Back to Journals » Therapeutics and Clinical Risk Management » Volume 15
Effectiveness and prescription pattern of lipid-lowering therapy and its associated factors among patients with type 2 diabetes mellitus in Malaysian primary care settings
Authors Elnaem MH , Nik Mohamed MH , Huri HZ, Mohd Shah AS
Received 5 August 2018
Accepted for publication 8 November 2018
Published 18 January 2019 Volume 2019:15 Pages 137—145
DOI https://doi.org/10.2147/TCRM.S182716
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Video abstract presented by Dr Mohamed Hassan Elnaem
Views: 345
Mohamed Hassan Elnaem,1 Mohamad Haniki Nik Mohamed,1 Hasniza Zaman Huri,2 Azarisman Shah Mohd Shah3
1Department of Pharmacy Practice, Faculty of Pharmacy, International Islamic University Malaysia, Kuantan, Pahang, Malaysia; 2Department of Pharmacy, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia; 3Department of Internal Medicine, Faculty of Medicine, International Islamic University Malaysia, Kuantan, Pahang, Malaysia
Background: Cardiovascular diseases (CVDs) are the main complication leading to morbidity and mortality among patients with type 2 diabetes mellitus (T2DM). There is a large amount of evidence to support the use of lipid-lowering therapy (LLT) for the prevention of CVD. This study aimed to assess the effectiveness and prescription quality of LLT among T2DM patients and to identify its associated factors.
Methods: A multicenter cross-sectional study included 816 T2DM patients from four different primary care centers in Pahang, Malaysia. We involved LLT-eligible T2DM patients as per the national clinical practice guidelines (CPG). The assessment of therapy effectiveness focused on the attainment of target lipid measures stated in the CPG. Evaluation of the prescription quality was classified into appropriate, potentially inappropriate, and inappropriate, based on the compliance with guidelines and existence of potential safety concerns. Binomial logistic regression was employed to identify the predictors of LLT effectiveness and prescription quality.
Results: The overall percentage of T2DM patients receiving statin therapy was 87.6% (715/816). Statin therapy was appropriately prescribed in 71.5% of the cases. About 17.5% of the LLT prescriptions have at least one significant drug interaction with co-prescribed medications. The achievement of the primary target of low-density lipoprotein cholesterol (LDL-C) levels was observed in only 37% of T2DM patients. The LLT indication and appropriateness of prescription were significantly associated with the attainment of LDL-C treatment goals. Primary prevention, Malay race, and hypertension were identified as predictors for appropriate prescribing of LLT among T2DM subjects.
Conclusion: There is a need to enhance the quality of LLT prescribing in the primary care setting to cover all eligible high-risk patients and ensure patient safety. Strategies to improve the achievement of LDL-C goals among patients with T2DM, such as investigating the potential role of the combination therapy and high-intensity statin therapy, are required.
Keywords: primary care, statin therapy, diabetes, dyslipidemia, Malaysia
Introduction
Cardiovascular diseases (CVD) are the principal cause of morbidity and mortality among patients with type 2 diabetes mellitus (T2DM).1 Strategies to prevent CVD among T2DM patients are well established in most clinical practice guidelines (CPGs).2,3 The implementation of lifestyle modifications and the use of CVD prophylaxis medications are the main pathways to achieve adequate CVD prophylaxis.4 Lipid-lowering therapy (LLT), particularly statin therapy, has been endorsed by most CPGs to help decrease the risk of developing CVD among T2DM patients who are 40–75 years old, regardless of their baseline lipid measures.2,4,5
It is well known that the management of T2DM is directed toward improving patients’ quality of life by reducing acute and chronic complications.6 Therefore, optimal management of diabetic dyslipidemia is paramount in preventing or delaying the incidence of diabetes-related cardiovascular complications. LLT, mainly statins, as well as additional lifestyle modifications, is the recommended approach for managing diabetic dyslipidemia.7 Furthermore, previous research focusing on reducing the CVD risk among patients with T2DM has demonstrated that the achievement of a low-density lipoprotein cholesterol (LDL-C) level of less than 2.6 mmol/L was associated with a substantial decrease in the overall CVD risk.2,8 Numerous studies have shown the clinical benefit of statin therapy in patients with T2DM.8,9
In real clinical practice, the prescribing of statin therapy for primary and secondary CVD prophylaxis has frequently been reported not to comply with the CPG recommendations. There are several reports of inadequate dyslipidemia treatment and the challenge of attaining LDL-C treatment goals in general practice.10,11 Moreover, it has been highlighted in previous research that a significant portion of the statin-eligible population is not receiving the treatment.12 Furthermore, it has been shown that the failure to reduce LDL-C with the recommended LLT has been related to an increased risk of cardiac events.13 The enhancement of the prescription efficiency of statin therapy and the use of high-intensity statins have been associated with improvements in clinical outcomes.14
There is a focus among health care providers on the thresholds for initiating CVD preventive treatment and the optimal targets of clinical parameters that can modify the CVD risk.15 Understanding the factors that may modulate the prescription of LLT for patients with T2DM is essential for optimal prescription practice.16 Ensuring the appropriate prescription of LLT in primary diabetes care is critical in evaluating the effectiveness of these medications.
In Malaysia, the underutilization of statin therapy for CVD prophylaxis has been reported in the periodical report on the national medicine use statistics.17 Moreover, a 2017 study highlighted the issue of underprescribing of LLT among T2DM patients in Malaysian hospital settings.18 The need to improve the management of diabetic dyslipidemia in the primary care setting has been underscored.19 Current data on LLT effectiveness and prescription quality among patients with T2DM in the Malaysian primary care setting are lacking. We carried out this work to assess the effectiveness and prescription quality of LLT among T2DM patients and to identify its associated factors.
Patients and methods
Study design
This work is a multicenter cross-sectional study involving four different primary care centers in the state of Pahang, Malaysia. The data were collected from the four sites through a two-stage process. The first stage took place from September to December 2016 and the second one was conducted between September and December 2017. The two-stage data collection was meant to allow for broader representativeness. Two primary centers were based in an urban area while the other two were located in a rural area to provide some comparisons of treatment use based on location.
Sampling method and inclusion criteria
The result of the sample size calculation was 369 per study stage. Therefore, in each stage, we planned to include at least 400 cases to achieve the required sample and compensate for any missed cases. Convenience sampling was employed to identify the clusters that provided the selection of study sites from both urban and rural areas. After identifying the clusters, a quota sampling method was followed for each study site to ensure that the required sample size was met, following the plan of including a quota of 100 cases per primary care center in each of the first and second stages.
Inclusion criteria were patients with T2DM aged 40–75 years without any contraindication for receiving LLT. Exclusion criteria were elderly patients aged >75 years or patients with severe renal impairment or acute liver transaminitis. The data collection procedure was based on examining patients’ files for the history of LLT use, modification of LLT, and the currently prescribed LLT. The data collection form also included the patient’s demographics, laboratory parameters related to lipid and glucose control, comorbid conditions, and co-prescribed medications.
Ethical considerations
Ethical approval for the study protocol was obtained from the medical research ethical committee (MREC) of the National Medical Research Registry (NMRR) before any data collection procedures began. The protocol approval ID is NMRR-16-713-29691 (IRR). Approval was first granted in May 2016 and an updated ethical approval letter was obtained in September 2017. Informed consent was waived because the data collection was planned and executed from medical records at the study sites with no direct patient involvement. The MREC allowed data collection from all four study sites following the guidelines and principles of the Declaration of Helsinki.20 Further administrative approvals were also obtained for each study site upon request.
Appropriateness of LLT prescription
We have classified our outcome of LLT prescription quality into three main classes. In the first class, the appropriate prescription refers to prescribing the statin therapy at the suitable intensity with no potential safety issues with co-administered drugs. The second class was the potentially inappropriate prescription. This refers to statin prescription at a suboptimal intensity or with safety concerns based on the necessary dose adjustment or potential risk of drug–drug interactions with other prescribed medications. The third class is the inappropriate prescription. This refers to those patients who did not receive the recommended statin therapy despite having no contraindications or those who received non-statin therapy without prior statin prescription.
Target lipid panels
Regarding the evaluation of the effectiveness of the prescribed statin therapy, the latest values of lipid parameters found at the time of data collection were compared against the target lipid values among patients with T2DM as stated in the national CPG.6 The current guidelines recommend statin therapy initiation in patients with T2DM and aged 40–75 years, regardless of the baseline LDL-C. The target LDL-C values were regarded as 2.6 and 1.8 mmol/L for primary and secondary CVD prevention, respectively. Lipid parameters such as triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C) were also recorded to offer a better picture of the control of diabetic dyslipidemia.
Statistical analysis
All completed data collection sheets were included in the data analysis. Descriptive statistics using frequencies and proportions were used to describe the prevalence of LLT prescription and its related effectiveness and quality among Malaysian patients with T2DM. Factors associated with the appropriate LLT prescribing and achievement of recommended LDL-C targets were initially investigated using the chi-squared test of independence. Some important variables, such as the most recently prescribed LLT, did not fully meet the assumptions required for the chi-squared test of independence regarding the number of the expected cell frequencies. Therefore, Goodman and Kruskal’s λ was run to determine whether the appropriate statin therapy prescription could be better predicted by knowledge of the types of the currently prescribed LLT in the primary care setting. Then, relevant variables were entered into a binomial logistic regression model to evaluate their influence on statin therapy. The level of significance was set at P<0.05. Data were analyzed using SPSS version 22 software (IBM Corporation, Armonk, NY, USA).
Results
Main characteristics of the study population
In total, 816 records were reviewed, comprising 404 records for the first stage and 412 records for the second. The mean ± SD age of study subjects was 59.05±8.44 years. Almost 60% of the study sample were females. The majority of subjects were of Malay ethnicity (84.8%), while the rest were Chinese (9.4%), Indian (5%), and others. About 80% of the study subjects had underlying hypertension. A considerable portion of the study participants had been diagnosed with dyslipidemia (44.2%). Approximately 40% of the subjects had been diagnosed with T2DM for at least 10 years. The vast majority of T2DM subjects (93%) in the primary care settings were eligible to receive statin therapy for primary CVD prophylaxis.
Patterns of LLT prescription
A considerable portion of the study subjects has been receiving moderate-intensity simvastatin therapy for more than 1 year. About 3.1% of subjects had been prescribed with combination LLT. Only 1.3% were initiated on high-intensity statin therapy. Table 1 provides a summary of the description of the prescribing of LLT for patients with T2DM in the primary care settings. A significant portion of statin therapy prescriptions (n=125, 17.5%) involved at least one medication that potentially interacts with statin therapy. Further recording of the interacting medications was carried out to evaluate their use and to assist in the planning of corrective intervention in some cases, if required. Amlodipine, gemfibrozil, diltiazem, verapamil, digoxin, and amitriptyline were the most commonly reported interacting medications.
  | Table 1 Patterns of LLT prescribing among subjects with type 2 diabetes mellitus in primary care |
Attainment of treatment goals and associated factors
As per the national CPG, LDL-C is considered a primary target for treating diabetic dyslipidemia, while HDL-C and TG are regarded as secondary targets. Categorization into two main groups according to the achievement of target treatment levels was carried out. The mean ± SD levels of LDL-C, HDL-C, and TG were 3.05±1.06, 1.35±0.57, and 1.71±0.98 mmol/L, respectively. Table 2 provides the percentage of achievement of primary and secondary treatment goals.
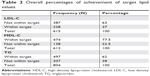  | Table 2 Overall percentages of achievement of target lipid values |
The achievement of target LDL-C levels was not significantly associated with the patient’s gender or race, the onset of T2DM, or the presence of hypertension as comorbidity. Nevertheless, LLT indication was significantly associated with the achievement of LDL-C target levels in the primary care settings (χ2(1)=10.653, P=0.001). Although the magnitude of the association was small, it was the largest one of all the variables (Cramer’s V=0.132). Similarly, there was a potential significant association between the achievement of LDL-C levels and the appropriateness of LLT prescription (P=0.011).
Evaluation of LLT prescription practice and its associated factors
Following the working definitions of appropriate, potentially inappropriate, and inappropriate prescription (see Appropriateness of LLT prescription, in the Patients and methods section), 71.5% of the study subjects were categorized under appropriate prescription since they had been prescribed with statin therapy with no issues related to drug interactions or dosing. About 16.2% were considered as potentially inappropriate, which means that they might need adjustment of their dose or concurrent medications. The rest (12.3%) had not been initiated on any LLT despite their eligibility. An overall summary of the tested independent variables, their P-values, and the strength of association is presented in Table 3.
There was a statistically significant association between the patients’ race and the appropriateness of statin therapy prescription (χ2(4)=27.647, P<0.0005). However, the association was relatively small (Cramer’s V=0.130). Table 4 shows the cross-tabulation of the patients’ race and the appropriateness of LLT prescription. Considering that the vast majority of T2DM subjects (approximately 85%) in primary care are entitled to receive LLT for primary CVD prevention, the appropriateness of LLT prescription was found to be significantly associated with the proposed indication to receive LLT in the primary care settings (χ2(2)=27.129, P<0.005). The magnitude of the association was relatively small (Cramer’s V=0.182). Table 5 shows the cross-tabulation of LLT indication and the appropriateness of LLT prescription.
Regarding the analysis, if the appropriate statin therapy prescription could be better predicted by knowledge of the types of currently prescribed LLT in the primary care settings, Goodman and Kruskal’s λ was 0.291. This was a statistically significant reduction in the proportion of errors due to the knowledge of the currently prescribed LLT as a predictor of evaluation of LLT prescription appropriateness (P<0.005).
Predictors of appropriate LLT prescription in the primary care settings
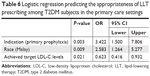
The logistic regression model was statistically significant (χ2(3)=43.935, P<0.0005). The model revealed a 10% (Nagelkerke R2) variance in appropriate statin therapy prescription among T2DM patients in the study. It also correctly classified 74.3% of the cases. This model revealed only three statistically significant independent variables, which were the patient’s race being “Malay” (P=0.009), an indication to receive statin therapy (P=0.003), and the achievement of target LDL-C level (P=0.021). Malay patients were found to have 2.5 times higher odds of receiving appropriate statin therapy prescription. In the primary care settings, patients with T2DM who did not have underlying CVD had 3.4 times higher odds of being considered for an appropriate statin therapy prescription. Table 6 shows the findings of binomial logistic regression to identify the predictors of the appropriateness of LLT prescription.
Discussion
Our study reported the achievement of a percentage of target LDL-C values that was markedly lower than the findings of previous studies involving patients with T2DM in the Netherlands and Thailand. In those studies, approximately 75% and 90% of the study subjects were able to attain their target LDL-C values, respectively.21,22 Nevertheless, the LDL-C attainment level in this study is higher than that found in a broad European cross-sectional survey that used data from 14 European regions and revealed that there was a 33% fulfillment of target LDL-C values in the study population.23 It is also higher than the reported percentage in the Polish primary care setting, where only 30% of the study population achieved the target LDL-C levels.24 The prevalence of LLT prescription in our setting seems to be higher than the previously reported estimates in Scotland and the Netherlands, where approximately two-thirds of the eligible candidates have been offered the treatment.25,26 Despite the high prevalence of LLT prescription, attainment of LDL-C targets was not observed in more than half of T2DM patients. This may highlight the need to consider further individualized interventions to improve adherence, which could be planned accordingly.27 It is worth highlighting that the results from EUROASPIRE III and IV showed that the control of well-defined modifiable risk factors in people at high CVD risk remains unsatisfactory.28 Moreover, the report from the EUROASPIRE IV survey underpinned the need for improving management among T2DM patients with coronary artery disease, where 60% of the study population received cardioprotective medications and at most 28% attained their guideline-endorsed treatment goals.29
In the literature, the type of the prescribed LLT has been reported to be linked to a higher chance of achieving target LDL-C. For instance, a Lebanese study reported that the use of statin combination therapy, preferably with ezetimibe, in addition to adherence to a low-fat diet, was linked to higher likelihoods of target LDL-C and non-HDL-C attainment.30 Similarly, another study showed that the fixed simvastatin–ezetimibe combination, followed by monotherapy with simvastatin, atorvastatin, and rosuvastatin, is more likely to achieve target LDL-C values.31 Compared to our study, the overall combination therapy was considered in 3% of the study population; of those, only 0.5% were offered statin–ezetimibe combination therapy. There is well-established evidence to support the claim that combinations of LLTs, particularly ezetimibe and PCSK9 inhibitors, are significantly linked to increased chances of attaining target LDL-C values. According to the European expert panel opinions regarding the use of the fenofibrate–statin combination, it has been reported that this combination may help to individualize CVD risk management in patients with atherogenic dyslipidemia, with a favorable benefit–risk profile.32,33 In our setting, the combination LLT seems not to be fully utilized. Therefore, it may be advisable to consider proper utilization of combination LLT in clinical practice, in light of the proposed efforts to improve the attainment of target LDL-C values among Malaysian populations with a high CVD risk. This may also boost the patient’s overall adherence to the prescribed LLT.34
The present study showed that the achievement of the LDL-C targets was not significantly associated with the duration of LLT. In contrast, the relatively prolonged duration of LLT was associated with a positive impact on the overall reduction in the LDL-C levels.31 Previous research has demonstrated that Malay ethnicity is a significant determinant for uncontrolled dyslipidemia among T2DM patients.19 Therefore, it is interesting to highlight that a higher portion of the Malay population which is at a higher risk of uncontrolled dyslipidemia was prescribed with appropriate statin therapy. However, our study did not reveal any significant differences based on race with regard to the achievement of LDL-C target values. It has been identified that receiving antihypertensive medication is an independent predictor not only for statin therapy prescription but also for initiating cholesterol treatment discussions.35 This is similar to our study findings and is related to the presence of hypertension comorbidity and its association with statin therapy prescription. Furthermore, our results did not reveal any differences based on gender regarding the achievement of LDL-C treatment goals or the appropriateness of the prescribed LLT. This contradicts the findings of a recent US study, which reported that women were less likely than men to attain their target LDL-C measures, despite receiving adequate statin therapy prescriptions.36 One important point highlighted by this study is that women with diabetes and high CVD risk were exhibiting better achievement of treatment goals in comparison with those who only had CVDs.
The findings showed that simvastatin-based regimens are the most commonly prescribed LLT regimens in the Malaysian primary care setting. Consistent with this, previous research showed simvastatin to be the most studied statin therapy in clinical trials with a recommended efficacy margin, according to pharmacodynamic reports of the estimated effective doses of different statin therapies.37 On the other hand, this finding seems to not coincide with the recommendation from the latest review of the National Drug Formulary, which includes a comparison of all locally available statins and concludes that atorvastatin should be offered as a first-line therapeutic choice in subjects with dyslipidemia.38 Moreover, the overall prevalence of simvastatin as the most prescribed LLT, followed by atorvastatin, is consistent with the previous US study, with results that provided a similar description of LLT use among a population aged 40 years and above.39
The percentage of potential drug interactions reported in our study is considered to be of average value compared with the previously reported incidence range of potential statin drug interactions among statin users (2%–33%) using different measures and drug databases.40 There are differences between statin therapies that could affect their potential drug-interaction profiles. Statin therapies affected by cytochrome P450 (CYP), such as simvastatin and lovastatin, are expected to be more susceptible to interactions. On the other hand, this metabolic pathway does not affect atorvastatin. Unlike other statin therapies, pravastatin and rosuvastatin do not affect the CYP3A4 pathway. As such, they are less likely to be influenced by drugs that inhibit this metabolic pathway, such as macrolide antibiotics and azole antifungals.41 According to the findings of a French study conducted in a university hospital, pravastatin and rosuvastatin were identified as the statin therapies least likely to be associated with significant drug interactions.42 The same study found that lipophilic statins, eg, simvastatin and atorvastatin, were the most probable statin therapies encountered in statin–drug interactions. Systematic, intensive screening of statin therapy prescriptions is warranted to identify and prevent potential interactions. The challenge in dealing with data on drug interactions is the presence of variations. There are still some discrepancies among the various drug databases.40 A consensus on having a drug database that would serve as the main reference for the evaluation of possible statin–drug interactions at a national level would be imperative in unifying the reported significance of a particular drug interaction across the whole country.
Our data showed that few cases experienced potential drug interactions because of the inappropriate selection of gemfibrozil as an adjunct combination therapy. Besides, it was frequently reported to be contraindicated with statin therapy. Moreover, the gemfibrozil add-on therapy was not associated with any further clinical benefit related to CVD prophylaxis.43 Therefore, it could be seen as essential to revise the therapeutic choices of statin-combination therapy to ensure appropriate and safe non-statin therapy in current clinical practice. Fenofibrate could be a more reasonable choice for statin–fibrate combination therapy if such a combination is needed in patients with mixed dyslipidemia. This refers to the national CPG on the treatment of dyslipidemia.5 Moreover, if the decision is made to prescribe a combination therapy, promotion of the rational use of relatively effective and safe add-on therapies, eg, ezetimibe, should be considered as it has shown to improve the CVD risk reduction outcomes.44
The strength of this study is the attempt to provide updates and outlines for understanding current challenges in CVD prophylaxis among T2DM patients, to enable future improvements to be made. However, this study has several limitations. First, the cross-sectional design is a constraint of our study, although it gives the opportunity to make the best use of the available resources. In addition, we acknowledge the fact that LDL-C treatment goals provided by the CPG are relatively subjective values regarding their clinical significance and feasibility to be attained. Although we included cases from more than one center to increase the representativeness of our data, the study was still limited to one state in Malaysia, which could limit the generalizability of our findings. Future prospective cohort studies are required to confirm our findings on a larger scale.
Conclusion
Our study outlined the need to enhance the quality of LLT prescribing in primary care settings to cover all eligible high-risk patients and ensure patient safety. The study revealed that LDL-C treatment goals were not achieved in a significant portion of T2DM subjects. Strategies to improve the achievement of LDL-C goals are required, such as investigating the potential role of combination therapy and high-intensity statin therapy. The findings also highlighted the need to explore thoroughly the underlying obstacles in attaining LDL-C treatment goals regarding the assessment of patients’ adherence and the evaluation of LLT quality prescription practice.
Acknowledgment
We are grateful for the support provided for this work by the Research Management Center of the International Islamic University Malaysia under grant number RIGS17-122-0697.
Disclosure
The authors report no conflicts of interest in this work.
References
Rydén L, Grant PJ, Anker SD. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2013;34(39):3035–3087. | ||
Stone NJ, Robinson JG, Lichtenstein AH; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–2934. | ||
Anderson TJ, Grégoire J, Pearson GJ, et al. 2016 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32(11):1263–1282. | ||
Catapano AL, Graham I, De Backer G, et al; ESC Scientific Document Group. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37(39):2999–3058. | ||
Ministry of Health Malaysia. Clinical Practice Guidelines. Management of Dyslipidaemia 2017. 5th edn. Putrajaya: Ministry of Health Malaysia; 2017. | ||
Ministry of Health Malaysia. Malaysian Clinical Practice Guidelines. Management of Type 2 Diabetes Mellitus. 5th edn. Putrajaya: Ministry of Health Malaysia; 2015:1–129. | ||
Bell DS, Al Badarin F, O’Keefe JH Jr. Therapies for diabetic dyslipidaemia. Diabetes Obes Metab. 2011;13(4):313–325. | ||
Fung CSC, Wan EYF, Chan AKC, Lam CLK. Statin use reduces cardiovascular events and all-cause mortality amongst Chinese patients with type 2 diabetes mellitus: a 5-year cohort study. BMC Cardiovasc Disord. 2017;17(1):166. | ||
Chen PH, Wang JS, Lin SY. Effects of statins on all-cause mortality at different low-density-lipoprotein cholesterol levels in Asian patients with type 2 diabetes. Curr Med Res Opin. 2018;34(11):1885–1892. | ||
Reiner Ž, De Bacquer D, Kotseva K, et al; EUROASPIRE III Study Group. Treatment potential for dyslipidaemia management in patients with coronary heart disease across Europe: findings from the EUROASPIRE III survey. Atherosclerosis. 2013;231(2):300–307. | ||
Finnikin S, Ryan R, Marshall T. Statin initiations and QRISK2 scoring in UK general practice: a THIN database study. Br J Gen Pract. 2017;67(665):e881–e887. | ||
Navar AM, Wang TY, Li S, et al. Lipid management in contemporary community practice: results from the Provider Assessment of Lipid Management (PALM) Registry. Am Heart J. 2017;193:84–92. | ||
Yeh YT, Yin WH, Tseng WK, et al; Taiwanese Secondary Prevention for Patients with AtheRosCLErotic Disease (T-SPARCLE) Registry Investigators. Lipid lowering therapy in patients with atherosclerotic cardiovascular diseases: Which matters in the real world? Statin intensity or low-density lipoprotein cholesterol level? – Data from a multicenter registry cohort study in Taiwan. PLoS One. 2017;12(10):e0186861. | ||
Bennie M, Godman B, Bishop I, Campbell S. Multiple initiatives continue to enhance the prescribing efficiency for the proton pump inhibitors and statins in Scotland. Expert Rev Pharmacoecon Outcomes Res. 2012;12(1):125–130. | ||
Hong KN, Fuster V, Rosenson RS, Rosendorff C, Bhatt DL. How low to go with glucose, cholesterol, and blood pressure in primary prevention of CVD. J Am Coll Cardiol. 2017;70(17):2171–2185. | ||
Elnaem MH, Mohamed MHN, Huri HZ, Azarisman SM, Elkalmi RM. Statin therapy prescribing for patients with type 2 diabetes mellitus: a review of current evidence and challenges. J Pharm Bioallied Sci. 2017;9(2):80–87. | ||
Siti Fauziah A, Kamarudin A, Nnan O, et al. Malaysian Statistics on Medicines 2009 & 2010. Malaysia: Pharmaceutical Services Division and Clinical Research Centre, Ministry of Health; 2014. | ||
Elnaem MMH, Nik Mohamed MMH, Huri HZ, Azarisman SM. Patterns of statin therapy prescribing among hospitalized patients with Type 2 diabetes mellitus in two Malaysian tertiary hospitals. Trop J Pharm Res. 2017;16(12):3005–3011. | ||
Chew BH, Ismail M, Lee PY, et al. Determinants of uncontrolled dyslipidaemia among adult type 2 diabetes in Malaysia: the Malaysian Diabetes Registry 2009. Diabetes Res Clin Pract. 2012;96(3):339–347. | ||
Czarkowski M. [Helsinki Declaration-next version]. Pol Merkur Lekarski. 2014;36(215):295–297. Polish. | ||
Gant CM, Binnenmars SH, Harmelink M, et al. Real-life achievement of lipid-lowering treatment targets in the DIAbetes and LifEstyle Cohort Twente: systemic assessment of pharmacological and nutritional factors. Nutr Diabetes. 2018;8(1):24. | ||
Lee B, Dumrongkitchaiporn K. Statin intensity regimens in Thai Type 2 diabetic patients who achieved LDL-C targets. J Med Assoc Thai. 2017;100(5):603–611. | ||
Kotseva K, De Bacquer D, De Backer G, et al; On Behalf Of The Euroaspire Investigators. Lifestyle and risk factor management in people at high risk of cardiovascular disease. A report from the European Society of Cardiology European Action on Secondary and Primary Prevention by Intervention to Reduce Events (EUROASPIRE) IV cross-sectional survey in 14 European regions. Eur J Prev Cardiol. 2016;23(18):2007–2018. | ||
Jozwiak J, Chrusciel P, Tomaszewski M. Lipids profile and efficacy of treatment of dyslipidemia in primary care in Poland. Results of lipidogram 5 years study (2004–2010). Atherosclerosis. 2017;263:e187. | ||
Jones NR, Fischbacher CM, Guthrie B, et al; Scottish Diabetes Research Network Epidemiology Group. Factors associated with statin treatment for the primary prevention of cardiovascular disease in people within 2 years following diagnosis of diabetes in Scotland, 2006–2008. Diabet Med. 2014;31(6):640–646. | ||
Kuiper JG, Sanchez RJ, Houben E, et al. Use of lipid-modifying therapy and LDL-C goal attainment in a high-cardiovascular-risk population in the Netherlands. Clin Ther. 2017;39(4):819–827.e1. | ||
Krumme AA, Franklin JM, Isaman DL, et al. Predicting 1-year statin adherence among prevalent users: a retrospective cohort study. J Manag Care Spec Pharm. 2017;23(4):494–502. | ||
De Backer G, De Bacquer D, Rydén L, et al; EUROASPIRE investigators. Lifestyle and risk factor management in people at high cardiovascular risk from Bulgaria, Croatia, Poland, Romania and the United Kingdom who participated in both the EUROASPIRE III and IV primary care surveys. Eur J Prev Cardiol. 2016;23(15):1618–1627. | ||
Gyberg V, De Bacquer D, De Backer G, et al; EUROASPIRE Investigators. Patients with coronary artery disease and diabetes need improved management: a report from the EUROASPIRE IV survey: a registry from the Euro Observational Research Programme of the European Society of Cardiology. Cardiovas Diabetol. 2015;14(1):1–11. | ||
Lama S, Souraya D, Youssef F. Statin prescription strategies and atherogenic cholesterol goals attainment in Lebanese coronary artery disease patients. Int J Clin Pharm. 2017;39(4):919–926. | ||
Guglielmi V, Bellia A, Pecchioli S, et al. Effectiveness of adherence to lipid lowering therapy on LDL-cholesterol in patients with very high cardiovascular risk: a real-world evidence study in primary care. Atherosclerosis. 2017;263:36–41. | ||
Aguiar C, Alegria E, Bonadonna RC, et al. A review of the evidence on reducing macrovascular risk in patients with atherogenic dyslipidaemia: a report from an expert consensus meeting on the role of fenofibrate-statin combination therapy. Atheroscler Suppl. 2015;19:1–12. | ||
Ferrari R, Aguiar C, Alegria E, et al. Current practice in identifying and treating cardiovascular risk, with a focus on residual risk associated with atherogenic dyslipidaemia. Eur Heart J Suppl. 2016;18(Suppl C):C2–C12. | ||
Russell C, Sheth S, Jacoby D. A clinical guide to combination lipid-lowering therapy. Curr Atheroscler Rep. 2018;20(4):19. | ||
Karmali KN, Lee JY, Brown T, Persell SD. Predictors of cholesterol treatment discussions and statin prescribing for primary cardiovascular disease prevention in community health centers. Prev Med. 2016;88:176–181. | ||
Schoen MW, Tabak RG, Salas J, Scherrer JF, Buckhold FR. Comparison of adherence to guideline-based cholesterol treatment goals in men versus women. Am J Cardiol. 2016;117(1):48–53. | ||
Dimmitt SB, Stampfer HG, Warren JB. The pharmacodynamic and clinical trial evidence for statin dose. Br J Clin Pharmacol. 2018;84(6):1128–1135. | ||
Ramli A, Aljunid SM, Sulong S, Md Yusof FA. National Drug Formulary review of statin therapeutic group using the multiattribute scoring tool. Ther Clin Risk Manag. 2013;9:491–504. | ||
Gu Q, Paulose-Ram R, Burt V, Kit B. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003–2012. NCHS Data Brief. 2014;177:1–8. | ||
Thai M, Reeve E, Hilmer SN, Qi K, Pearson SA, Gnjidic D. Prevalence of statin-drug interactions in older people: a systematic review. Eur J Clin Pharmacol. 2016;72(5):513–521. | ||
Maghsoodi N, Wierzbicki AS. Statins: general safety profile and association with myopathy. Clin Pharm. 2016;8(5):1–25. | ||
Morival C, Westerlynck R, Bouzillé G, Cuggia M, Le Corre P. Prevalence and nature of statin drug-drug interactions in a university hospital by electronic health record mining. Eur J Clin Pharmacol. 2018;74(4):525–534. | ||
American Diabetes Association. Standards of medical care in diabetes-2016. Am Diabetes Assoc. 2016;39(Suppl 1):S1–S112. | ||
Grundy SM. Primary prevention of cardiovascular disease with statins: assessing the evidence base behind clinical guidance. Clin Pharm. 2016;8(2). |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.