Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Effectiveness and Safety of the PD-1 Inhibitor Lenvatinib Plus Radiotherapy in Patients with HCC with Main PVTT: Real-World Data from a Tertiary Centre
Authors Li G , Zhao Y, Li K, Yang S, Xiang C, Song J, Yang Y, Li G, Dong J
Received 22 August 2023
Accepted for publication 3 November 2023
Published 9 November 2023 Volume 2023:10 Pages 2037—2048
DOI https://doi.org/10.2147/JHC.S432542
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Manal Hassan
Guangxin Li,1,* Ying Zhao,1,* Keren Li,2,* Shizhong Yang,2 Canhong Xiang,2 Jiyong Song,3 Yanmei Yang,1 Gong Li,1 Jiahong Dong2,4
1Department of Radiation Oncology, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, People’s Republic of China; 2Hepatopancereatobiliary Center, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, People’s Republic of China; 3Department of Liver Transplantation, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, People’s Republic of China; 4Research Unit of Precision Hepatobiliary Surgery Paradigm, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Gong Li; Jiahong Dong, Email [email protected]; [email protected]
Background: Patients with hepatocellular carcinoma (HCC) with portal vein tumour thrombus (PVTT), especially type Vp-4, usually have a poor prognosis. However, the vast majority of Phase III clinical trials exclude this population based on the inclusion criteria. Lenvatinib plus a PD-1 inhibitor has shown promising antitumour activity and tolerable safety in patients with unresectable HCC in Asian populations. Radiotherapy has also demonstrated high response rates and favourable survival for HCC patients with PVTT. This study aimed to explore the preliminary clinical efficacy and safety of lenvatinib plus the PD-1 inhibitor combined with radiotherapy for HCC patients with main portal vein tumour thrombus.
Methods: Between 1 March 2018 and 31 October 2020, HCC patients with main PVTT who received lenvatinib plus a PD-1 inhibitor (pembrolizumab, nivolumab or sintilimab) combined with radiotherapy from Beijing Tsinghua Changgung Hospital in China were reviewed for eligibility. The efficacy was evaluated by the survival and PVTT response rate, and the safety was evaluated by the frequency of key adverse events (AEs).
Results: In total, 39 eligible HCC patients with type Vp-4 PVTT who received triple therapy were included in this study. The 2-year OS rate was 15.4%, which was the primary end-point of our study. The median overall survival (OS) and progression-free survival (PFS) were 9.4 months (range 2.3 to 57.1) and 4.9 months (range 1.4 to 36.1), respectively. The objective response rate (ORR) of PVTT based on mRECIST was 61.5%. AFP dropped to normal 3 months after radiotherapy and was an independent risk factor associated with OS. All AEs were controlled, and no treatment-related deaths occurred.
Conclusion: Lenvatinib plus PD-1 inhibitor combined with radiotherapy had a significant therapeutic effect and manageable AEs in HCC patients with type Vp-4 PVTT and may be a potential treatment option for advanced HCC.
Keywords: hepatocellular carcinoma, portal vein tumour thrombus, lenvatinib, PD-1 inhibitor, radiotherapy
Introduction
Portal vein tumour thrombus (PVTT) occurs very frequently in patients with hepatocellular carcinoma (HCC) because the biological behaviour of HCC results in a strong likelihood of vascular invasion. PVTT incidence varies across countries and regions, and ranges from 13% to 45%.1 HCC patients with PVTT have poor prognosis, with a median survival time associated with the best supportive care of only 4 to 6 months.2,3 The American and ESMO guidelines for the diagnosis and treatment of HCC, which is based on the Barcelona Clinical Liver Cancer (BCLC) staging system, recommend systematic treatment regimens such as targeted drugs and immune drugs.4,5 However, guidelines for the diagnosis and treatment of HCC in Asia such as Asian-Pacific guidelines6 and guidelines from mainland China,7 Korea8 and Taiwan9 all indicate that local therapies (eg, hepatic resection, radiotherapy, TACE and HAIC) may be used as an optional regimen for patients with PVTT in addition to targeted drugs and immunotherapy as the first-line treatment.
In recent years, more treatment options have been developed for patients with HCC with PVTT which has led to significant improvements in patient prognosis.10–13 For example, advancements in radiotherapy technology have realized the use of local radiotherapy as an effective treatment option for PVTT. A study by a Chinese research team confirmed that, compared with surgery alone, surgery after radiotherapy prolonged the progression-free survival (PFS) and overall survival (OS) of liver cancer patients with PVTT.14 Another study from South Korea showed that radiotherapy combined with immunotherapy was associated with longer OS than immunotherapy alone.15 Given these promising results, the Chinese Expert Consensus on cases of HCC with PVTT and the CSCO HCC Treatment Guidelines both regard radiotherapy as the recommended method for PVTT.
New clinical data on antiangiogenic targeted drugs combined with immunotherapy for PVTT have been published. Since the publication of the 2008 SHARP study, there has been a rapid development of systematic drugs for advanced or unresectable HCC. The IMbrave150 study16 included 501 patients with unresectable HCC who received atezolizumab plus bevacizumab or sorafenib. In the subgroup of people with PVTT, the objective response rate (ORR) of the combined treatment regimen reached 23%, and the median PFS and OS were 5.4 months and 7.6 months, respectively, which were superior to the results achieved by sorafenib monotherapy. In addition, some real-world studies have also shown that antiangiogenic targeted drugs combined with PD-1 inhibitors improve the efficacy and survival of HCC patients with PVTT.
However, clinical benefits in patients with type Vp-4 PVTT is unsatisfactory. A real-world study from Japan reported that the postoperative recurrence-free survival according to the degree of PVTT and Vp-4 was only 0.38 years (Vp-1, Vp-2 and Vp-3 were 1.23 years, 0.82 years and 0.56 years, respectively).17 In the IMbrave 150 study, 129 patients with macrovascular invasion demonstrated that the median OS (mOS) was 14.2 months vs 9.7 months (HR: 0.68), which confirmed the effectiveness of immune checkpoint inhibitors (ICIs) combined with VEGF inhibitors in patients with PVTT. However, in 73 patients with Vp-4 PVTT, the OS was 7.6 months.18
An evaluation of radiotherapy combined with lenvatinib plus a PD-1 inhibitor as a treatment regimen for patients with HCC with main PVTT has not yet been conducted. Herein, we aimed to explore the efficacy and safety of triple therapy as the first-line treatment in HCC with main PVTT (type Vp-4).
Materials and Methods
Study Design and Participants
This real-world study investigated the activity and safety of the PD-1 inhibitor lenvatinib plus radiotherapy in patients with HCC with main PVTT at the Beijing Tsinghua Changgung Hospital. The trial protocol, which is available in Figure 1, was approved by the Research Ethics Board of Beijing Tsinghua Changgung Hospital (No. 23223-6-01). All participants provided written informed consent. The primary inclusion criteria were as follows: aged eighteen years or older; HCC a diagnosis based on the American Association for the Study of Liver Diseases practice guideline;19 PVTT diagnosis with Vp4 classification system of Japan, and a Child‒Pugh score≤7. Patients with lymph node and extrahepatic metastases were not excluded. The primary exclusion criteria were as follows: hypersensitivity to Lenvatinib or PD-1 inhibitor components; patients with myocardial ischemia or myocardial infarction of grade II or above and poorly controlled arrhythmias; abnormal coagulation function (prothrombin time>ULN+4 seconds or activated partial thromboplastin time>1.5 ULN, with bleeding tendency or receiving thrombolytic or anticoagulation therapy); pregnant or breastfeeding women; have a history of mental illness or abuse of psychotropic substances; combined HIV-infected; history of liver transplantation; patients with active infection; with contraindications to radiotherapy.
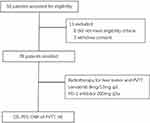 |
Figure 1 Protocol of this study. Abbreviations: OS, overall survival; PFS, progression-free survival; ORR, objective response rate; PVTT, portal vein tumour thrombus; AE, adverse event. |
Procedures
All participants underwent treatment with external beam radiation therapy at the beginning of study enrolment. A CT scan (Discovery 590 RT, GE, USA) was performed with the patient in the supine position using chest-abdominal thermoplastic mask immobilization to reduce uncertainty and to restrain organ motion caused by abdominal breathing. Monaco 5.11 treatment planning systems (Elekta, Sweden) were used to optimize target and normal structure delineation. External RT with volumetric modulated arc therapy (VMAT) treatment plans were used to target the PVTT and primary tumour. The gross tumour volume (GTV) of the PVTT was defined as the tumour volume that was shown as a filling defect in the portal venous phase of the CT scan. The planning target volume (PTV) of the PVTT was expanded to include a margin of 5 mm in the transaxial direction and abdominal-dorsal direction and 10 mm in the cranio-caudal direction. The GTV of the primary tumour was defined as the liver tumour volume that was enhanced in the arterial phase of the CT scan. The clinical tumour volume (CTV) of the primary tumour was generated by adding 5 mm to the GTV in all directions. The PTV of the primary tumour was expanded to include a 5- to 10-mm margin from the CTV to compensate for internal physiologic movements and variations in the size, shape, and position of the CTV. The total dose to the PTV was 32.4–50 Gy, with a fractional size of 1.8–3.0 Gy in 36 participants, and another 3 participants were given 24–30 Gy to PTV with a fractional size of 8–10 Gy using 6-MV X-rays with a linear accelerator (Synergy; Elekta, Sweden). The mean dose to the normal liver (volume of total liver minus GTV) was limited to ≤23 Gy. The maximum allowable point dose to the duodenum and stomach was less than 54 Gy. The maximum allowable point dose to the colon was less than 55 Gy. The maximum point dose of the spinal cord was less than 45 Gy. The kidney volume receiving a dose ≥20 Gy (V20) was <20%.
Participants received the first cycle of PD-1 inhibitor (pembrolizumab, nivolumab or sintilimab) at a fixed dose of 200 mg within 3 days after the completion of radiotherapy. The PD-1 inhibitor was then given every 3 weeks until disease progression, the development of grade 3 or worse immune-related adverse events (AEs), or if the patient withdrew consent. Lenvatinib was started on the first day the PD-1 inhibitor was given after radiotherapy. The daily dose was determined according to body weight (8 mg for bodyweight < 60 kg and 12 mg for bodyweight ≥ 60 kg) until disease progression, the development of grade 3 or worse targeted-related AEs, or withdrawal of consent. Lenvatinib was used until disease progression or the patients reported the symptoms were intolerable, and the PD-1 inhibitors were used for up to 2 years.
All patients underwent baseline contrast-enhanced abdominal MRI or CT. The treatment response evaluation was assessed with MRI/CT after cycle 3 and cycle 6 of the PD-1 inhibitor and every 3 months thereafter (plus or minus 7 days). The Modified Response Evaluation Criteria in Solid Tumours (mRECIST) was used to report radiological imaging. Safety assessments were documented throughout the treatment period. AEs were graded according to the US National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE; version 4.01). AFP concentration and the Child‒Pugh score were measured at baseline, after radiotherapy and every 4 weeks thereafter.
Outcomes
The primary endpoint was the OS rate at 2 years, which was defined as the proportion of participants alive at 2 years. The secondary endpoints were OS, PFS, ORR, and AEs. Tumour and PVTT assessments were evaluated by two independent radiologists according to the mRECIST, and the best responses were documented. The responses were confirmed by assessment after 9 weeks of radiotherapy. OS was defined as the time from radiotherapy to the date of death from any cause; PFS was defined as the time from radiotherapy to the first documented disease progression according to the mRECIST or death from any cause; the ORR was expressed as the percentage of patients who had a complete response or partial response of PVTT 2 months after radiotherapy. Safety was evaluated according to NCI CTCAE, version 4.01. Immune-related AEs were evaluated according to the consensus recommendations from the Society for Immunotherapy of Cancer Toxicity Management Working Group.
Statistical Analysis
Tumour response, survival, and AEs were assessed and analysed in patients who received at least one cycle PD-1 inhibitor after radiotherapy. Baseline characteristics and response rates are expressed in terms of frequencies and percentages, and variables are indicated as either the mean (standard deviation) or median (range). The Kaplan–Meier method was used to estimate PFS, and univariate and multivariate regression analyses were used to analyse the prognostic factors of PFS. All analyses were performed using SPSS version 25.0.
Results
Between 1st March 2018 and 31st October 2020, 50 participants were screened. Eight participants did not meet the eligibility criteria, and 3 participants withdrew consent. Thirty-nine of 50 (78.0%) screened patients were enrolled and received the study treatment (Figure 1). The baseline characteristics of the study participants are shown in Table 1. Most of the patients recruited were male and were Child‒Pugh class A. All participants had main PVTT, and 25.6% of participants had extrahepatic spread. Participants infected with hepatitis B virus (92.3%) were given preemptive antiviral therapy.
 |
Table 1 Patient Demographics and Clinical Characteristics |
At the time of the enrolment period cut-off (Oct 31st, 2022), all patients had completed study treatment. Three patients received a median dose of 24.0 Gy (range 24.0–30.0) in 3 fractions with stereotactic body radiotherapy (SBRT). Thirty-six patients received VMAT with a single dose from 1.8 Gy to 3.0 Gy, and the median biological effective dose (BED) and equivalent dose in 2 Gy/f (EQD2) were 48.0 Gy (range 39.0–78.0) and 40 Gy (range 32.5–65.0), respectively. There were 16, 10 and 13 patients who received pembrolizumab, nivolumab and sintilimab, respectively. The median number of cycles that patients received PD-1 inhibitors was 11 (range 2–34). The median duration that participants received lenvatinib was 33 weeks (range 6–94).
Efficacy
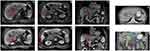
Among 39 participants, the OS of 6 participants was longer than 2 years, and the 2-year OS rate was 15.4%, which was the primary endpoint of our study. The 6 months, 1-year and 2-year OS rates were 71.8%, 33.3% and 15.4%, respectively. The median OS and PFS were 9.4 months (range 2.3 to 57.1) and 4.9 months (range 1.4 to 36.1), respectively (Figures 2 and 3). The PVTT response is shown in Table 2. The confirmed ORR of PVTT was 61.5%, according to a per investigator review. The complete response rate was 5.1%, and the partial response rate was 56.4%. Eight (15.4%) patients had stable disease, and 9 (23.1%) patients had progressive disease. The disease control rate of PVTT was 76.9%. Two patients were evaluated as CR based on the mRECIST criteria after treatment with radiotherapy combined with lenvatinib plus a PD-1 inhibitor, and the results of one patient is shown in Figure 4.
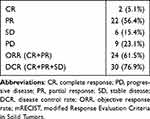 |
Table 2 Radiological Response of PVTT According to mRECIST |
 |
Figure 2 OS and PFS. |
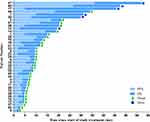 |
Figure 3 Patients survival information. Abbreviations: PFS, progression-free survival; OS, overall survival. |
Safety
We analysed safety data for all 39 patients. Thirty-seven patients (94.9%) experienced at least one treatment-related AE. The main treatment-related AEs (TRAEs) of patients are shown in Table 3. The most common treatment-related AEs of any grade were lymphopenia (84.6%), fatigue (53.8%), nausea/anorexia (43.6%), elevated aminotransferases (38.5%) and leukopenia (35.9%). Treatment-related grade 3 or 4 AEs occurred in 7 (17.9%) participants, and the most common AE was lymphopenia (10.3%). No treatment-related deaths occurred. Immune-related AEs of any grade were observed in 12 (30.8%) of patients. The most common immune-related AE was immune-related hypothyroidism (12.8%).
 |
Table 3 Treatment Related Adverse Events |
Prognostic Factor Analysis
The prognostic factors for OS and PFS are shown in Table 4. Univariate analysis showed that GTV and AFP levels returning to normal 3 months after radiotherapy, and that a reduction in AFP by half 1 month after radiotherapy were factors that influenced OS A multivariate analysis showed that AFP returning to normal 3 months after radiotherapy was an independent risk factor for OS (Figure 5). Patients whose AFP levels returned to normal 3 months after radiotherapy had longer OS than those whose AFP remained higher than normal.
 |
Table 4 Univariate and Multivariate Analyses of Prognostic Factors Affecting OS |
 |
Figure 5 Independent risk factor for OS. Abbreviations: AFP, α-fetoprotein; HR, Hazard Ratio; CI, Confidence Interval. |
Discussion
PVTT is a common phenomenon in HCC patients and usually shows a poor prognosis, particularly in the Vp-4 type patient population. Radiotherapy-antiangiogenesis-immune checkpoint blockade (RAICB) combination therapy has shown apparent clinical efficacy in HCC with PVTT. This real-world study investigated the OS rate at 2 years after a triple combination regimen of systemic therapy (lenvatinib plus PD-1 inhibitor) and locoregional therapy (radiotherapy). The OS was observed to be 15.4%, and there was no serious adverse reactions.
More recently, immune checkpoint blockade (ICB) has emerged as a promising therapeutic option for advanced HCC patients.19 Phase III ICB trials, such as the IMbrave 150 rial and the HIMALAYA trials, which have used antibodies against programmed cell death protein 1 (PD-1), programmed cell death-Ligand 1 (PD-L1) and cytotoxic T-lymphocyte–associated antigen-4 (CTLA-4), have demonstrated clinical benefit and a lower incidence of serious treatment-related AEs in patients with advanced HCC.20,21 Despite the initial success observed with ICB therapies across a broad range of tumours, reduced efficacy and acquired resistance were reported following initial responses to ICB.22,23 Therefore, immunotherapy in combination with other therapies is particularly important.
Radiotherapy (RT) for treating HCC has traditionally been linked to suboptimal results due to limited tolerance of whole liver irradiation and the inability to conform radiation doses to tumours.24 However, recent improved techniques allow for high doses of radiation to be delivered to the tumour while limiting the damage to surrounding healthy tissues. Meanwhile, clinical studies have shown that radiotherapy is sensitive to PVTT. A 2007 clinical study in Japan concluded that preoperative RT applied to PVTT in the main trunk or first branch improves patient prognosis in cases of HCC with PVTT and could be a promising new modality in the treatment of these patients.25 RT has demonstrated good tumour control with 2-year local control rates between 84% and 95%.26,27 However, OS is limited by out-of-field intra- and extrahepatic disease progression,28 highlighting the need for concurrent systemic disease control.
Numerous findings have shown that RT can convert an otherwise “cold” tumour that has low immunogenicity and poorly infiltrated immune cells to an immune-reactive “hot” tumour, which is well infiltrated by immune cells. Traditionally, the rationale behind RT for cancer treatment is to induce lethal DNA damage to tumour cells with high-energy particles, leading to subsequent cell death.29 However, the ability of RT to elicit an immune-mediated antitumour response, a phenomenon known as the “abscopal effect” denoted by the downsizing of nontargeted distant tumours following ionizing radiation treatment, has gained increased prominence in the last decade.30 RT causes immunogenic cell death and cellular stress, which increases the pool of tumour-associated antigens and damage-associated molecular patterns (DAMPs).31 These in turn activate dendritic cells, professional antigen-presenting cells (APCs) that prime tumour-specific CD8+ T cells to further enhance antitumour responses and promote immune cell infiltration into the tumor micro-environment (TME).32
In addition, the VEGF pathway is also an important mechanism for resistance to anti-PD-1 therapy, as it could hamper tumour infiltration and the functions of T effector cells.33,34 Tumours with higher transcriptomic diversity were associated with worse OS in patients treated with ICB, and these tumour cells also expressed a significantly higher level of VEGF-A in patients with HCC.35 A phase Ib clinical study showed that ensartinib, which can block VEGF, combined with a PD-1 inhibitor, has demonstrated good efficacy in the treatment of advanced HCC.36 Although the phase III randomized controlled study (Leap-002) failed to meet the study endpoint, lenvatinib in combination with pembrolizumab resulted in the longest first-line treatment OS (21.2 m) observed to date,37 and remains an important systematic option for treating advanced liver cancer in clinical settings in China.
In this real-world study, we combined radiotherapy with lenvatinib plus PD-1 inhibitors in HCC patients with main PVTT, and observed an objective response rate at 3 months of 63.8%, which showed good local tumour control. The median OS and PFS were 9.4 months and 4.9 months, respectively, which demonstrated good long-term survival. Patients whose AFP returned to normal 3 months after radiotherapy had longer OS. In addition, adverse effects of the triple therapy regimen were acceptable The most serious adverse reactions were lymphopenia, fatigue, nausea/anorexia, elevated aminotransferases and leukopenia.
To our knowledge this is the first study which reports real-world clinical results of treatment using lenvatinib + PD-1 inhibitors combined with radiotherapy for HCC with Vp4 PVTT. The results may provide clinical evidence for future prospective trials. However, there were some limitations to this study. First, this study was conducted in China at a single institution, with a limited sample size and, thus, the findings may be influenced by potential bias. Therefore, further study is required in larger populations in China or other countries and regions. Second, the types of PD-1 inhibitors, the dose of radiotherapy and the radiation pattern were not uniform, which required the results of this study to be validated using well-designed prospective multicentre randomized clinical trials. Third, this study mainly focused on HBV-related HCC, and whether triple therapy can be applied to patients with HCC with other aetiologies needs further research.
In summary, lenvatinib plus PD-1 inhibitors combined with radiotherapy had a significant therapeutic effect and resulted in manageable AEs in HCC patients with main PVTT and, thus, this particular combination therapy may be a promising alternative treatment for this specific subpopulation of HCC patients.
Abbreviations
PD-1, programmed cell death-1; RT, radiotherapy; HCC, hepatocellular carcinoma; PVTT, portal vein tumour thrombus; AEs, adverse events; OS, overall survival; PFS, progression-free survival; ORR, objective response rate; mRECIST, modified response evaluation criteria in solid tumours; AFP, α-fetoprotein; ESMO, European society for medical oncology; BCLC, Barcelona clinical liver cancer; TACE, transcatheter arterial chemoembolization; HAIC, hepatic artery infusion chemotherapy; CSCO, Chinese society of clinical oncology; HR, hazard ratio; ICIs, immune checkpoint inhibitors; VEGF, vascular endothelial growth factor; ULN, upper limit of normal; HIV, human immunodeficiency virus; CT, computed tomography; VMAT, volumetric modulated arc therapy; GTV, gross tumour volume; PTV, planning target volume; CTV, clinical tumour volume; MRI, Magnetic resonance imaging; NCI CTCAE, National cancer institute common terminology criteria for adverse events; SBRT, stereotactic body radiotherapy; BED, biological effective dose; EQD2, equivalent dose in 2 Gy/f; CR, complete response; TAREs, treatment-related adverse events; RAICB, radiotherapy-antiangiogenesis-immune checkpoint blockade; PD-L1, programmed cell death-Ligand 1; CTLA-4, cytotoxic T-lymphocyte–associated antigen-4 (CTLA-4), ICB, immune checkpoint blockade; DNA, Deoxyribonucleic acid; DAMPs, damage-associated molecular patterns; APCs, antigen-presenting cells; TME, tumour micro-environment.
Ethics Statement
This study has been approved by the ethics committee of Beijing Tsinghua Changgung Hospital (No. 23223-6-01). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Acknowledgement
Guangxin Li, Ying Zhao, and Keren Li are co-first authors for this study. We would like to thank Wenting Chen from MSD medical affairs for his scientific support on this manuscript and thank all the other investigators who have contributed to this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Precision Medicine Research Program of Tsinghua University (No. 10001020111).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Deng ZJ, Li L, Teng YX, et al. Treatments of hepatocellular carcinoma with portal vein tumor thrombus: current status and controversy. J Clin Transl Hepatol. 2022;10(1):147–158. doi:10.14218/JCTH.2021.00179
2. Chan SL, Chong CC, Chan AW, Poon DM, Chok KS. Management of hepatocellular carcinoma with portal vein tumor thrombosis: review and update at 2016. World J Gastroenterol. 2016;22(32):7289–7300. doi:10.3748/wjg.v22.i32.7289
3. Xiang X, Lau WY, Wu ZY, et al. Transarterial chemoembolization versus best supportive care for patients with hepatocellular carcinoma with portal vein tumor thrombus: a multicenter study. Eur J Surg Oncol. 2019;45(8):1460–1467. doi:10.1016/j.ejso.2019.03.042
4. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
5. Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):871–873. doi:10.1093/annonc/mdy510
6. Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. doi:10.1007/s12072-017-9799-9
7. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
8. Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver cancer association-national cancer center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver. 2019;13(3):227–299. doi:10.5009/gnl19024
9. Shao YY, Wang SY, Lin SM. Diagnosis Systemic Therapy G. Management consensus guideline for hepatocellular carcinoma: 2020 update on surveillance, diagnosis, and systemic treatment by the Taiwan liver cancer association and the gastroenterological society of Taiwan. J Formos Med Assoc. 2021;120(4):1051–1060. doi:10.1016/j.jfma.2020.10.031
10. Jiang JF, Lao YC, Yuan BH, et al. Treatment of hepatocellular carcinoma with portal vein tumor thrombus: advances and challenges. Oncotarget. 2017;8(20):33911–33921. doi:10.1038/s41575-020-00395-0
11. Josep ML, Thierry DB, Laura K, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(5):293–313. doi:10.1038/s41575-020-00395-0
12. Francesco T, Giulia N, Francesca B, Chiara F, Elisabetta G, Alessandro G. Systemic treatments for hepatocellular carcinoma: challenges and future perspectives. Hepat Oncol. 2018;5(1):HEP01. doi:10.2217/hep-2017-0020
13. Yin J, Bo WT, Sun J, et al. New evidence and perspectives on the management of hepatocellular carcinoma with portal vein tumor thrombus. J Clin Transl Hepatol. 2017;5(2):169–176. doi:10.14218/JCTH.2016.00071
14. Wei XB, Jiang YB, Zhang XP, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol. 2019;37(24):2141–2151. doi:10.1200/JCO.18.02184
15. Yu JI, Lee SJ, Lee JY, et al. Clinical significance of radiotherapy before and/or during nivolumab treatment in hepatocellular carcinoma. Cancer Med. 2019;8(6):6986–6994. doi:10.1002/cam4.2570
16. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
17. Kokudo T, Hasegawa K, Matsuyama Y, et al. Liver cancer study group of Japan. survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016:
18. Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi:10.1016/j.jhep.2021.11.030
19. Sangro B, Park J-W, Cruz CMD, et al. A randomized, multicenter, Phase 3 study of nivolumab vs sorafenib as first line treatment in patients (pts) with advanced hepatocellular carcinoma (HCC): checkMate-459. J Clin Oncol. 2016;34(15_suppl):TPS4147–TPS4147. doi:10.1200/JCO.2016.34.15_suppl.TPS4147
20. Cheng A-L, Qin S, Ikeda M, et al. LBA3IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30(Supplement9):ix183–202. doi:10.1093/annonc/mdz446.002
21. Yau T, Kang Y-K, Kim T-Y, et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J Clin Oncol. 2019;37(15_suppl):4012. doi:10.1200/JCO.2019.37.15_suppl.4012
22. Wang Q, Wu X. Primary and acquired resistance to PD-1/PD-L1 blockade in cancer treatment. Int Immunopharmacol. 2017;46:210–219. doi:10.1016/j.intimp.2017.03.015
23. Flynn MJ, Larkin JMG. Novel combination strategies for enhancing efficacy of immune checkpoint inhibitors in the treatment of metastatic solid malignancies. Expert Opin Pharmacother. 2017;18(14):1477–1490. doi:10.1080/14656566.2017.1369956
24. Ohri N, Dawson LA, Krishnan S, et al. Radiotherapy for Hepatocellular Carcinoma: new Indications and Directions for Future Study. J Natl Cancer Inst. 2016;108(9):djw133. doi:10.1093/jnci/djw133
25. Kamiyama T, Nakanishi K, Yokoo H, et al. Efficacy of preoperative radiotherapy to portal vein tumor thrombus in the main trunk or first branch in patients with hepatocellular carcinoma. Int J Clin Oncol. 2007;12(5):363–368. doi:10.1007/s10147-007-0701-y
26. Wahl DR, Stenmark MH, Tao Y, et al. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J Clin Oncol off J Am Soc Clin Oncol. 2016;34(5):452–459. doi:10.1200/JCO.2015.61.4925
27. Kang J-K, Kim M-S, Cho CK, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012;118(21):5424–5431. doi:10.1002/cncr.27533
28. Bujold A, Massey CA, Kim JJ, et al. Sequential Phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31(13):1631–1639. doi:10.1200/JCO.2012.44.1659
29. ArnoldKM F, RabenA RL, YuY DAP, et al. The Impact of Radiation on the Tumor Microenvironment: effect of Dose and Fractionation Schedules. Cancer Growth Metastasis. 2018;11:1179064418761639. doi:10.1177/1179064418761639
30. Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41(6):503–510. doi:10.1016/j.ctrv.2015.03.011
31. Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4–dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi:10.1038/nm1622
32. Lee YH, Tai D, Yip C, Choo SP, Chew V. Combinational immunotherapy for hepatocellular carcinoma: radiotherapy, immune checkpoint blockade and beyond. Front Immunol. 2020;11(1):1–13. doi:10.3389/fimmu.2020.568759
33. Ohm JE, Gabrilovich DII, Sempowski GD, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101(12):4878–4886. doi:10.1182/blood-2002-07-1956
34. Motz GT, Santoro SP, Wang LP, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20(6):607–615. doi:10.1038/nm.3541
35. Ma L, Hernandez MO, Zhao Y, et al. Tumor Cell Biodiversity Drives Microenvironmental Reprogramming in Liver Cancer. Cancer Cell. 2019;36(4):418–430.e6. doi:10.1016/j.ccell.2019.08.007
36. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38:2960–2970. doi:10.1200/JCO.20.00808
37. Finn RS, Kudo M, Merle P, et al. Primary Results from the Phase 3 LEAP-002 Study: lenvatinib Plus Pembrolizumab Versus Lenvatinib as First-line Therapy for Advanced Hepatocellular Carcinoma. Ann Oncol. 2022;33:S1401.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.