasreh E, Moles R, Chen TF. The medication reconciliation process and classification of discrepancies: a systematic review. Br J Clin Pharmcol. 2016;82(3):645–658. doi:10.1111/bcp.13017
7. Fernandes BD, Almeida P, Foppa A, et al. Pharmacist-led medication reconciliation at patient discharge: a scoping review. Res Social Adm Pharm. 2020;16(5):605–613. doi:10.1016/j.sapharm.2019.08.001
8. Etchells E, Fernandes O. Medication reconciliation: ineffective or hard to implement? BMJ Qual Saf. 2018;27:947. doi:10.1136/bmjqs-2018-008605
9. Pevnick JM, Shane R, Schnipper JL. The problem with medication reconciliation. BMJ Qual Saf. 2016;25:726. doi:10.1136/bmjqs-2015-004734
10. Dufay É, Doerper S, Michel B, et al. High 5s initiative: implementation of medication reconciliation in France a 5 years experimentation. Saf Health. 2017;3(1):6. doi:10.1186/s40886-017-0057-6
11. Guo Q, Guo H, Song J, et al. The role of clinical pharmacist trainees in medication reconciliation process at hospital admission. Int J Clin Pharm-Net. 2020;42(2):796–804. doi:10.1007/s11096-020-01015-2
12. The Joint Commission. Using medication reconciliation to prevent errors. Jt Comm J Qual Patient Saf. 2006;32(4):230–232. doi:10.1016/s1553-7250(06)32030-2
13. Leotsakos A, Zheng H, Croteau R, et al. Standardization in patient safety: the WHO High 5s project. Int J Qual Health C. 2014;26(2):109–116. doi:10.1093/intqhc/mzu010
14. Institute for Healthcare Improvement. Medication reconciliation to prevent adverse drug events; 2022. Available from: https://www.ihi.org/Topics/ADEsMedicationReconciliation/Pages/default.aspx.
15. World Health Organization. Action on Patient Safety (High 5s) - Medication Reconciliation SOP Version 3, September 2014; 2014. Available from: https://cdn.who.int/media/docs/default-source/patient-safety/high5s/h5s-sop.pdf?sfvrsn=594d8e49_4.
16. Federico F. Implementation of medication reconciliation. Am J Health Syst Pharm. 2020;77(2):67–68. doi:10.1093/ajhp/zxz279
17. Ebbens MM, Gombert-Handoko KB, Wesselink EJ, et al. The effect of medication reconciliation via a patient portal on medication discrepancies: a randomized noninferiority study. J Am Med Dir Assoc. 2021;22(12):2553–2558.e1. doi:10.1016/j.jamda.2021.03.022
18. Katoue MG, Ker J. Implementing the medicines reconciliation tool in practice: challenges and opportunities for pharmacists in Kuwait. Health Policy (New York). 2018;122(4):404–411. doi:10.1016/j.healthpol.2017.12.011
19. Hammad EA, Bale A, Wright DJ, et al. Pharmacy led medicine reconciliation at hospital: a systematic review of effects and costs. Res Soc Admin Pharm. 2016;13(2):300–312. doi:10.1016/j.sapharm.2016.04.007
20. Meguerditchian AN, Krotneva S, Reidel K, et al. Medication reconciliation at admission and discharge: a time and motion study. BMC Health Serv Res. 2013;13:485. doi:10.1186/1472-6963-13-485
21. Merandi J, Sapko M, Catt C, et al. Medication reconciliation. Pediatr Rev. 2017;38:54–55. doi:10.1542/pir.2015-0153
22. Yu A, Wei G, Chen F, et al. Study protocol for the evaluation of pharmacist-participated medication reconciliation at county hospitals in China: a multicentre, open-label, assessor-blinded, non-randomised, controlled study. BMJ Open. 2022;12:e053741. doi:10.1136/bmjopen-2021-053741
23. Lloyd S, Long K, Oshni Alvandi A, et al. A National Survey of EMR Usability: comparisons between medical and nursing professions in the hospital and primary care sectors in Australia and Finland. Int J Med Inform. 2021;154:104535. doi:10.1016/j.ijmedinf.2021.104535
24. Rungvivatjarus T, Kuelbs CL, Miller L, et al. Medication reconciliation improvement utilizing process redesign and clinical decision support. Jt Comm J Qual Patient Saf. 2020;46(1):27–36. doi:10.1016/j.jcjq.2019.09.001
25. Lin H-L, Wu D-C, Cheng S-M, Chen C-J, Wang M-C, Cheng C-A. Association between electronic medical records and healthcare quality. Medicine. 2020;99(31):e21182. doi:10.1097/md.0000000000021182
26. Institute for Healthcare Improvement. Medication reconciliation to prevent adverse drug events. Available from: http://www.ihi.org/topics/adesmedicationreconciliation/Pages/default.aspx.
27. The Joint Commission. Using medication reconciliation to prevent errors; 2006. Available from: http://www.jointcommission.org/assets/1/18/SEA_35.pdf.
28. Koprivnik S, Albiñana-Pérez MS, López-Sandomingo L, Taboada-López RJ, Rodríguez-Penín I. Improving patient safety through a pharmacist-led medication reconciliation programme in nursing homes for the elderly in Spain. Int J Clin Pharm. 2020;42(2):805–812. doi:10.1007/s11096-020-00968-8
29. Petrovich B, Sweet M, Gillian S, Copenhaver J. Assessing the impact of a pharmacist-managed discharge medication reconciliation pilot at a community hospital system. J Healthcare Qual. 2020;43(2):e26–e32. doi:10.1097/jhq.0000000000000282
30. Schnipper JL, Liang CL, Hamann C, et al. Development of a tool within the electronic medical record to facilitate medication reconciliation after hospital discharge. J Am Med Inform Assoc. 2011;18(3):309–313. doi:10.1136/amiajnl-2010-000040
31. Prey JE, Polubriaginof F, Grossman LV, et al. Engaging hospital patients in the medication reconciliation process using tablet computers. J Am Med Inform Assoc Jamia. 2018;25:1460–1469. doi:10.1093/jamia/ocy115
32. Fernandes O. Medication Reconciliation in the Hospital: what, WHY, WHERE, WHEN, WHO AND HOW? Nurs Leadersh Tor Ont. 2012;25 Spec No 2012:42–49.
33. Sim J, Lewis M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J Clin Epidemiol. 2012;65(3):301–308. doi:10.1016/j.jclinepi.2011.07.011
34. World Health Organization. Medication Safety in Polypharmacy (WHO/UHC/SDS/2019.11); 2019. https://apps.who.int/iris/bitstream/handle/10665/325454/WHO-UHC-SDS-2019.11-eng.pdf?ua=1.
35. González-García L, Salmerón-García A, García-Lirola M, et al. Medication reconciliation at admission to surgical departments. J Eval Clin Pract. 2015;22(1):20–25. doi:10.1111/jep.12403
36. Sardaneh AA, Burke R, Ritchie A, et al. Pharmacist-led admission medication reconciliation before and after the implementation of an electronic medication management system. Int J Med Inform. 2017;101:41–49. doi:10.1016/j.ijmedinf.2017.02.001
37. Roure Nuez C, González Navarro M, González Valdivieso J, et al. Efectividad de un programa de conciliacio´n perioperatoria de la medicacio´n cro´nica en pacientes de cirugı´a programada [Effectiveness of a perioperative chronic medication reconciliation program in patients scheduled for elective surgery]. Med Clin-Barcelona. 2012;139(15):662–667. Spanish. doi:10.1016/j.medcli.2012.04.032
38. Poon EG, Blumenfeld B, Hamann C, et al. Design and implementation of an application and associated services to support interdisciplinary medication reconciliation efforts at an integrated healthcare delivery network. J Am Med Inform Assn. 2006;13:581–592. doi:10.1197/jamia.m2142
39. Kabir R, Liaw S, Cerise J, et al. Obtaining the best possible medication history at hospital admission: description of a pharmacy technician-driven program to identify medication discrepancies. J PHARM PRACT. 2021;36(1):19–26. doi:10.1177/08971900211021254
40. Cai T, Zhang L, Yang N, et al. EXTraction of EMR numerical data: an efficient and generalizable tool to EXTEND clinical research. BMC Med Inform Decis Mak. 2019;19(1):226. doi:10.1186/s12911-019-0970-1
41. Dainton C, Chu C. A review of electronic medical record keeping on mobile medical service trips in austere settings. Int J Med Inform. 2017;98:33–40. doi:10.1016/j.ijmedinf.2016.11.008
42. Wu G, Wang S, Ning Z, Zhu B. Privacy-preserved EMR information publishing and sharing: a blockchain-enabled smart healthcare system. IEEE J Biomed Health Inform. 2021. doi:10.1109/jbhi.2021.3123643
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
