Back to Journals » Infection and Drug Resistance » Volume 15
Effectiveness and Safety of Sofosbuvir-Velpatasvir in Patients with Cirrhosis Associated with Genotype 3 Hepatitis C Infection in Xinjiang, China
Authors Abulitifu Y, Lian J, Adilijiang M, Liu L, Zhao F, Qian W, Zhang Y
Received 5 August 2022
Accepted for publication 20 October 2022
Published 3 November 2022 Volume 2022:15 Pages 6463—6470
DOI https://doi.org/10.2147/IDR.S385071
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Yilihamu Abulitifu,1 Jiangshan Lian,2 Munire Adilijiang,1 Lan Liu,1 Fengcong Zhao,1 Wen Qian,1 Yongping Zhang1
1Department of Infectious Diseases, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, Xinjiang, People’s Republic of China; 2Department of Infectious Diseases, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China
Correspondence: Yongping Zhang, Department of Infectious Diseases, People’s Hospital of Xinjiang Uygur Autonomous Region, No. 91, Tianchi Road, Urumqi, Xinjiang, 830001, People’s Republic of China, Tel +86 13999233386, Fax +86 991 8563585, Email [email protected]
Purpose: Patients with cirrhosis from genotype 3 (GT3) hepatitis C virus (HCV) infection are difficult to cure. This study investigated the effectiveness and safety of sofosbuvir-velpatasvir (SOF/VEL) with and without ribavirin (RBV) in patients with GT3 HCV-infection-related cirrhosis from Xinjiang, China.
Patients and Methods: This study included 33 patients with GT3 HCV infected cirrhosis, who were treated with either SOF/VEL+RBV for 12 weeks (n = 27) or SOF/VEL alone for 24 weeks (n = 6) between January 2019 and June 2021. The primary endpoint was a sustained virological response at 12 weeks (SVR12), post-treatment. Secondary endpoints included changes from baseline in Child-Pugh-Turcotte scores, clinical results, hepatic-encephalopathy status, ascites, and gastrointestinal bleeding at 12 weeks, post-treatment.
Results: Out of the 33 patients, 18 (54.6%) were diagnosed with GT3a, 15 (45.4%) with GT3b, 16 (48.5%) with compensated cirrhosis, and 17 (51.5%) with decompensated cirrhosis. SVR12 was 87.9% (compensated cirrhosis: 93.8%, decompensated cirrhosis: 82.4%). The Child-Pugh-Turcotte scores improved at 12 weeks (p < 0.05). Total bilirubin, albumin, and alanine transaminase levels, as well as hepatic-encephalopathy were significantly improved among patients with compensated and decompensated cirrhosis (p < 0.05). The blood cell count and serum creatinine levels did not deteriorate.
Conclusion: SOF/VEL, with and without RBV, was effective, safe, and well-tolerated as a treatment for GT3 HCV associated cirrhosis.
Keywords: hepatitis C, genotype 3, cirrhosis, sofosbuvir-velpatasvir, ribavirin
Introduction
Chronic hepatitis C virus (HCV) infection is a major global health problem. Approximately 58 million people are infected with HCV worldwide, with about 1.5 million new infections occurring per year, and the mortality rate is approximately 290,000 per year, mostly from cirrhosis and hepatocellular carcinoma.1 In mainland China, HCV prevalence in the general population was 1.0%.2 Infected individuals are at risk of progressive liver disease, liver cirrhosis, and hepatocellular carcinoma. Genotype data are widely collected because HCV genotype is important for predicting disease progression. In China, the most prevalent genotypes and subtypes are 1b (52.18%), 2a (28.69%), 3b (7.06%), 6a (6.41%), and 3a (4.62%), accounting for 98.84% of the tested patient samples.3 In Xinjiang, China, the most prevalent genotypes and subtypes are 1b (53.1%), 2a (24.1%), 3a (14.3%), 3b (7.9%), and 6a (0.6%).4 Genotype 3 (GT3) HCV is associated with a higher risk of liver fibrosis, cirrhosis, and cancer, compared with other HCV genotypes.5–7 Although the recent discovery of direct-acting antivirals (DAAs) has revolutionized treatment, that results in most patients achieving a sustained virologic response (SVR) greater than 95%.8,9 Despite these advances, GT3 HCV infected individuals, particularly those with cirrhosis, still exhibit an impaired SVR rate.10 Therefore, real-world data are crucial for a better understanding of treatment outcomes.
Various international guidelines recommend two pan-genotypic DAA regimens, glecaprevir/pibrentasvir (GLE/PIB) and sofosbuvir/velpatasvir (SOF/VEL), as first-line treatments for GT3 HCV patients. In China, SOF/VEL and sofosbuvir/ledipasvir are DAAs approved by the National Health Insurance Agency for treating these patients,11–13 and GLE/PIB is not eligible for insurance reimbursements. Hence, SOF/VEL administration is dependent on physician decisions in accordance with drug availability and price. Previous real-world data from Western cohorts reported high SVR (80–100%) among GT3 HCV-infected cirrhotic patients treated with SOF/VEL ± RBV.14–17 A recent real-world study from Singapore showed that the SVR12 was 99.3% and 88.2% for GT3 HCV-related compensated cirrhosis and decompensated cirrhosis, respectively, when treated with SOF/VEL ± RBV.18 In China, treatment strategies for GT3a-infected patients can be formulated based on recommendations in international guidelines and current clinical data, but there are insufficient data to make recommendations for GT3b-infected patients, especially patients with GT3b HCV-related cirrhosis as a difficult-to-treat population.19 More clinical trials needed to evaluate various regimens and then to determine the optimal ones in this group. To address the relative lack of real-world studies that assess the effectiveness and safety of SOF/VEL, with or without RBV, in these patients, particularly from non-Western populations, we investigated this question in a cohort from Xinjiang.
Materials and Methods
Patients and Treatment
The study enrolled patients older than 18 years of age with GT3 HCV infected cirrhosis, who received either SOF/VEL alone (Epclusa®, fixed-dose combination 400/100 mg per tablet; Gilead Sciences, Carrigtohill, Ireland) or including ribavirin (1000 mg/day if weight <75 kg, 1200 mg/day if weight ≥75 kg) for 24 and 12 weeks, respectively. Participants were admitted to the People’s Hospital of Xinjiang Uygur Autonomous Region between January 2019 and June 2021. Treatment regimens were in accordance with the guidelines from the European Association for the Study of the Liver, 2020, AASLD-IDSA Hepatitis C Guidance, 2020, and Chinese Society of Hepatology and Chinese Society of Infectious Diseases.11–13 Patients who met one of the following criteria were excluded: <18 years old, diagnosed with chronic hepatitis C without cirrhosis, coinfection with hepatitis A/B/E or human immunodeficiency virus, autoimmune hepatitis, primary biliary cholangitis or primary sclerosing cholangitis, non-alcoholic fatty liver cirrhosis, alcoholic fatty liver cirrhosis, drug-induced cirrhosis, and genetic liver diseases.
The study was approved by the Ethics Committee of the People’s Hospital of the Xinjiang Uygur Autonomous Region and performed in accordance with the Declaration of Helsinki. The requirement for written informed consent was waived because of the retrospective design, anonymous data use, and elimination of patient-identifying information.
Measurements
Detailed medical history was recorded for each patient. All patients underwent a clinical examination, and baseline laboratory values were collected at the beginning of treatment. The following data were recorded at baseline, end of treatment, and during week 12 post-treatment: baseline demographics (age, sex, and nationality), leukocytes, hemoglobin (lower limit of normal [LLN], 130 g/L for men and 115 g/L for women), platelets, serum albumin, total bilirubin (upper limit of normal [ULN], 26 μmol/L for men and 21 μmol/L for women), alanine transaminase (ALT) (ULN, 60 U/L for men and 45 U/L for women), creatinine (ULN, 97 μmol/L for men and 73 μmol/L for women), plasma prothrombin time, and HCV RNA. Serum HCV RNA was quantified using reverse transcription-polymerase chain reaction (RT-PCR) using the Cobas AmpliPrep/COBAS TaqMan HCV Test (Roche Diagnostics, Branchburg, NJ, USA), which has a lower limit of quantitation at 15 IU/mL. The HCV genotype was determined using RT-PCR with genotype-specific primers from the five noncoding regions of the virus. The presence of cirrhosis was confirmed with liver biopsy showing cirrhosis, transient elastography (FibroScan) higher than 12.5kPa or FibroTest higher than 0.75 and an aspartate aminotransaminase to platelet ratio index (APRI) higher than 2 or Fibrosis-4 (FIB-4) higher than 3.25. Hepatic imaging examinations which included ultrasonography, computed tomography or magnetic resonance imaging were also used for diagnosis of cirrhosis. Ascites was confirmed with clinical features or hepatic imaging. Hepatic-encephalopathy (HE) was diagnosed following Chinese guidelines on HE management in cirrhosis.20 Esophageal and gastric variceal bleeding (EGVB) was diagnosed using gastroscopy. Child-Pugh-Turcotte (CPT) scores were calculated for all patients.
Efficacy Assessments
The primary endpoint was SVR12. The secondary endpoint was a change in CPT scores at week 12 post-treatment from baseline, along with undetectable levels of HCV RNA, defined as <15 IU/mL at the end of treatment (SVR0) and post-treatment week 12 (SVR12).
Safety Assessments
Adverse events associated with SOF/VEL with or without RBV in real-world settings were examined, and adverse hematological reactions including neutropenia, anemia, etc. were recorded. Changes of HE, ascites, and gastrointestinal bleeding before and after treatment were recorded.
Statistical Analysis
Categorical (nominal or ordinal) data were expressed as percentages, and continuous data as means ± standard deviation (SD). Continuous variables were analyzed using Student’s paired or unpaired t-tests. Nominal variables were analyzed using the χ2 test or McNemar’s test. Ordinal variables were analyzed using the Wilcoxon signed-rank test. All statistical tests were two-tailed. Significance was set at p < 0.05. Analyses were performed in SPSS Statistics version 23.0 (IBM Corp., Armonk, NY, USA). Graphs were constructed in GraphPad Prism 5.1 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Patient Characteristics
The mean age of the enrolled patients (n = 33) was 45.64 ± 4.75 years, and 72.7% (n = 24) were Male (Table 1). Out of the 33 patients, seven (21.2%) were Han, 16 (48.5%) were Uygur, nine (27.3%) were Hui, and one (3.0%) was Kazakh. Among the enrolled patients (n = 33), 18 (54.6%) had GT3a, 15 (45.4%) had GT3b, 16 (48.5%) had compensated cirrhosis, and 17 (51.5%) had decompensated cirrhosis. Six patients (18.2%) received SOF/VEL for 24 weeks and the remaining patients received SOF/VEL+RBV for 12 weeks (Table 1).
 |
Table 1 Baseline Characteristics of GT3 HCV Infected Cirrhosis |
Treatment Effectiveness
Virologic Response
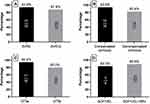
The SVR0 was 93.9% (31/33) and overall SVR12 was 87.9% (29/33). For compensated and decompensated cirrhosis, SVR12 was 93.8% (15/16) and 82.4% (14/17), respectively. The SVR12 for GT3a and GT3b patients was 94.4% (17/18) and 80.0% (12/15), respectively. Among patients treated with SOF/VEL, SVR12 was 83.3% (5/6); among patients treated with SOF/VEL+RBV, SVR12 was 88.9% (24/27) (Figure 1). Four patients experienced virological failure; one had GT3a and decompensated cirrhosis treated with SOF/VEL, while another had GT3b and decompensated cirrhosis treated with SOF/VEL+RBV, in both these cases, HCV RNA remained detectable at the end of treatment. The remaining two were GT3b patients, one with compensated cirrhosis and the other with decompensated. Both were treated with SOF/VEL+RBV for 12 weeks and achieved SVR0 but relapsed at week 12 post-treatment (Table 2).
 |
Table 2 Characteristics of 4 Virologic Failure Patients |
Changes in Liver Function
The proportion of CPT class A patients increased from 51.5% to 75.8%, whereas proportions of class B and C patients decreased from 30.3% to 18.2% and from 18.2% to 6.1%, respectively (Table 3). Among the 17 decompensated cirrhotic patients, six individuals, who were class B at baseline, improved to class A, and four baseline class C individuals improved to class B or A (Table 4). Mean CPT score significantly improved, from 7.12 ± 2.40 points at baseline to 5.85 ± 1.50 points at 12 weeks, post-treatment (Table 5). No patient regressed in their CPT class.
 |
Table 3 Comparison of GT3 HCV Infected Cirrhosis Before and After Treatment |
 |
Table 4 Comparison of GT3 HCV Decompensated Cirrhosis Before and After Treatment |
 |
Table 5 Comparison of GT3 HCV Cirrhosis and Decompensated Cirrhosis Before and After Treatment |
Safety
Patient groups did not differ significantly in pre- and post-treatment values of leukocytes, hemoglobin, platelets, creatinine, ascites, and EGVB. At 12 weeks post-treatment, total bilirubin, albumin, ALT levels, and HE improved significantly from baseline for cirrhotic patients, including those with decompensated cirrhosis (P < 0.05) (Table 5). No deaths, hepatocellular carcinoma, severe adverse events, drug-related adverse events, or drug-related discontinuation were recorded.
Discussion
In this study, SOF/VEL ± RBV was highly effective, generally safe, and well-tolerated in Chinese patients with GT3 HCV-related cirrhosis. Out of a total of 33 patients, 27 (81.8%) were treated with SOF/VEL+RBV for 12 weeks, and six (18.2%) received SOF/VEL only for 24 weeks because of RBV intolerance. The decision to add RBV or extend treatment duration was at the physician’s discretion, with consideration of cirrhotic status. We found that RBV addition resulted in an SVR12 of 88.9%, whereas the lack of RBV led to an SVR12 of 83.3%; therefore, RBV seems to improve the treatment effectiveness of SOF/VEL for GT3 HCV-infected cirrhotic patients. Our findings indicated that SOF/VEL+RBV 12 weeks should be the first choice for these patients, SOF/VEL+RBV 24 weeks are worth exploring further.
The presence of cirrhosis seems to influence the effectiveness of SOF/VEL. Previous study revealed that despite the availability of pan genotypic, strong therapeutic options, GT3 infected patients with cirrhosis still remain difficult to treat.21 Though GLE/PIB could be a good option in HCV GT3 patients including HCV GT3 with compensated cirrhosis patients,21,22 it is clearly forbidden to be administered in decompensated cirrhosis patients due to the presence of protease inhibitor, hence, SOF/VEL remains the only option. To achieve a high SVR rate for those relatively difficult to treat population, extending the duration of treatment to 24 weeks or adding ribavirin are worth confirming. Esteban et al reported that a higher rate of patients with GT3 HCV infection and compensated cirrhosis achieved SVR12 with SOF/VEL+RBV 12 weeks than SOF/VEL monotherapy (96% vs 91%) 12 weeks, and RBV addition could ameliorate the negative influence of baseline NS5A RASs.23 A recent Phase III study, testing SOF/VEL in Chinese patients with GT3, showed that the SVR12 was 90% and 100% for GT3a patients with and without compensated cirrhosis, respectively. The percentages dropped to 50% and 96%, respectively, for GT3b patients with and without compensated cirrhosis.24 Our SVR12 rates were much higher, at 94.4% and 80.0% for GT3a HCV cirrhosis and GT3b HCV cirrhosis, respectively, suggesting that RBV might be needed to improve SVR among GT3 HCV cirrhotic patients. To date, only two-phase III studies are available regarding the administration of SOV/VEL with or without RBV for patients with decompensated cirrhosis: ASTRAL-4 for CPT B patients and another from Japan. The ASTRAL-4 study found that overall SVR12 was 94% for those treated with 12 weeks of SOF/VEL+RBV, and 86% for those treated with 24 weeks of SOF/VEL; these rates dropped to 85% and 50% among GT3 patients.25 Our results were similar with an SVR12 of 82.4% for GT3 HCV-decompensated cirrhosis, indicating that SOF/VEL with or without RBV is highly effective in patients with this condition. In the Japanese phase III study, overall SVR12 was 92% for 12 weeks of SOF/VEL with or without RBV.26 However, the previous study focused on patients with GT1b or GT2 HCV, our study only focused on GT3 HCV patients. A recent real-world study from Singapore18 showed that 163 HCV GT3 cirrhosis patients (10% decompensated cirrhosis) were treated with SOF/VEL ± RBV, the SVR12 was 88.2% (15/17) for GT3 HCV-decompensated cirrhosis, interestingly, this study comprised a low percentage of GT3b (0.4%). Although the total sample size in our study was relatively small, the number of patients with GT3 HCV-decompensated cirrhosis was 17 (51.5%) in our study, consistently with the prior Singapore study, and the number of HCV GT3b in our study was 15 contributed to 45.4% of all the patients, which were much higher than that of the above study. With the approval of Sofosbuvir/Velpatasvir/Voxilaprevir (SOF/VEL/VOX) in China, SOF/VEL/VOX has become accessible and affordable, providing more options for the treatment of HCV GT3 patients. POLARIS-3 study had also shown that SOF/VEL/VOX 8 weeks treatment could achieve 96% SVR12 in patients with HCV GT3 with compensated cirrhosis,27 but physicians should note that SOF/VEL/VOX contains protease inhibitor (VOX) and is contraindicated in patients with decompensated disease or a history of prior decompensation. In addition, the approved indication of SOF/VEL/VOX in China is for the patients who have experienced DAA treatment failure.
Interferon-free DAA-based regimens have only recently become available for treating HCV infection. Thus, their clinical benefits in patients with decompensated cirrhosis are still being characterized. In our study overall mean CPT score significantly improved post-treatment, including 4 patients with virological failure; these findings are consistent with previous research.28 Achieving SVR in patients with decompensated cirrhosis should have long-term benefits, with several studies reporting a decrease in mortality after comparing the survival rates of patients treated with sofosbuvir-based regimens to matched controls from transplant waitlists.29
Considering that the safety of the treatment is important due to the advanced nature of liver disease with high morbidity and mortality, we found no significant difference in leukocyte, hemoglobin, platelet, and creatinine levels from baseline to week 12. Furthermore, these patients exhibited a significant improvement in the levels of total bilirubin, albumin, and ALT, along with a significant improvement in HE. The antiviral treatment can restore liver function; therefore, cirrhosis-related complications may also be ameliorated.
Our study has several limitations. First, inherent bias could not be excluded owing to the retrospective nature of the study, which was limited by the sample size. Therefore, these results should be verified by conducting a prospective clinical study with a larger cohort. Second, although we observed early improvements in liver function, the mid- and long-term clinical benefits of achieving SVR12 in patients with cirrhosis remain unclear and need to be further explored.
Conclusion
In conclusion, GT3 HCV patients with cirrhosis, including decompensated cirrhosis, achieved high SVR12 when subjected to 12 or 24 weeks of SOF/VEL with or without RBV, respectively. These treatment regimens also improved liver function while being safe and well-tolerated.
Acknowledgments
We would like to acknowledge: Department of Infectious Diseases of People’s Hospital of Xinjiang Uygur Autonomous Region, administrators and staff of each data collectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. Geneva, Switzerland: World Health Organization; 2016. Available from: https://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-chi.pdf.
2. Cui Y, Jia J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol. 2013;28(Suppl 1):7–10. doi:10.1111/jgh.12220
3. Chen Y, Yu C, Yin X, et al. Hepatitis C virus genotypes and subtypes circulating in Mainland China. Emerg Microbes Infect. 2017;6(11):e95. doi:10.1038/emi.2017.77
4. Yilihamu A, Weng Q, Zhang Y. Genotyping characteristics of hepatitis C virus and its relation with the progress of hepatitis C in Xinjiang. Man Xing Bing Xue Za Zhi. 2020;2020(5):670–673.
5. Gondeau C, Pageaux GP, Larrey D. Hepatitis C virus infection: are there still specific problems with genotype 3? World J Gastroenterol. 2015;21(42):12101–12113. doi:10.3748/wjg.v21.i42.12101
6. Probst A, Dang T, Bochud M, et al. Role of hepatitis C virus genotype 3 in liver fibrosis progression--a systematic review and meta-analysis. J Viral Hepat. 2011;18(11):745–759. doi:10.1111/j.1365-2893.2011.01481.x
7. Shahnazarian V, Ramai D, Reddy M, et al. Hepatitis C virus genotype 3: clinical features, current and emerging viral inhibitors, future challenges. Ann Gastroenterol. 2018;31(5):541–551. doi:10.20524/aog.2018.0281
8. Chung RT, Ghany MG, Kim AY; AASLD-IDSA HCV Guidance Panel. Hepatitis C guidance 2018 update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin Infect Dis. 2018;67(10):1477–1492. doi:10.1093/cid/ciy585
9. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69(2):461–511. doi:10.1016/j.jhep.2018.03.026
10. Tapper EB, Afdhal NH. Is 3 the new 1: perspectives on virology, natural history and treatment for hepatitis C genotype 3. J Viral Hepat. 2013;20(10):669–677. doi:10.1111/jvh.12168
11. Chinese Society of Hepatology, & Chinese Society of Infectious Diseases, Chinese Medical Association. Guidelines for the prevention and treatment of hepatitis C (2019 version). Zhonghua Gan Zang Bing Za Zhi. 2019;27:962–979. doi:10.3760/cma.j.issn.1007-3418.2019.12.008
12. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: final update of the series. J Hepatol. 2020;73(5):1170–1218. doi:10.1016/j.jhep.2020.08.018
13. Ghany MG, Morgan TR; AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2020;71(2):686–721. doi:10.1002/hep.31060
14. Belperio PS, Shahoumian TA, Loomis TP, et al. Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3. J Hepatol. 2019;70(1):15–23. doi:10.1016/j.jhep.2018.09.018
15. Mangia A, Cenderello G, Copetti M, et al. SVR12 higher than 97% in GT3 cirrhotic patients with evidence of portal hypertension treated with SOF/VEL without ribavirin: a nation-wide cohort study. Cells. 2019;8(4):313. doi:10.3390/cells8040313
16. von Felden J, Vermehren J, Ingiliz P, et al. High efficacy of sofosbuvir/velpatasvir and impact of baseline resistance-associated substitutions in hepatitis C genotype 3 infection. Aliment Pharmacol Ther. 2018;47(9):1288–1295. doi:10.1111/apt.14592
17. Wilton J, Wong S, Yu A, et al. Real-world effectiveness of sofosbuvir/velpatasvir for treatment of chronic hepatitis C in British Columbia, Canada: a population-based cohort study. Open Forum Infect Dis. 2020;7(3):ofaa055. doi:10.1093/ofid/ofaa055
18. Wong YJ, Thurairajah PH, Kumar R, et al. Efficacy and safety of sofosbuvir/velpatasvir in a real-world chronic hepatitis C genotype 3 cohort. J Gastroenterol Hepatol. 2021;36(5):1300–1308. doi:10.1111/jgh.15324
19. Wang X, Wei L. Direct-acting antiviral regimens for patients with chronic infection of hepatitis C virus genotype 3 in China. J Clin Transl Hepatol. 2021;9(3):419–427. doi:10.14218/JCTH.2020.00097
20. Xu XY, Ding HG, Li WG, et al. Chinese guidelines on management of hepatic encephalopathy in cirrhosis. World J Gastroenterol. 2019;25(36):5403–5422. doi:10.3748/wjg.v25.i36.5403
21. Zarębska-Michaluk D, Jaroszewicz J, Parfieniuk-Kowerda A, et al. Effectiveness and safety of pangenotypic regimens in the most difficult to treat population of genotype 3 HCV infected cirrhotics. J Clin Med. 2021;10(15):3280. doi:10.3390/jcm10153280
22. Soria A, Fava M, Bernasconi DP, et al. Comparison of three therapeutic regimens for genotype-3 hepatitis C virus infection in a large real-life multicentre cohort. Liver Int. 2020;40(4):769–777. doi:10.1111/liv.14386
23. Esteban R, Pineda JA, Calleja JL, et al. Efficacy of Sofosbuvir and Velpatasvir, with and without ribavirin, in patients with hepatitis C virus genotype 3 infection and cirrhosis. Gastroenterology. 2018;155(4):1120–1127.e4. doi:10.1053/j.gastro.2018.06.042
24. Wei L, Lim SG, Xie Q, et al. Sofosbuvir-velpatasvir for treatment of chronic hepatitis C virus infection in Asia: a single-arm, open-label, Phase 3 trial. Lancet Gastroenterol Hepatol. 2019;4(2):127–134. doi:10.1016/S2468-1253(18)30343-1
25. Curry MP, O’Leary JG, Bzowej N, et al. Sofosbuvir and Velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015;373(27):2618–2628. doi:10.1056/NEJMoa1512614
26. Takehara T, Sakamoto N, Nishiguchi S, et al. Efficacy and safety of sofosbuvir-velpatasvir with or without ribavirin in HCV-infected Japanese patients with decompensated cirrhosis: an open-label phase 3 trial. J Gastroenterol. 2019;54(1):87–95. doi:10.1007/s00535-018-1503-x
27. Jacobson IM, Lawitz E, Gane EJ, et al. Efficacy of 8 weeks of Sofosbuvir, Velpatasvir, and Voxilaprevir in patients with chronic HCV infection: 2 phase 3 randomized trials. Gastroenterology. 2017;153(1):113–122. doi:10.1053/j.gastro.2017.03.047
28. Flamm SL, Lawitz E, Borg B, et al. High efficacy and improvement in CPT class with sofosbuvir/velpatasvir plus ribavirin for 12 weeks in patients with CPT C decompensated cirrhosis. Gastroenterology. 2019;156:S–1219. doi:10.1016/S0016-5085(19)40032-2
29. Kwong A, Kim WR, Mannalithara A, et al. Decreasing mortality and disease severity in hepatitis C patients awaiting liver transplantation in the United States. Liver Transpl. 2018;24(6):735–743. doi:10.1002/lt.24973
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.