Back to Journals » ClinicoEconomics and Outcomes Research » Volume 15
Effectiveness of Disinfecting Caps for Intravenous Access Points in Reducing Central Line-Associated Bloodstream Infections, Clinical Utilization, and Cost of Care During COVID-19
Authors Hou Y, Griffin LP, Ertmer K, Bernatchez SF , Kärpänen TJ, Palka-Santini M
Received 8 February 2023
Accepted for publication 13 June 2023
Published 21 June 2023 Volume 2023:15 Pages 477—486
DOI https://doi.org/10.2147/CEOR.S404823
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xing Lin Feng
Yuefeng Hou,1 Leah P Griffin,2 Kari Ertmer,3 Stéphanie F Bernatchez,3 Tarja J Kärpänen,4 Maria Palka-Santini4
1 3M Health Care, 3M Company, Austin, TX, USA; 2 3M Health Care, 3M Company, San Antonio, TX, USA; 3 3M Health Care, 3M Company, St, Paul, MN, USA; 4 3M Health Care, 3M Deutschland GmbH, Neuss, Germany
Correspondence: Yuefeng Hou, Email [email protected]
Purpose: Intravenous (IV) access point protectors, serving as passive disinfection devices and a cover between line accesses, are available to help reduce the risk of central line-associated bloodstream infections (CLABSIs). This low-maintenance disinfection solution is particularly valuable in situations with excessive workloads. This study examined the effect of a disinfecting cap for an IV access point on CLABSI rates, hospital length of stay, and cost of care in an inpatient setting during the coronavirus disease 2019 (COVID-19) pandemic.
Methods: The study utilized data from the Premier Healthcare Database, focusing on 200,411 hospitalizations involving central venous catheters between January 2020 and September 2020. Among these cases, 7423 patients received a disinfecting cap, while 192,988 patients did not use any disinfecting caps and followed the standard practice of hub scrubbing. The two cohorts, Disinfecting Cap and No-Disinfecting Cap groups, were compared in terms of CLABSI rates, hospital length of stay (LOS), and hospitalization costs. The analysis accounted for baseline group differences and random clustering effects by employing a 34-variable propensity score and mixed-effect multiple regression, respectively.
Results: The findings demonstrated a significant 73% decrease in CLABSI rates (p= 0.0013) in the Disinfecting Cap group, with an adjusted CLABSI rate of 0.3% compared to 1.1% in the No-Disinfecting Cap group. Additionally, the Disinfecting Cap group exhibited a 0.5-day reduction in hospital stay (9.2 days versus 9.7 days; p = 0.0169) and cost savings of $6703 ($35,604 versus $42,307; p = 0.0063) per hospital stay compared to the No-Disinfecting Cap group.
Conclusion: This study provides real-world evidence that implementing a disinfecting cap to protect IV access points effectively reduces the risk of CLABSIs in hospitalized patients compared to standard care, ultimately optimizing the utilization of healthcare resources, particularly in situations where the healthcare system is under significant strain or overloaded.
Keywords: comparative effectiveness, real world evidence, Premier Healthcare Database, inpatient, cohort study, central venous catheters
Introduction
Central lines are commonly used for central venous access and for the administration of drugs or fluids in critically ill patients. However, central line-associated bloodstream infections (CLABSIs) impose a significant burden on the US healthcare system. In 2021, the Centers for Disease Control and Prevention (CDC) reported a total of 30,389 CLABSIs across various inpatient settings.1 These infections result in a high attributable cost of approximately $48,000 per episode, along with a 12–25% increase in mortality and prolonged hospital stays.2 Furthermore, CLABSIs are linked to a 2.75-fold increased risk of in-hospital death and incur an additional $32,000 in variable inpatient costs.3
The advent of the coronavirus disease 2019 (COVID-19) pandemic further compounded the challenges posed by CLABSIs, as hospitals faced heightened patient volumes, urgent care demands, and challenges related to staffing and supply shortages. Consequently, an increase in device utilization and CLABSI cases was observed throughout 2020.4–6 Remarkably, during the first quarter of 2021, a substantial 45.3% rise in the CLABSI Standardized Infection Ratio (comparing actual to predicted numbers and reflecting differences in risk between populations)7 was documented across 3394 hospitals in the United States compared to the corresponding period in 2019.8
To combat the alarming prevalence of CLABSIs, various interventions have been explored, including the use of an intravenous (IV) access point protector known as disinfecting caps. These caps securely luer-lock to the central line hub and act as passive disinfection devices. They have demonstrated their ability to decrease CLABSI rates and associated clinical costs when compared to the standard practice of scrubbing the hub. For instance, a pre- and post-intervention study revealed that the implementation of alcohol-impregnated disinfecting caps resulted in a reduction of CLABSI incidence from 7.3 to 3.0 per 1000 line-days among burn patients.9 Similarly, the utilization of alcohol-impregnated disinfecting caps in a tertiary care hospital increased adherence rates to safe practices from 67% to 94% within a span of 9 months and contributed to a decrease in CLABSI rates from 1.36 to 0.87 per 1000 device days. This reduction resulted in a total cost savings of $1,636,792 due to 27 fewer CLABSIs, after considering the added cost of the port protectors.10 Furthermore, meta-analyses have consistently shown that disinfecting caps can reduce CLABSI rates by 41–57% compared to scrubbing the hub and generate median cost savings of $21,890 per CLABSI.11–14 Additionally, studies have indicated that disinfecting caps can enhance the effectiveness of CLABSI prevention bundles.15,16
Despite the extensive data provided by previous studies, limited literature exists regarding the clinical and economic evidence of disinfecting caps specifically in times of healthcare system overload, where the advantages of the caps in terms of time-saving passive disinfection, improved compliance, and optimized utilization of healthcare resources have a more pronounced impact. Therefore, the present study aims to examine the impact of a disinfecting cap on CLABSI rates, hospital length of stay, and hospitalization costs when compared to the standard practice of scrubbing the hub during the first three quarters of the COVID-19 pandemic. Real-world evidence extracted from national data across 664 inpatient facilities in the United States from January 2020 to September 2020 is utilized, with a specific focus on periods characterized by overwhelming stress on healthcare institutions.
Materials and Methods
Data Source
This study utilized data obtained from the Premier Healthcare Database (PHD) focusing on inpatients with central venous catheters (CVCs) between January 1, 2020, and September 30, 2020. The PHD is a comprehensive repository of real-world data collected since 2000, encompassing a large and diverse population that reflects clinical practices in the general population. The database comprises medical records from over 1041 contributing hospitals/healthcare systems, encompassing more than 231 million patients.17 The authors’ institutional review board determined that this study, involving de-identified clinical data, was exempt from IRB approval according to the Code of Federal Regulations, title 45 CFR 46.18 The study was subject to continuous monitoring and oversight to ensure adherence to the ethical principles outlined in the Declaration of Helsinki. The privacy and confidentiality of medical record data were rigorously maintained throughout the study using encrypted drives as a secure storage method.
Study Population
Hospitalizations involving CVCs were selected using pre-identified Current Procedural Terminology (CPT) codes (Supplemental Table 1). The Premier hospital chargemaster data was utilized to identify 7423 inpatient cases with CVCs that received a disinfecting cap (3M™ Curos™ Disinfecting Port Protectors, 3M, St. Paul, MN) exclusively for disinfecting and protecting the IV access point. These cases did not use any other disinfecting caps and were assigned to the “Disinfecting Cap” experimental cohort.
For the control cohort, a group of 192,988 CVC inpatient cases without any disinfecting caps was identified. These cases followed the standard practice of scrubbing the hub for disinfection. They were assigned to the “No-Disinfecting Cap” control cohort for comparative analysis.
Endpoints
The effectiveness of the two approaches to disinfect the IV access point was compared by measuring the incidence of CLABSI, hospital length of stay (LOS), and hospitalization cost as endpoints.
To identify cases of CLABSI, all patients were screened using ICD-10 Code T80.211A due to the absence of laboratory data, which limited the ability to rely on microbiological criteria for CLABSI identification. Following a similar approach to a previous retrospective cohort study, the authors utilized the diagnosis code that indicates the initial occurrence of bloodstream infection due to a CVC during hospitalization and not present upon admission to determine whether a patient developed CLABSI after admission.19
Given that data on the number of CVC insertions and line days were not available in the PHD, the CLABSI rate was calculated as the percentage of hospitalizations during which CLABSI was developed. Hospital length of stay (LOS) and costs were obtained from the PHD’s inpatient billing and hospital encounter data. These data included the recorded length of hospitalization and the estimated cost of care per hospitalization provided by the Premier facilities.
Statistical Analysis
To examine cohort differences in each of the endpoints, a statistical analysis was conducted using mixed-effect multiple regression. Given the nature of the comparative cohort study, it is crucial to account for baseline group differences and underlying clustering effects that arise from non-randomized sampling. To address this, logistic, Poisson, and linear mixed-effect regression models were developed to examine the CLABSI rate, LOS, and hospitalization cost, respectively. These models were specifically designed to control for baseline group differences and random clusters associated with the endpoints, thereby minimizing estimation bias, and enhancing the confidence of inference.
Baseline group differences were assessed using a propensity score approach. Logistic multiple regression was conducted on the cohort using a total of 34 variables (see Table 1) that encompassed baseline patient demographics, comorbidities, and hospital characteristics. Additionally, the severity of a patient’s illness as well the specific treatment received were considered as important factors influencing their risk of CLABSI, clinical utilization, and costs. To account for variations in the endpoints attributed to different patient pathologies and services received, the study also incorporated the Diagnosis-Related Group (DRG) system in the analysis. The DRG system categorizes hospital cases with similar resource utilization patterns based on clinical coherence, taking into account factors such as organ systems, surgical procedures, critical care, and clinical specialties. By including DRG in the regression models, the analysis aimed to control for confounding factors associated with different patient case mixes.
 |
Table 1 Patient Demographics, Comorbidities, and Hospital Characteristics in the Disinfecting Cap and No-Disinfecting Cap Groups |
The propensity score obtained from the logistic regression was included as a covariate in each regression model. In addition, to address potential clustering effects due to variations in patient case mixes, services, wards, and hospitals, the models incorporated random effects for DRG and hospital in the intercept. All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
The cohort comparison on the 34 baseline characteristics is presented in Table 1. Both groups were comprised of approximately 50% elderly patients who were at high risk, with an average Charlson Comorbidity Index (CCI) exceeding 3.5 and an average age above 60. The No-Disinfecting Cap group exhibited higher rates of blood disorders (65% versus 49%), renal disease (41% versus 28%), and obesity (26% versus 19%). Conversely, the Disinfecting Cap group had a higher percentage of intensive care unit (ICU) admissions (19% versus 6%). Notably, there were clear geographic disparities between the two groups. The Disinfecting Cap group primarily comprised patients from hospitals located in the southern region (99%), while the Non-Disinfecting Cap group had 50% of patients from the South, and the remaining 50% from the West, Midwest, and Northeast. In addition, the two cohorts differed in terms of hospital type and costing methods. All Disinfecting Cap patients were admitted to non-teaching hospitals, and 99% of these hospitalizations adopted the cost-to-charge ratio (RCC) costing method. In contrast, 52% of No-Disinfecting Cap cases were admitted to teaching hospitals, with 72% of them utilizing the relative value unit (procedural) costing method. In summary, the No-Disinfecting Cap group exhibited a higher number of comorbidities (CCI = 4.6 ± 3.4) compared to the Disinfecting Cap group (CCI = 3.8 ± 3.4), while the Disinfecting Cap group had a greater proportion of critically ill patients requiring intensive care.
After comparing the two cohorts using mixed-effect multiple regression models, the Disinfecting Cap group demonstrated a significant 73% decrease in CLABSI rates (OR=0.27, 95% CI: 0.12–0.60, p = 0.0013), with an adjusted CLABSI rate of 0.30% compared to 1.11% for the No-Disinfecting Cap group (Table 2 and Figure 1). Furthermore, patients who received a disinfecting cap experienced a 0.5-day reduction in hospital stay, with a mean length of stay of 9.20 days, compared to 9.71 days for those without a cap (p < 0.0169) (Table 2 and Figure 2). The hospitalization costs were reduced by $6703 per hospital stay in the Disinfecting Cap group (p = 0.0063), with a mean cost of $35,604 compared to $42,307 in the No-Disinfecting Cap group (Table 2 and Figure 3).
 |
Table 2 Comparison of Endpoints Between the Disinfecting Cap and No-Disinfecting Cap Groups |
 |
Figure 1 Central line-associated bloodstream infection incidence comparison. |
 |
Figure 2 Hospital length of stay comparison (days). |
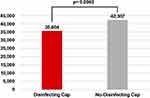 |
Figure 3 Hospitalization cost comparison (US dollars). |
Discussion
The COVID-19 pandemic had a profound impact on US hospitalizations, leading to increased patient risk and strained hospital resources and operations. Consequently, healthcare systems reported substantial increases in CLABSIs.4,5 Hospitals faced unprecedented challenges in infection prevention, including increased patient morbidity, higher catheter utilization, urgent line operations, intensive immunosuppressive treatments, staffing shortages, and supply constraints.20 In such circumstances, the use of disinfecting caps, simplifying the disinfection process and ensuring better compliance, has proven highly effective in mitigating the contamination risk, especially when resources are limited. The findings of the present study underscore the significant benefits of implementing disinfecting caps, showing a remarkable 73% decrease in CLABSI rates, a reduction in LOS by 0.5 days, and cost savings of $6703 per hospitalization compared to the standard practice during the COVID-19 pandemic.
Increased compliance with disinfection is associated with lower CLABSI rates, as suggested by Merrill et al in their study, where a 10% increase in compliance using the disinfecting cap resulted in a 7% reduction in infection.15 Disinfecting caps effectively protect IV access points from contamination by maintaining prolonged contact with the disinfectant, thus eliminating the need for frequent catheter hub scrubbing.11 This simplifies daily tasks, promoting higher compliance compared to procedures that are not easily observed through visual surveillance.15 Monitoring the “scrub the hub” technique is time-consuming and unrealistic, which has been unreliable in ensuring compliance and eliminating variance.9,15,21 Implementation of protective disinfecting caps for needleless connectors has shown significant improvement in compliance rates. For CVC patients in two US ICU units, compliance rates increased from 63% to 80%,22 while in a UK hospital trust, they increased from 27% to 80%, resulting in a time-saving of 45 seconds per disinfection process or potentially 659.4 hours per year.23
According to the epic3 guidelines, scrubbing the hub as an “active disinfection” method is required each time IV access points are accessed, with a minimum of 15 seconds for decontamination and 30 seconds for drying.24 However, the results of the UK national IV hub cleaning survey indicated that 63% of IV hubs were accessed within 25 seconds or less after decontamination.25 Similarly, a US hospital observed less than 10% compliance with the disinfection protocol when using the manual method of scrubbing with alcohol.26 The implementation of passive disinfection caps has shown improved compliance, which reduces the risk of contamination through the intraluminal route and the formation of biofilm that can occur when IV catheter hub disinfection is compromised. In addition to providing superior cleaning options that result in higher reduction rates of microorganisms, disinfecting caps have the potential to free up clinical time by replacing the traditional “active disinfection” method with a “passive” one. This ensures constant protection of devices through the use of antiseptic barriers for up to 7 days or until they are next accessed.9,23 By simplifying the disinfection process, these caps offer a more efficient and time-saving solution for healthcare professionals.
The findings of the present study demonstrate a remarkable reduction in CLABSI rates, compared to the majority of published data.9,15,22,27,28 These results highlight the exceptional effectiveness of disinfecting caps in mitigating the risks associated with CLABSI, particularly in the challenging context of the COVID-19 pandemic. It is evident that the pandemic has exerted substantial strain on the management of central lines in hospitalized patients, leading to decreased compliance with essential practices such as bedside checks and scrub-the-hub procedures.4 During these demanding times, the utilization of disinfecting caps assumes an even more critical role in combating the prevailing risk of CLABSI compared to the pre-pandemic period. The time-saving nature and easy application of these caps offer a practical solution that can significantly alleviate the growing burden of CLABSI. By simplifying the disinfection process, disinfecting caps not only enhance compliance but also contribute to the overall reduction of CLABSI incidence.
Moreover, the implementation of disinfecting caps yields significant benefits beyond reducing CLABSIs. It liberates valuable clinical hours, enabling healthcare providers to deliver a higher quality of care and achieve improved patient outcomes. This, in turn, leads to reduced length of stay and decreased hospitalization costs for patients who receive the caps. The observed shortened LOS by 0.5 days and cost savings of $6703 within the Disinfecting Cap group could be attributed not only to the direct impact of CLABSI reduction but also to the optimized utilization of clinical resources during this demanding period. Such optimization is of utmost importance in effectively managing the stress that COVID-19 has imposed on CLABSI rates and the overall healthcare system.
Current clinical guidelines prioritize manual disinfection as the recommended method for central line maintenance.29,30 However, despite the evolving circumstances, these guidelines typically view the use of disinfecting caps as a supplementary approach.31,32 It is worth noting that recent changes in the healthcare landscape have sparked renewed interest in the role of disinfecting caps as a potential alternative to traditional disinfection methods. Fakih et al have emphasized the critical importance of maintaining optimal line care and providing regular feedback on performance, particularly in high-stress environments where the risk of line contamination is amplified.4 The demanding nature of such environments often leads to suboptimal aseptic practices, further underscoring the need for innovative solutions. In this context, the discussion surrounding the use of disinfecting caps as an alternative to traditional disinfection methods has become increasingly relevant, reigniting the discourse after years of accumulating evidence. The growing body of evidence and the challenges posed by demanding healthcare settings call for a reevaluation of the role of disinfecting caps. As healthcare professionals strive to optimize patient outcomes and ensure the highest standard of care, exploring alternative approaches, such as disinfecting caps, becomes essential in adapting to evolving circumstances and improving patient safety.
Limitations
The present study relied on retrospective data to conduct measures and analyses. There are limitations to this study that could have influenced the results and interpretation. While efforts were made to account for baseline group differences in important risk factors for bloodstream infections, such as asthma,33 liver disease,34,35 cancer,36–38 peripheral vascular disease,39,40 rheumatic disease,41 renal disease,34,38 systemic inflammatory response syndrome,38 blood disorders,36 ICU admission,42 and Charlson Comorbidity Index (CCI) score,34,43 the study was limited by the lack of information regarding the type of CVC and catheter dwell time. Incorporating this information would have provided a more comprehensive assessment of patient risk profiles across the cohorts, even though the DRG clusters had accounted for partial variations associated with these two factors.
Furthermore, the study was constrained by the absence of microbiological criteria to precisely define CLABSIs, which might have led to an underestimation of the true incidence of CLABSIs. Additionally, we had no data available on the duration of cap application. In cases where the cap was not applied throughout the entire catheter dwell time, scrubbing the hub disinfection was assumed to be the only alternative. Not accounting for the “dosage” of intervention could potentially result in an underestimation of the effectiveness of the intervention.
While leveraging the advantages of a substantial study population to maximize study power, it is essential to exercise additional caution when interpreting statistically significant small effects. The adjusted 0.5-day difference in LOS between the two cohorts suggests that it may not be as clinically significant as the considerable cost savings of $6703 associated with reduced hospitalization.
Although the study incorporated hospital and DRG as random intercepts in the models to accommodate variations attributed to service types and hospital-specific characteristics, it is important to acknowledge that the cost structures across hospitals were not standardized. This lack of standardization could have introduced additional uncertainty in estimating cost savings. Considering the evident geographic disparities, different hospital types, and divergent costing methods between the two cohorts, relying solely on statistical adjustment may not be sufficient to fully eliminate all potential confounding factors. As such, substantial randomized trials are necessary to corroborate the conclusions drawn from this study.
Conclusion
The COVID-19 pandemic has placed unprecedented stress on healthcare systems, resulting in elevated CLABSI rates. This study provides real-world evidence demonstrating that implementing disinfecting caps in hospitalized patients with CVCs is more effective in reducing CLABSI risk and optimizing the allocation of healthcare resources than the standard care during this challenging period. This intervention emerges as a valuable strategy to mitigate the escalating burden of CLABSIs, particularly in high-risk and high-stress environments, ensuring enhanced patient safety and improved overall healthcare outcomes.
Acknowledgments
The authors would like to thank Eliza Searcy, BSN, RN, VA-BC (3M) for technology consulting.
Disclosure
All authors are employees of 3M. The authors report no other conflicts of interest in this work.
References
1. CDC. National and state healthcare-associated infections progress report; 2021. Available from: https://www.cdc.gov/hai/data/portal/progress-report.html.
2. Chopra V, Felix K, Johns K, Mermel L, Olmsted R, Patel P. Central Line-Associated Bloodstream Infection (CLABSI): an introduction. centers of disease control and prevention; 2020. Available from: https://www.cdc.gov/infectioncontrol/pdf/strive/CLABSI101-508.pdf.
3. Stevens V, Geiger K, Concannon C, Nelson RE, Brown J, Dumyati G. Inpatient costs, mortality and 30-day re-admission in patients with central-line-associated bloodstream infections. Clin Microbiol Infect. 2014;20(5):O318–324. doi:10.1111/1469-0691.12407
4. Fakih MG, Bufalino A, Sturm L, et al. Coronavirus disease 2019 (COVID-19) pandemic, central-line-associated bloodstream infection (CLABSI), and catheter-associated urinary tract infection (CAUTI): the urgent need to refocus on hardwiring prevention efforts. Infect Control Hosp Epidemiol. 2022;43(1):26–31. doi:10.1017/ice.2021.70
5. Patel PR, Weiner-Lastinger LM, Dudeck MA, et al. Impact of COVID-19 pandemic on central-line-associated bloodstream infections during the early months of 2020, national healthcare safety network. Infect Control Hosp Epidemiol. 2022;43(6):790–793. doi:10.1017/ice.2021.108
6. Wu H, Soe MM, Konnor R, et al. Hospital capacities and shortages of healthcare resources among US hospitals during the coronavirus disease 2019 (COVID-19) pandemic, National Healthcare Safety Network (NHSN), March 27-July 14, 2020. Infect Control Hosp Epidemiol. 2022;43(10):1473–1476. doi:10.1017/ice.2021.280
7. CDC. The national healthcare safety network standardized infection ratio (SIR); 2015. Available from: https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/nhsn-sir-guide.pdf.
8. Lastinger LM, Alvarez CR, Kofman A, et al. Continued increases in the incidence of healthcare-associated infection (HAI) during the second year of the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. 2022;2022:1–5. doi:10.1017/ice.2022.116
9. Martino A, Thompson L, Mitchell C, et al. Efforts of a Unit Practice Council to implement practice change utilizing alcohol impregnated port protectors in a burn ICU. Burns. 2017;43(5):956–964. doi:10.1016/j.burns.2017.01.010
10. Beeler C, Kerley D, Davis C, et al. Strategies for the successful implementation of disinfecting port protectors to reduce CLABSI in a large tertiary care teaching hospital. Am J Infect Control. 2019;47(12):1505–1507. doi:10.1016/j.ajic.2019.05.016
11. Voor In ‘t Holt AF, Helder OK, Vos MC, et al. Antiseptic barrier cap effective in reducing central line-associated bloodstream infections: a systematic review and meta-analysis. Int J Nurs Stud. 2017;69:34–40. doi:10.1016/j.ijnurstu.2017.01.007
12. Voor In ‘t Holt AF, Helder OK, Vos MC, et al. Corrigendum to ‘Antiseptic barrier cap effective in reducing central line-associated bloodstream infections: a systematic review and meta-analysis’. Int J Nurs Stud. 2018;84:79–80. doi:10.1016/j.ijnurstu.2017.08.001
13. Tejada S, Leal-Dos-Santos M, Pena-Lopez Y, Blot S, Alp E, Rello J. Antiseptic barrier caps in central line-associated bloodstream infections: a systematic review and meta-analysis. Eur J Intern Med. 2022;99:70–81. doi:10.1016/j.ejim.2022.01.040
14. Flynn JM, Larsen EN, Keogh S, Ullman AJ, Rickard CM. Methods for microbial needleless connector decontamination: a systematic review and meta-analysis. Am J Infect Control. 2019;47(8):956–962. doi:10.1016/j.ajic.2019.01.002
15. Merrill KC, Sumner S, Linford L, Taylor C, Macintosh C. Impact of universal disinfectant cap implementation on central line-associated bloodstream infections. Am J Infect Control. 2014;42(12):1274–1277. doi:10.1016/j.ajic.2014.09.008
16. Grigonis AM, Dawson AM, Burkett M, et al. Use of a central catheter maintenance bundle in long-term acute care hospitals. Am J Crit Care. 2016;25(2):165–172. doi:10.4037/ajcc2016894
17. PINC AI™ Healthcare Data White Paper. Data that informs and performs, September 14, 2021. PINC AI™ Applied Sciences, Premier Inc; 2021. Available from: https://offers.premierinc.com/rs/381-NBB-525/images/Premier-Healthcare-Database-Whitepaper-Final.pdf.
18. US Dept of Health and Human Services. Code of Federal Regulations, title 45 CFR 46; 2018. Available from: https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/common-rule-subpart-a-46104/index.html.
19. Toor H, Farr S, Savla P, Kashyap S, Wang S, Miulli DE. Prevalence of central line-associated bloodstream infections (CLABSI) in intensive care and medical-surgical units. Cureus. 2022. doi:10.7759/cureus.22809
20. Kurt AF, Mete B, Urkmez S, et al. Incidence, risk factors, and prognosis of bloodstream infections in COVID-19 patients in intensive care: a single-center observational study. J Intensive Care Med. 2022;37(10):1353–1362. doi:10.1177/08850666221103495
21. Stango C, Runyan D, Stern J, Macri I, Vacca M. A successful approach to reducing bloodstream infections based on a disinfection device for intravenous needleless connector hubs. J Infus Nurs. 2014;37(6):462–465. doi:10.1097/NAN.0000000000000075
22. Ramirez C, Lee AM, Welch K. Central venous catheter protective connector caps reduce intraluminal catheter-related infection. J Assoc Vasc Access. 2012;17(4):210–213. doi:10.1016/j.java.2012.10.002
23. Cameron-Watson C. Port protectors in clinical practice: an audit. Br J Nurs. 2016;25(8):S25–31. doi:10.12968/bjon.2016.25.8.S25
24. Loveday HP, Wilson JA, Pratt RJ, et al. epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect. 2014;86(1):S1–70. doi:10.1016/S0195-6701(13)60012-2
25. Rawlinson L. National IV port cleaning survey results; 2014. Available from: https://issuu.com/vygonuk/docs/ps304_-_email_national_port_cleanin.
26. Lee J. Disinfection cap makes critical difference in central line bundle for reducing CLABSIs (Abstract). Am J Infect Control. 2011;39(5). doi:10.1016/j.ajic.2011.04.124
27. Kamboj M, Blair R, Bell N, et al. Use of disinfection cap to reduce central-line-associated bloodstream infection and blood culture contamination among hematology-oncology patients. Infect Control Hosp Epidemiol. 2015;36(12):1401–1408. doi:10.1017/ice.2015.219
28. Wright MO, Tropp J, Schora DM, et al. Continuous passive disinfection of catheter hubs prevents contamination and bloodstream infection. Am J Infect Control. 2013;41(1):33–38. doi:10.1016/j.ajic.2012.05.030
29. American Society of Anesthesiologists Task Force on Central Venous A. Practice guidelines for central venous access 2020. An updated report by the American Society of Anesthesiologists Taskforce on central venous access. Anesthesiology. 2020;132:8–43. doi:10.1097/ALN.0000000000002864
30. Institute PS. Prevent central line infections. Getting Started Kit; 2012. Available from: https://www.patientsafetyinstitute.ca/en/toolsResources/Documents/Interventions/Central%20Line-Associated%20Bloodstream%20Infection/CLI%20Getting%20Started%20Kit.pdf.
31. Marschall J, Mermel LA, Fakih M, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(7):753–771. doi:10.1086/676533
32. Buetti N, Marschall J, Drees M, et al. Strategies to prevent central line-associated bloodstream infections in acute-care hospitals: 2022 Update. Infect Control Hosp Epidemiol. 2022;2022:1–17.
33. Bang DW, Yang HJ, Ryoo E, et al. Asthma and risk of non-respiratory tract infection: a population-based case–control study. BMJ open. 2013;3(10):e003857. doi:10.1136/bmjopen-2013-003857
34. Pepin CS, Thom KA, Sorkin JD, et al. Risk factors for central-line–associated bloodstream infections: a focus on comorbid conditions. Infect Control Hosp Epidemiol. 2015;36(4):479–481. doi:10.1017/ice.2014.81
35. Bartoletti M, Giannella M, Lewis RE, Viale P. Bloodstream infections in patients with liver cirrhosis. Virulence. 2016;7(3):309–319. doi:10.1080/21505594.2016.1141162
36. Mollee P, Jones M, Stackelroth J, et al. Catheter-associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011;78(1):26–30. doi:10.1016/j.jhin.2011.01.018
37. Gudiol C, Aguado JM, Carratalà J. Bloodstream infections in patients with solid tumors. Virulence. 2016;7(3):298–308. doi:10.1080/21505594.2016.1141161
38. Aliyu S, Cohen B, Liu J, Larson E. Prevalence and risk factors for bloodstream infection present on hospital admission. J Infect Prev. 2018;19(1):37–42. doi:10.1177/1757177417720998
39. Budzyński J, Wiśniewska J, Ciecierski M, Kędzia A. Association between bacterial infection and peripheral vascular disease: a review. Int J Angiol. 2015;25(1):3–13. doi:10.1055/s-0035-1547385
40. Shanmugam L, Priyadarshi K, Kumaresan M, et al. A rare case report of non-toxigenic corynebacterium diphtheriae bloodstream infection in an uncontrolled diabetic with peripheral vascular disease. Cureus. 2021. doi:10.7759/cureus.14947
41. Mehta B, Pedro S, Ozen G, et al. Serious infection risk in rheumatoid arthritis compared with non-inflammatory rheumatic and musculoskeletal diseases: a US national cohort study. RMD Open. 2019;5(1):e000935. doi:10.1136/rmdopen-2019-000935
42. Tao XB, Qian LH, Li Y, et al. Hospital-acquired infection rate in a tertiary care teaching hospital in China: a cross-sectional survey involving 2434 inpatients. Int J Infect Dis. 2014;27:7–9. doi:10.1016/j.ijid.2014.05.011
43. Edwards F, Glen K, Harris PNA, Paterson DL, Laupland KB. Determinants and outcomes of bloodstream infections related to obesity. Eur J Clin Microbiol Infect Dis. 2022;41(11):1347–1353. doi:10.1007/s10096-022-04501-9
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
