Back to Journals » Lung Cancer: Targets and Therapy » Volume 15
Effectiveness and Safety of Anlotinib Combined with PD-1 Blockades in Patients with Previously Immunotherapy Treated Advanced Non-Small Cell Lung Cancer: A Retrospective Exploratory Study
Authors Dou XJ, Ma RY, Ren DW, Liu Q, Yan P
Received 15 October 2023
Accepted for publication 18 February 2024
Published 25 March 2024 Volume 2024:15 Pages 29—40
DOI https://doi.org/10.2147/LCTT.S444884
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sai-Hong Ignatius Ou
Xue-Jun Dou,1,* Run-Yang Ma,1,* De-Wang Ren,1 Qiang Liu,2 Peng Yan3
1Department of Thoracic Surgery, Aerospace Center Hospital, Beijing, 100049, People’s Republic of China; 2Department of Thoracic Surgery, Peking University International Hospital, Beijing, 102206, People’s Republic of China; 3Department of Respiratory Medicine, China Aerospace Science & Industry Corporation 731 Hospital, Beijing, 100071, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiang Liu, Department of Thoracic Surgery, Peking University International Hospital, Beijing, 102206, People’s Republic of China, Tel +86 15901472799, Email [email protected] Peng Yan, Department of Respiratory Medicine, China Aerospace Science & Industry Corporation 731 hospital, Beijing, 100071, People’s Republic of China, Email [email protected]
Objective: This study aimed to investigate the effectiveness and tolerability of anlotinib plus PD-1 blockades in patients with previously immunotherapy treated advanced non-small-cell lung cancer (NSCLC).
Methods: A total of 67 patients with previously immunotherapy treated advanced NSCLC who received anlotinib plus PD-1 blockades in clinical practice were screened retrospectively. All the PD-1 blockades used in this study were approved in China and consisted of sintilimab, camrelizumab, tislelizumab and pembrolizumab. Effectiveness and safety of anlotinib plus PD-1 blockades were assessed, and all patients were followed up regularly. Clinical significance between response status to previous immune-related treatment regimens and therapeutic outcomes of anlotinib plus PD-1 blockades was further explored.
Results: The best overall response among the 67 patients suggested that a partial response was observed in 16 patients, stable disease was noted in 41 patients and progressive disease was found in 10 patients, which yielded an objective response rate of 23.9% (95% CI: 14.3– 35.9%) and a disease control rate of 85.1% (95% CI: 74.3– 92.6%). Prognostic outcomes indicated that the median progression-free survival (PFS) was 6.1 months (95% CI: 2.37– 9.83) and the median overall survival (OS) was 16.5 months (95% CI: 10.73– 22.27). Exploratory analysis highlighted that patients who were intolerant to previous immune-related regimens (17 patients) might have a superior prognosis (median OS: 22.3 months vs 12.5 months, P=0.024). Additionally, adverse reactions with any grades during anlotinib plus PD-1 blockades administration were observed in 62 patients (92.5%), of which 31 patients (46.3%) had ≥grade 3 adverse reactions. Most common adverse reactions were fatigue, hypertension, diarrhea and hepatotoxicity.
Conclusion: Anlotinib plus PD-1 blockades demonstrated promising effectiveness and tolerable safety in patients with previously immunotherapy treated advanced NSCLC. Those who were intolerant to previous immune-related regimens might benefit significantly from treatment with anlotinib plus PD-1 blockades. This conclusion should be confirmed in future studies.
Keywords: previously immunotherapy treated NSCLC, anlotinib, PD-1 blockades, effectiveness, safety
Introduction
Lung cancer is the second most common malignant tumor worldwide with the highest mortality globally.1 In China, lung cancer is the most common malignant tumor with 815,000 new cases and 715,000 deaths annually. Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for approximately 85%, resulting in about 693,000 new cases and 608,000 new deaths in China each year.2 Considerable driver gene in NSCLC and the respective targeted drugs have been developed consecutively recently, rendering NSCLC with positive driver gene mutations the most successful cancer in precision medicine.3 However, almost 50% of patients with advanced NSCLC with negative driver genes have to receive chemotherapy as the first-line therapeutic regimens; 5-year overall survival (OS) rate of these patients improves from 5% to 20% with the combination of immunotherapy currently.4
Immunotherapy represented by programmed cell death protein 1 (PD-1), including PD-L1 and CTLA-4, was one of the most important breakthroughs in the field of oncology in recent years, indicating that immunotherapy might provide a new therapeutic strategy for solid tumors clinically.5 Among patients with advanced NSCLC, the Keynote and Checkmate series of clinical trials demonstrated that pembrolizumab and nivolumab had surpassed chemotherapy and became the standard second-line therapy recently.6 Subsequently, pembrolizumab, sintilimab, camrelizumab, tislelizumab and toripalimab were also approved as first-line treatment for patients with advanced NSCLC in China,7 which indicated that PD-1 blockades had become the therapeutic trend for NSCLC in China and accessibility of the drugs might significantly improve with the price reductions. As a result, a considerable number of patients with advanced NSCLC might receive PD-1 plus chemotherapy regimen as the first-line therapy in clinical practice.8 Still and all, many patients might discontinue PD-1 blockades therapy in clinical practice, such as disease progression, intolerance of immune-related adverse events or completion of a prespecified therapeutic course.9 However, there was still no consensus regarding whether PD-1-related regimens might be kept in subsequent treatment among patients who had failed the previous immunotherapy-related treatment currently.10 Noteworthily, some researchers believed that rechallenge of PD-1-related regimens was also a promising therapeutic strategy that might benefit patients consecutively.11 Unfortunately, no sufficient evidence-based results were available on this topic, and more research data was needed to support the feasibility and rationality of PD-1 blockade rechallenge for patients with previously immunotherapy treated advanced NSCLC in clinical practice.12
It seemed that rechallenge strategy originated from 1970s and achieved promising potentiality in certain circumstance such as bevacizumab rechallenge in patients with metastatic colorectal cancer.13 Interestingly, previous studies highlighted that based on clinical experience, patients who discontinued primary immunotherapy due to toxicity or clinical decision or the completion of a fixed therapeutic course might be more likely to benefit from immunotherapy rechallenge.14 The potential rationality for immunotherapy rechallenge for these patients might be the fact that immunotherapy responded to these subjects clinically. However, there was still no clinical guideline to provide a specific treatment of immunotherapy rechallenge for NSCLC in terms of efficacy and safety currently.
Furthermore, inhibiting angiogenesis has been an important therapeutic option for patients with advanced NSCLC over the past years.15 It should be noted that anlotinib exhibited outstanding efficacy and acceptable safety profile as a third-line treatment for patients with advanced NSCLC, according to the ALTER0303 clinical trial by comprehensively inhibiting various targets such as VEGFR1-3, PDGFRα-β, FGFR1-4, c-FMS, c-Kit and RET.16 Therefore, anlotinib became the standard of care as third-line treatment for advanced NSCLC patients in China, and then anlotinib was included in the national medical insurance program successfully. Therefore, anlotinib could be widely used for patients with advanced NSCLC in clinical practice.17 Interestingly, previous work highlighted that the abnormality in tumor vasculature might be one of the potential mechanisms of resistance to PD-1/PD-L1 blockades, which resulted in immunosuppressive effect and compromised the efficacy of immunotherapy in vivo.18,19 Therefore, the combination of PD-1/PD-L1 blockades with antiangiogenic targeted drugs demonstrated synergistic action in cancer therapy recently.20 Consequently, we noticed that the phase III clinical trial of bevacizumab plus atezolizumab (PD-L1 blockade) achieved remarkable therapeutic activity in hepatocellular carcinoma and was approved as the first-line indication in hepatocellular carcinoma accordingly.21 However, it remained to be elucidated whether the combination of anlotinib plus PD-1 blockades might result in potential synergistic action for patients with previously immunotherapy treated advanced NSCLC clinically.
As a result, this study aimed to investigate the feasibility and tolerability of anlotinib plus PD-1 blockades in patients with previously immunotherapy treated advanced NSCLC retrospectively.
Patients and Methods
Design of This Study and Eligibility Criteria
Given the widespread administration of PD-1 blockades in combination with chemotherapy and the approval of the third-line indication for anlotinib in advanced NSCLC, a considerable number of patients had received treatment for anlotinib in combination with PD-1 blockades in clinical practice. Therefore, this study retrospectively screened patients with previously immunotherapy (including PD-1 and PD-L1 blockades) treated advanced NSCLC who received anlotinib plus PD-1 blockades in clinical practice from February 2019 to December 2022 at the Aerospace Center Hospital. The detailed inclusion criteria were as follows: (1) pathologically confirmed NSCLC with staging of IIIB or IV; (2) age ≥ 18 years; (3) Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 score; (4) failure to the previous immunotherapy-related regimens, including PD-1 or PD-L1 blockades (progression or intolerance to these regimens), and subsequent treatment with anlotinib plus PD-1 blockades in clinical practice; (5) measurable target lesions according to RECISTv1.1 criteria to assess the therapeutic response.22 Exclusion criteria were as follows: (1) history of autoimmune diseases or treatment with steroids or other immunosuppressive agents; (2) diagnosis of one more tumor or a serious disease that might compromise their life; (3) patients received other systemic treatment than anlotinib combined with PD-1 blockades; (4) efficacy assessment data were not available. The study profile is illustrated in Figure 1, and ultimately, a total of 67 patients with previously immunotherapy treated advanced NSCLC met the study screening criteria and were included in this study. The main objective of this study was to identify the feasibility and tolerability of combination treatment with anlotinib plus PD-1 blockades in patients with advanced NSCLC who previously received immunotherapy. The primary endpoints included objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), OS and exploratory analysis between OS and baseline subgroups. The protocol and appendants used in this study were approved by the ethics committee of Aerospace Center Hospital. Written informed consent was provided by patients prior to study commencement according to a statement from the Declaration of Helsinki.
 |
Figure 1 Study profile of this retrospective study. |
Therapeutic Regimens and Efficacy Evaluation Protocol in Clinical Practice
All the 67 patients included in this study had advanced NSCLC that progressed or were intolerant to previous immunotherapy regimens and then received a combination treatment of anlotinib plus PD-1 blockades in clinical practice. Anlotinib was administered orally at 12 or 10 mg once daily for 14 consecutive days, discontinued for 7 days, and every 21 days was deemed as one therapeutic cycle. PD-1 blockades used in this study had been marketed in China, including sintilimab, camrelizumab, tislelizumab, and pembrolizumab. PD-1 blockades were administered at a dose of 200 mg on the first day, intravenous infusion for more than half an hour, and every 21 days as one therapeutic cycle. Combination regimens continued until disease progressed or intolerable adverse reactions. The longest treatment for PD-1 blockades in this study was 2 years. Patients who were intolerant to anlotinib plus PD-1 blockades might switch to single-drug treatment with one of the two drugs. PD-1 blockades might not be used for dose adjustment. However, anlotinib could be adjusted from 12 mg to 10 mg or from 10 mg to 8 mg.
CT or MRI was adopted to evaluate the therapeutic response based on target lesions of the patients every two treatment cycles. However, given that this study was designed as a retrospective analysis, the compliance of some patients was disappointing, and the on-demand efficacy evaluation protocols based on their actual conditions were also accepted. The radiological efficacy evaluation results of the patients were assessed using RECISTv1.1 criteria accordingly.
Collection for Baseline and Safety Data and Follow-Up Plan of the Patients
Before the implementation of the study, relevant case collection forms were designed to collect patients’ baseline clinical characteristics and their entire treatment process through the hospital’s electronic medical record system. Additionally, given that this study also used OS as a primary endpoint, patients were followed up regularly and evaluated for adverse reactions after disease progression. Safety profile of patients treated with anlotinib plus PD-1 blockades was evaluated using Common Terminology Criteria for Adverse Events (CTCAE) 5.0. The highest levels of adverse reactions that occurred during treatment were recorded in the safety analysis in this study.
Follow-up was conducted monthly via mobile phone. Regular telephone communication with the patients or their family members was performed to obtain prognostic data of the patients after disease progression, mainly the survival status and date of death. The data cutoff date for this study was April 15, 2023.
Statistical Analysis
The definition of ORR and DCR in this study was adopted according to the previous study.23 Statistical analysis of the data was performed using SPSS (version 25.0). Continuous variables were presented as median and range, categorical variables were presented as frequency (percentage). Kaplan–Meier curves for PFS and OS data were generated using Stata software (version 14.0). The definition of PFS and OS in this study was adopted from the previous study.23 Noteworthily, the association between baseline clinical characteristics and OS was performed using Log rank test. Those who failed to experience disease progression or death at the date of data cutoff were treated as censored data. P<0.05 was considered as statistically significant.
Results
Baseline Characteristics of the 67 Patients with Previously Immunotherapy Treated Advanced NSCLC
Baseline characteristics of 67 patients with previously immunotherapy treated advanced NSCLC are presented in Table 1. The median age of the patients was 63 years (range: 19–82 years). Patients with age ≥63 and <63 years were found in 36 and 31 cases, respectively. Male and female were observed in 43 and 24 patients, respectively. An ECOG performance status of 0–1 score was noted in 44 patients. Most patients (94.0%) had stage IV disease. Fifteen patients were nonsmokers. Furthermore, 41 and 26 patients had adenocarcinoma and squamous cell carcinoma, respectively. Interestingly, EGFR positive mutation and negative mutation were found in 9 and 58 patients, respectively. Patients had ≤3 metastatic lesions and >3 metastatic lesions were found in 45 and 22 cases, respectively. Interestingly, lines of previous first- and second-line treatments were observed in 28 and 39 patients, respectively. Most of the patients were treated with PD-1 related regimens (86.6%). Progression and intolerance to immunotherapy were detected in 50 and 17 patients, respectively. Noteworthily, a total of 29 patients (43.3%) were administered with the same PD-1 blockades that were used previously. The median interval from prior immunotherapy termination to rechallenge of PD-1 blockades was 0.92 months (ranging from 0.26 months to 9.81 months). In addition, the initial dosages of anlotinib (12 and 10 mg) were noted in 54 and 13 patients, respectively. Four PD-1 blockers were used in this study: sintilimab, camrelizumab, tislelizumab, and pembrolizumab were administered to 23, 20, 15, and 9 patients, respectively.
 |
Table 1 Baseline Characteristics of the 67 Patients with Previously Immunotherapy Treated Advanced NSCLC |
Efficacy of the 67 Patients with Previously Immunotherapy Treated Advanced NSCLC Who Received Anlotinib Plus PD-1 Blockades
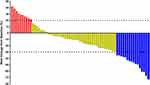
Radiological results of the 67 patients who underwent anlotinib combined with PD-1 blockades treatment were collected and evaluated retrospectively. Each patient was assessed based on the best overall response during the treatment, which showed that none of the patients achieved complete response, partial response (PR) was detected in 16 patients, stable disease (SD) was observed in 41 patients and progressive disease was noted in 10 patients, which yielded an ORR of 23.9% (95% CI: 14.3–35.9%) and a DCR of 85.1% (95% CI: 74.3–92.6%). A waterfall plot of target lesion changes (percentage) among the 67 patients with previously immunotherapy treated advanced NSCLC who received anlotinib combined with PD-1 blockades treatment is shown in Figure 2, it was evident that most patients demonstrated varying degrees of shrinkage in the target lesions after treatment of anlotinib combined with PD-1 blockades.
Prognosis of the 67 Patients with Previously Immunotherapy Treated Advanced NSCLC Who Received Anlotinib Plus PD-1 Blockades
The median time interval between the last immunotherapy treatment and the initiation of second-time immunotherapy among the 67 patients was 0.92 months (ranging from 0.26 months to 9.81 months). Additionally, to identify the therapeutic outcomes of anlotinib combined with PD-1 blockades among patients with previously immunotherapy treated advanced NSCLC, this study performed regular follow-up and collected radiological results for each patient to evaluate their PFS data. At the data cutoff date of April 15, 2023, 11 patients were still receiving treatment for anlotinib combined with PD-1 blockades, whereas other 56 patients terminated the treatment. The median follow-up duration of the 67 patients was 15.3 months (range: 0.8–33.5 months). At the data cutoff date, 48 patients experienced disease progression or death events, resulting in a data maturity of 71.6% for PFS data. Kaplan–Meier survival curve for PFS is presented in Figure 3, which exhibited that the median PFS of the 67 previously immunotherapy treated patients who received anlotinib plus PD-1 blockades was 6.1 months (95% CI: 2.37–9.83). Furthermore, the 6-month PFS rate was 50.1% (95% CI: 37.6–1.4%) and the 12-month PFS rate was 31.1% (95% CI: 20.0–42.9%) among the 67 patients.
 |
Figure 3 Progression-free survival of the 67 patients with previously immunotherapy treated advanced NSCLC who received anlotinib plus PD-1 blockades administration. |
Additionally, a total of 45 patients died by the data cutoff date, resulting in a data maturity of 67.2% for OS data. Kaplan–Meier survival curve for OS is illustrated in Figure 4, the median OS of the 67 patients who received anlotinib plus PD-1 blockades was 16.5 months (95% CI: 10.73–22.27). The 12-month OS rate was 58.0% (95% CI: 44.8–69.0%), and the 24-month OS rate was 28.0% (95% CI: 16.9–40.1%).
 |
Figure 4 Overall survival of the 67 patients with previously immunotherapy treated advanced NSCLC who received anlotinib plus PD-1 blockades administration. |
Interestingly, exploratory analysis in our study focused on the association between OS and the response to previous immunotherapy, which is illustrated in Figure 5, the median OS of patients with intolerance and progression of previous immunotherapy was 22.3 (95% CI: 14.24–30.36) months and 12.5 (95% CI: 4.59–20.41) months, respectively, and the difference was statistically significant (χ2=5.122, P=0.024).
 |
Figure 5 Comparison of overall survival in the 67 patients with advanced NSCLC who received anlotinib plus PD-1 blockades administration according to the response status of previous immunotherapy. |
Safety Profile of the 67 Patients with Previously Immunotherapy Treated Advanced NSCLC Who Received Anlotinib Plus PD-1 Blockades
The maximum grade of adverse reactions in each patient who received anlotinib combined with PD-1 blockades was collected and assessed, and the results are shown in Table 2. Obviously, adverse reactions were observed in 62 patients of any grade, yielding an incidence of 92.5%; of these, 31 patients had adverse reactions of ≥ grade 3 with an incidence of 46.3%. Common adverse reactions included fatigue (61.2%), hypertension (53.7%), diarrhea (40.3%), hepatotoxicity (29.9%), dermal toxicity (25.4%), hand-foot syndrome (17.9%), pneumonia (11.9%) and myelosuppression (7.5%). Grade ≥ 3 adverse reactions manifested as hypertension (14.9%), fatigue (9.0%), hepatotoxicity (9.0%), diarrhea (7.5%), dermal toxicity (3.0%), hand-foot syndrome (3.0%) and pneumonia (1.5%). However, it should be noted that grade 5 adverse reaction occurred in one patient who died of liver failure after two cycles of anlotinib combined with camrelizumab treatment. The overall tolerability of anlotinib combined with PD-1 blockades among patients with previously immunotherapy treated advanced NSCLC was acceptable and manageable.
 |
Table 2 Safety Profile of the 67 Patients with Previously Immunotherapy Treated Advanced NSCLC Who Received Anlotinib Plus PD-1 Blockades |
Discussion
In this retrospective study, the feasibility and tolerability of anlotinib plus PD-1 blockades among patients with previously immunotherapy treated advanced NSCLC were analyzed and presented in real-world clinical practice, which suggested that this combination regimen demonstrated promising efficacy and tolerable safety profile in patients with previously immunotherapy treated advanced NSCLC. Notably, those who were intolerant to previous immunotherapy might benefit profoundly from treatment with the combination of anlotinib and PD-1 blockades.
Recent years have witnessed that immunotherapy represented by PD-1 blockades had made great breakthrough in the treatment of advanced NSCLC. PD-1 blockades monotherapy and PD-1 blockades combined with chemotherapy achieved dramatically promising results as the second-line and first-line treatments for advanced NSCLC, respectively, thus changing the therapeutic landscape of advanced NSCLC recently.24 Therefore, PD-1 blockades combined with chemotherapy became the new standard of care as the first-line treatment for advanced NSCLC, and more patients in the first-line setting were able to receive this regimen with PD-1 blockades being covered in medical insurance system.25 Still and all, considerable patients might experience disease progression or intolerable adverse reactions within 8 months of this regimen, suggesting that it was necessary to further explore novel therapeutic strategy in clinical practice.26 The current standard treatment for patients who experienced disease progression after previous immunotherapy was still chemotherapy-based regimens. However, chemotherapy regimens were typically associated with moderate efficacy and intolerable adverse reactions, failing to meet current clinical needs.27 Anlotinib was the standard treatment for patients with advanced NSCLC who failed at least two lines of treatment in China. Previous studies demonstrated the potential synergistic action of anlotinib combined with PD-1 blockades clinically. Therefore, it was necessary to identify the feasibility and tolerability of anlotinib plus PD-1 blockades in patients with previously immunotherapy treated advanced NSCLC.28
In this study, a total of 67 patients with previously immunotherapy treated advanced NSCLC were administered with a combination of anlotinib and PD-1 blockades, resulting in an ORR of 23.9%, DCR of 85.1% and median PFS of 6.1 months. Compared to the efficacy of anlotinib monotherapy, this combination regimen in our study demonstrated numerically superior efficacy (anlotinib group: ORR=9.2%, DCR=81.0% and median PFS=5.4 months), highlighting the potential synergistic action of anlotinib in combination with PD-1 blockades in clinical practice.29 It should be noted that no standard therapeutic regimens are currently available among patients with previously immunotherapy treated advanced NSCLC and whether PD-1 rechallenge regimens for patients with advanced NSCLC made a difference is still a debatable research direction clinically.30 We noticed that a previous retrospective post-hoc study of the phase III OAK trial demonstrated that those who were treated with atezolizumab after progression conferred superior OS numerically, which showed that the median OS in atezolizumab arm, other anticancer therapy arm and no cancer treatment arm were 12.7 months vs 8.8 months vs 2.2 months, respectively,31 suggesting that PD-L1 blockades rechallenge for patients with immunotherapy treated advanced NSCLC might be a promising therapeutic strategy that warranted to be elucidated subsequently. Furthermore, another exploratory study initiated by Xu et al included a total of 40 patients with metastatic NSCLC who were rechallenged with mainly PD-1 blockades based regimens after they progressed to previous immunotherapy-related regimens.32 These PD-1 blockades based regimens achieved an ORR of 22.5%, a DCR of 85%, a median PFS of 6.8 months and the OS data were immature, which was consistent with the therapeutic outcomes in our study. These studies demonstrated that immunotherapy rechallenge might be a promising therapeutic option for patients with previously immunotherapy treated advanced NSCLC in clinical practice. Additionally, results of our study were consistent with those of two recently reported single-arm clinical studies regarding anlotinib combined with PD-1 blockades in patients with advanced NSCLC.18,33 Interestingly, another exploratory study investigated the efficacy and safety of anlotinib combined with PD-1 blockades compared to anlotinib alone in patients with previously treated advanced NSCLC.34 They recruited a total of 139 patients who received either anlotinib plus PD-1 blockades combination therapy or anlotinib monotherapy for previously treated advanced NSCLC. The ORR in the two groups was 20.5% vs 18.2%, and the median PFS was 5.8 months versus 4.2 months, respectively (P=0.022). The efficacy of anlotinib plus PD-1 blockades combination therapy in this study was consistent with that in our study, suggesting that the combination therapy might yield synergistic therapeutic action in vivo. The high neovascularization status in the tumor microenvironment might partially prevent the immune response in vivo and attenuate the clinical efficacy of PD-1 blockades clinically.35 Therefore, the administration of anlotinib improved the angiogenesis state of tumor tissues, promoted the normalization of blood vessels, and increased the immune response of tumor tissues, which might be the rationality of the synergistic action regarding anlotinib combined with PD-1 blockades in vivo to some extent.36 Noteworthily, bevacizumab combined with atezolizumab made an amazing breakthrough in the treatment of hepatocellular carcinoma and obtained indications accordingly,21 which also suggested that anlotinib combined with PD-1 blockades demonstrated promising therapeutic significance and valuable exploration implication in the treatment of solid tumors.
Furthermore, this study also performed OS analysis due to the relatively long follow-up duration, which showed that the median OS of 67 patients with advanced NSCLC who received the combination of anlotinib plus PD-1 blockades treatment was 16.5 months. Interestingly, this regimen produced a longer OS compared to anlotinib monotherapy or PD-1 blockades monotherapy (anlotinib: 9.6 months and PD-1 blockades: 12.5 months).37,38 We speculated that one possible explanation was that targeted drugs and immune checkpoint inhibitors were approved in China since 2018, making patients with advanced NSCLC in our study who progressed on anlotinib combined with PD-1 blockades receive subsequent treatments with other targeted drugs or PD-L1 blockades, providing some patients with survival benefits consecutively.39
Interestingly, our study further analyzed the difference in efficacy according to the response status of patients with previous immunotherapy: progression or immune treatment intolerance, which was currently a valuable and urgent issue to be addressed in clinical practice regarding whether immunotherapy might continue to be used after previous immunotherapy exposure. Our study suggested that patients who were intolerant to previous immunotherapy seemed to confer a longer OS than those who progressed the previous immunotherapy (median OS: 22.3 months vs 12.5 months, P=0.024), which was consistent with a previous study initiated by Santini et al.40 They included a total of 68 patients with advanced NSCLC who discontinued previous immunotherapy due to severe adverse reactions. Of these, 38 patients received immunotherapy rechallenge and 30 patients discontinued treatment. Results of that study suggested that those who failed to achieve an objective response before initial immunotherapy intolerance conferred superior PFS and OS after immunotherapy rechallenge. However, no improvement in efficacy was observed in patients who achieved an objective response before the initial immunotherapy intolerance. Nevertheless, given that the number of previous immunotherapy-intolerant patients included in this study was relatively limited (only 17 patients), further large-sample clinical trials were warranted to thoroughly elucidate these conclusions.
Safety profile in our study showed that the incidence of grade ≥3 adverse reactions was 46.3% of the 67 patients included, which was slightly higher than the incidence of grade ≥3 adverse reactions among patients with advanced NSCLC who were treated with anlotinib plus PD-1 blockades as the subsequent line therapy (approximately 40%).18 This result suggested that the combination of anlotinib plus PD-1 blockades in patients with previously immunotherapy treated advanced NSCLC might potentiate the incidence of grade ≥3 adverse reactions to some extent. Nevertheless, the overall safety profile of anlotinib plus PD-1 blockades was acceptable and manageable. Hypertension and hand-foot syndrome might be caused by anlotinib, whereas liver toxicity, skin toxicity and pneumonia might result from PD-1 blockades.41 Therefore, for patients with previously immunotherapy treated advanced NSCLC, the combination of anlotinib and PD-1 blockades might increase the incidence of liver toxicity. It should be noted that one patient in this study died of liver failure. Therefore, attention should be paid to this therapeutic regimen for patients with underlying liver dysfunction.42 However, given that the sample size of our study was relatively small, this conclusion was still needed to be confirmed in large-scale clinical trials subsequently. Collectively, the safety profile of anlotinib plus PD-1 blockades in patients with previously immunotherapy treated advanced NSCLC was tolerable and manageable.
Potential limitations of this study should be acknowledged. First, selection biases were inevitable owing to the non-randomized and retrospective design of our study, and physician discretion regarding PD-1 blockades discontinuation or rechallenge might also compromise the objective of the results. Second, the heterogeneous PD-1 blockades used in our study and the absence of PD-L1 expression detection might also confound the conclusions regarding the efficacy and safety of anlotinib combined with PD-1 blockades in patients with previously immunotherapy treated advanced NSCLC in clinical practice. Third, the sample size in our study was relatively small, and the results of our study were still needed to be confirmed in more patients in the future. Undoubtedly, these unresolved critical issues should be emphasized in future clinical studies.
Collectively, our study might be valuable for clinicians and patients regarding the reintroduction of PD-1 blockades in the care of this population. Therapeutic regimens with anlotinib plus PD-1 blockades provided encouraging real-world effectiveness and an acceptable safety profile in patients with previously immunotherapy treated advanced NSCLC, highlighting a potentially feasible treatment option. Large-scale prospective clinical trials were necessary to validate these findings and to determine the role of rechallenge with PD-1 blockades plus anlotinib in this population in the future.
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi:10.3322/caac.21763
2. Tao Q, Li X, Zhu T, et al. Lactate transporter SLC16A3 (MCT4) as an onco-immunological biomarker associating tumor microenvironment and immune responses in lung cancer. Int J Gen Med. 2022;15:4465–4474. doi:10.2147/ijgm.s353592
3. Jahanzeb M, Lin HM, Pan X, et al. Immunotherapy treatment patterns and outcomes among ALK-positive patients with non-small-cell lung cancer. Clin Lung Cancer. 2021;22(1):49–57. doi:10.1016/j.cllc.2020.08.003
4. Borghaei H, Gettinger S, Vokes EE, et al. Five-year outcomes from the randomized, Phase III trials CheckMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021;39(7):723–733. doi:10.1200/jco.20.01605
5. Huang MY, Jiang XM, Wang BL, Sun Y, Lu JJ. Combination therapy with PD-1/PD-L1 blockade in non-small cell lung cancer: strategies and mechanisms. Pharmacol Ther. 2021;219:107694. doi:10.1016/j.pharmthera.2020.107694
6. Franchi M, Pellegrini G, Corrao G. Effectiveness and cost-effectiveness profile of second-line treatments with nivolumab, pembrolizumab and atezolizumab in patients with advanced non-small cell lung cancer. Pharmaceuticals. 2022;15(4):489. doi:10.3390/ph15040489
7. Wang L, Yang Y, Yu J, et al. Efficacy and safety of anti-PD-1/PD-L1 in combination with chemotherapy or not as first-line treatment for advanced non-small cell lung cancer: a systematic review and network meta-analysis. Thorac Cancer. 2022;13(3):322–337. doi:10.1111/1759-7714.14244
8. Wu HX, Pan YQ, He Y, et al. Clinical benefit of first-line programmed death-1 antibody plus chemotherapy in low programmed cell death ligand 1-expressing esophageal squamous cell carcinoma: a post hoc analysis of JUPITER-06 and meta-analysis. J Clin Oncol. 2023;41(9):1735–1746. doi:10.1200/jco.22.01490
9. Zhou ZC, Chen KY, Li N, et al. Real-world utilization of PD-1/PD-L1 inhibitors with palliative radiotherapy in patients with metastatic non-small cell lung cancer. Thorac Cancer. 2022;13(16):2291–2300. doi:10.1111/1759-7714.14553
10. Giaj Levra M, Cotté FE, Corre R, et al. Immunotherapy rechallenge after nivolumab treatment in advanced non-small cell lung cancer in the real-world setting: a national data base analysis. Lung Cancer. 2020;140:99–106. doi:10.1016/j.lungcan.2019.12.017
11. Kitagawa S, Hakozaki T, Kitadai R, Hosomi Y. Switching administration of anti-PD-1 and anti-PD-L1 antibodies as immune checkpoint inhibitor rechallenge in individuals with advanced non-small cell lung cancer: case series and literature review. Thorac Cancer. 2020;11(7):1927–1933. doi:10.1111/1759-7714.13483
12. Gobbini E, Toffart AC, Pérol M, et al. Immune checkpoint inhibitors rechallenge efficacy in non-small-cell lung cancer patients. Clin Lung Cancer. 2020;21(5):e497–e510. doi:10.1016/j.cllc.2020.04.013
13. Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised Phase 3 trial. Lancet Oncol. 2013;14(1):29–37. doi:10.1016/s1470-2045(12)70477-1
14. Feng Y, Tao Y, Chen H, et al. Efficacy and safety of immune checkpoint inhibitor rechallenge in non-small cell lung cancer: a systematic review and meta-analysis. Thorac Cancer. 2023;14(25):2536–2547. doi:10.1111/1759-7714.15063
15. Hall RD, Le TM, Haggstrom DE, Gentzler RD. Angiogenesis inhibition as a therapeutic strategy in non-small cell lung cancer (NSCLC). Transl Lung Cancer Res. 2015;4(5):515–523. doi:10.3978/j.issn.2218-6751.2015.06.09
16. Xie C, Wan X, Quan H, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018;109(4):1207–1219. doi:10.1111/cas.13536
17. Fei Z, Rui M, Wang Y, Ma A. Cost-effectiveness of anlotinib vs. pembrolizumab and nivolumab as third-line treatment in recurrent small cell lung cancer in China. Expert Rev Pharmacoecon Outcomes Res. 2023;23(1):79–87. doi:10.1080/14737167.2023.2144837
18. Wang P, Fang X, Yin T, et al. Efficacy and safety of anti-PD-1 plus anlotinib in patients with advanced non-small-cell lung cancer after previous systemic treatment failure-a retrospective study. Front Oncol. 2021;11:628124. doi:10.3389/fonc.2021.628124
19. Jin R, Liu C, Zheng S, et al. Molecular heterogeneity of anti-PD-1/PD-L1 immunotherapy efficacy is correlated with tumor immune microenvironment in East Asian patients with non-small cell lung cancer. Cancer Biol Med. 2020;17(3):768–781. doi:10.20892/j.issn.2095-3941.2020.0121
20. Sun X, Xu J, Xie L, Guo W. Effectiveness and tolerability of anlotinib plus PD-1 inhibitors for patients with previously treated metastatic soft-tissue sarcoma. Int J Gen Med. 2022;15:7581–7591. doi:10.2147/ijgm.s379269
21. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
22. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
23. Yu L, Xu J, Qiao R, et al. Efficacy and safety of anlotinib combined with PD-1/PD-L1 inhibitors as second-line and subsequent therapy in advanced small-cell lung cancer. Cancer Med. 2023;12(5):5372–5383. doi:10.1002/cam4.5360
24. Cascone T, Fradette J, Pradhan M, Gibbons DL. Tumor immunology and immunotherapy of non-small-cell lung cancer. Cold Spring Harb Perspect Med. 2022;12(5):a037895. doi:10.1101/cshperspect.a037895
25. Ren S, Chen J, Xu X, et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-Sq): a Phase 3 trial. J Thorac Oncol. 2022;17(4):544–557. doi:10.1016/j.jtho.2021.11.018
26. Lu Y, Xu M, Guan L, et al. PD-1 inhibitor plus chemotherapy versus chemotherapy as first-line treatment for advanced esophageal cancer: a systematic review and meta-analysis. J Immunother. 2022;45(5):243–253. doi:10.1097/cji.0000000000000420
27. Bersanelli M, Buti S, Giannarelli D, et al. Chemotherapy in non-small cell lung cancer patients after prior immunotherapy: the multicenter retrospective CLARITY study. Lung Cancer. 2020;150:123–131. doi:10.1016/j.lungcan.2020.10.008
28. Chu T, Zhong R, Zhong H, et al. Phase 1b study of sintilimab plus anlotinib as first-line therapy in patients with advanced NSCLC. J Thorac Oncol. 2021;16(4):643–652. doi:10.1016/j.jtho.2020.11.026
29. Cheng JD, Chai LX, Zhao ZP, Hao YY, Li S. Efficacy and safety of anlotinib for patients with advanced NSCLC who progressed after standard regimens and the preliminary analysis of an efficacy predictor. Cancer Manag Res. 2020;12:5641–5650. doi:10.2147/cmar.s253366
30. Gobbini E, Charles J, Toffart AC, et al. Literature meta-analysis about the efficacy of re-challenge with PD-1 and PD-L1 inhibitors in cancer patients. Bull Cancer. 2020;107(11):1098–1107. doi:10.1016/j.bulcan.2020.07.009
31. Gandara DR, von Pawel J, Mazieres J, et al. Atezolizumab treatment beyond progression in advanced NSCLC: results from the randomized, Phase III OAK Study. J Thorac Oncol. 2018;13(12):1906–1918. doi:10.1016/j.jtho.2018.08.2027
32. Xu Z, Hao X, Yang K, et al. Immune checkpoint inhibitor rechallenge in advanced or metastatic non-small cell lung cancer: a retrospective cohort study. J Cancer Res Clin Oncol. 2022;148(11):3081–3089. doi:10.1007/s00432-021-03901-2
33. Zhai C, Zhang X, Ren L, et al. The efficacy and safety of anlotinib combined with PD-1 antibody for third-line or further-line treatment of patients with advanced non-small-cell lung cancer. Front Oncol. 2020;10:619010. doi:10.3389/fonc.2020.619010
34. Zhang W, Zhang C, Yang S, et al. Immune checkpoint inhibitors plus anlotinib versus anlotinib alone as third-line treatment in advanced non-small-cell lung cancer: a retrospective study. Future Oncol. 2021;17(31):4091–4099. doi:10.2217/fon-2021-0649
35. Lamplugh Z, Fan Y. Vascular microenvironment, tumor immunity and immunotherapy. Front Immunol. 2021;12:811485. doi:10.3389/fimmu.2021.811485
36. Guo F, Cui J. Anti-angiogenesis: opening a new window for immunotherapy. Life Sci. 2020;258:118163. doi:10.1016/j.lfs.2020.118163
37. Han B, Li K, Wang Q, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 Phase 3 randomized clinical trial. JAMA Oncol. 2018;4(11):1569–1575. doi:10.1001/jamaoncol.2018.3039
38. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi:10.1016/s0140-6736(15)01281-7
39. Jiang HT, Li W, Zhang B, Gong Q, Qie HL. Efficacy and safety of anlotinib monotherapy as third-line therapy for elderly patients with non-small cell lung cancer: a real-world exploratory study. Int J Gen Med. 2021;14:7625–7637. doi:10.2147/ijgm.s334436
40. Santini FC, Rizvi H, Plodkowski AJ, et al. Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res. 2018;6(9):1093–1099. doi:10.1158/2326-6066.cir-17-0755
41. Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–2471. doi:10.1016/s0140-6736(17)31827-5
42. Vitale G, Lamberti G, Comito F, et al. Anti-programmed cell death-1 and anti-programmed cell death ligand-1 immune-related liver diseases: from clinical pivotal studies to real-life experience. Expert Opin Biol Ther. 2020;20(9):1047–1059. doi:10.1080/14712598.2020.1762562
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.