Back to Journals » Drug Design, Development and Therapy » Volume 16
Effect of Systemic Lidocaine on Postoperative Early Recovery Quality in Patients Undergoing Supratentorial Tumor Resection
Authors Zhao K , Dong Y , Su G, Wang Y, Ji T, Wu N , Cui X, Li W , Yang Y, Chen X
Received 5 February 2022
Accepted for publication 6 April 2022
Published 22 April 2022 Volume 2022:16 Pages 1171—1181
DOI https://doi.org/10.2147/DDDT.S359755
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Kai Zhao,1,* Yushan Dong,1,* Gaowei Su,1 Yaolin Wang,1 Tao Ji,1 Nanling Wu,1 Xiaojie Cui,1 Wenzhan Li,1 Yanming Yang,1 Xiuxia Chen1,2
1Jiangsu Province Key Laboratory of Anesthesiology, Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China; 2Department of Anesthesiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiuxia Chen, Department of Anesthesiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou Medical University, Xuzhou, Jiangsu, 221000, People’s Republic of China, Tel +86 18052268332, Fax +0516-8346-9496, Email [email protected]
Purpose: Lidocaine has been gradually used in general anesthesia. This study was designed to investigate the effect of systemic lidocaine on postoperative quality of recovery (QoR) in patients undergoing supratentorial tumor resection, and to explore its brain-injury alleviation effect in neurosurgical anesthesia.
Patients and Methods: Sixty adult patients undergoing elective supratentorial tumor resection. Patients were randomly assigned either to receive lidocaine (Group L: 1.5 mg/kg bolus completed 10 min before anesthesia induction followed by an infusion at 2.0 mg/kg/h) or to receive normal saline (Group C: received volume-matched normal saline at the same infusion rate). Primary outcome measures were Quality of Recovery-40 (QoR-40) scores on postoperative day (POD) 1 and 2. Plasma concentrations of S100B protein (S100B), neuron specific enolase (NSE), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) before anesthesia induction and at the end of surgery were assessed. Visual Analogue Scale (VAS) scores were assessed at 1, 2, 6, 12, 24 and 48 h after surgery. Perioperative parameters and adverse events were also recorded.
Results: Patients between two groups had comparable baseline characteristics. Global QoR-40 scores on POD 1 and POD 2 were significantly higher (P < 0.001) in group L (165.5± 3.8 vs 173.7± 4.7) than those in group C (155.6± 4.0 vs 163.2± 4.5); and scores of physical comfort, emotional state, and pain in group L were superior to those in group C (P < 0.05). In group L, patients possessed lower plasma concentration of pro-inflammatory factors (IL-6, TNF-α) and brain injury-related factors (S100B, NSE) (P < 0.05), consumed less remifentanil and propofol, and experienced lower pain intensity. Multiple linear regression analysis demonstrated age and pain were correlated with postperative recovery quality.
Conclusion: Systemic lidocaine improved early recovery quality after supratentorial tumor resection with general anesthesia, and had certain brain-injury alleviation effects. These benefits may be attributed to the inflammation-alleviating and analgesic properties of lidocaine.
Keywords: lidocaine, brain neoplasm, supratentorial tumor resection, recovery quality, brain protection, postoperative pain
Introduction
The brain neoplasm, as a common neurosurgical disease, has various clinical manifestations due to different pathological types, locations and development rates. In terms of supratentorial tumor treatment, craniotomy is presently the first choice. Prolonged intracranial surgery, likely resulting in delayed postoperative recovery, may lead to ischemia and hypoxia in brain tissue, explosive increase of inflammatory factors, postoperative pain, postoperative nausea and vomiting (PONV), etc. Anesthesia acts as a key link during the surgery, including optimizing brain metabolism and improving brain oxygenation. With most attention being paid to high rates of disability after brain tumor resection, such as hemiplegia and aphasia, however, patients’ subjective feelings have not yet been taken seriously, and have even been ignored by clinicians. Among the bulk of recovery-evaluation scales, the QoR-40 scale is unique for its applicability, reliability, validity, responsiveness, accuracy, acceptability, interpretability and feasibility. This questionnaire was designed to assess health status as a whole and recovery quality from specific dimensions, and has been successfully used in cranial surgery patients.1–4 In our study, QoR-40 scores were set as primary outcome measures.
As the mainstay of pain treatment, opioids have become an integral part of surgery, despite inevitably causing “nausea and vomiting”, “hyperalgesia”, “drug resistance”, “delirium”, etc. Therefore, an intraoperative opioid-sparing strategy combined with the nerve block technique and the adjuvant becomes a wise choice.5–8 Sumathi et al. found that lidocaine could improve the threshold of airway stimulation response, thus inhibiting excessive increase in intracranial pressure caused by choking during extubation, which was of great benefit to patients with brain neoplasm.9 During general anesthesia, systemic lidocaine (1.5 mg/kg or 1.5–2 mg/kg/h, intravenously) contributed to lowering intraoperative opioids consumption and postoperative pain intensity; it also had additional advantages of inhibiting inflammatory response by reducing pro-inflammatory factors, such as IL-6 and TNF-α, and maintaining immune function.10–12 Lidocaine can reduce cerebral infarction scopes within 24 h after focal cerebral ischemia, confirming its brain-protection effect.13 Niiyama et al. demonstrated that lidocaine maintained adenosine triphosphate content of hippocampal CA1 pyramidal neurons during and after ischemia by protecting mitochondria, thus playing a neuroprotective role.14 However, there are few studies validating lidocaine’s effects on recovery quality and brain protection in patients after neurosurgery. In this study, we chose two typical brain injury-related factors, S100B and NSE, as our secondary outcomes, in order to observe severity of central nervous system injury.15,16
This randomized, double-blind, placebo-controlled clinical trial was aimed at investigating effects of lidocaine on postoperative recovery quality and its brain injury alleviation effect in supratentorial tumor resection. We hypothesized that systemic lidocaine would first, significantly improve postoperative recovery quality and second, that the improvement of postoperative recovery quality would correlate with stress alleviation, inflammatory response reduction, and brain injury alleviation effect.
Materials and Methods
This study was a single-center, prospective, randomized, assessor-blinded, placebo-controlled clinical trial between 1 November 2020 and 30 June 2021, approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University and registered in the Chinese Clinical Trial Center (ChiCTR2000040209). Our research complied with the Declaration of Helsinki and followed guidelines of CONSORT. Written informed consents were obtained from all patients or family authorized before enrollment.
Patients
Sixty patients (29 with gliomas and 31 with meningiomas) scheduled for supratentorial tumor resection with general anesthesia were included, with age over 18, American Society of Anesthesiologists (ASA) physical status II–III, and 15 points on the preoperative Glasgow Coma Score. The exclusion criteria included: metastatic supratentorial tumor; preoperative radiotherapy or chemotherapy; a history of craniotomy tumor resection; preoperative usage of anti-inflammatory agents or analgesics; known lidocaine intolerance; allergies to any drugs used in this study; abnormal liver or kidney function; severe cardiopulmonary failure; involvement in other local anaesthetic interventions; low body weight (<40 kg); with short (<2 h) or long (>6 h) surgery duration; and refusing to sign written informed consent. Patients with severe hemodynamic disturbances or other life-threatening complications during the operation, or those transferred to the intensive care unit (ICU) after surgery, were eliminated.
Randomization and Blinding
Sixty patients were randomly assigned to the lidocaine group (Group L) and the control group (Group C) in a 1:1 ratio by a computer-generated random sequence. Group numbers were randomly stored in a closed envelope, and experimental drugs were prepared by nurses who did not participate in the anesthesia operation after entering the operating room. Doctors and other personnel involved in anesthesia management did not know about the grouping.
Study Intervention
All patients underwent fasting and water abstaining to prepare for surgery. After entering the scheduled operating room, peripheral venous access was routinely opened, and radial artery puncture (after Allen test was confirmed to be negative; otherwise non-invasive blood pressure monitoring would be selected) was completed. Then, blood pressure, heart rate (HR), electrocardiogram (ECG), pulse oxygen saturation (SpO2), end-expiratory CO2 (PETCO2), and bispectral index (BIS) were monitored. Anesthesia induction was performed after pure oxygen inhalation nearly amounting to 5 min: midazolam 0.05 mg/kg, sufentanil 0.5 μg/kg, etomidate 0.3 mg/kg, and rocuronium 0.6–1.0 mg/kg. Before induction, patients in group L were given 1.5 mg/kg lidocaine (iv.) within 10 min (≤100 mg), followed by 2.0 mg/kg/h lidocaine (iv.) after intubation until the end of surgery. In Group C, patients received volume-matched normal saline loading dose and infusion as placebo. When BIS <50, endotracheal intubation was performed with muscle relaxation. Mechanical ventilation parameters settings were: tidal volume (VT) 6–8 mL/kg, respiratory rate (RR) 12–16 beats/min, the ratio of inspiratory and exhalation time (I:E) 1:1.5, PETCO2 30–35 mmHg. Anesthesia maintenance was: sevoflurane 1%, remifentanil 0.1–0.3 μg/kg/min, propofol 3–5 mg/kg/h, cis-atracurium 0.01 mg/kg/h, and BIS 40–60. Vasoactive agents would be used when intraoperative blood pressure and HR fluctuated dramatically (at least 20% above or below baseline). All patients were given 12.5 mg dolasetron (iv.) 15 min before the end of surgery. Anesthetics were stopped after skin closure.
After surgery, patients were transferred to postanesthesia care unit (PACU) for further observation. When extubation indications were met, the endotracheal tube would be removed and the surgeon would perform a neurological evaluation.
Considering that postoperative pain may affect physical comfort, emotional state, and physical independence, we adopted postoperative analgesia (diclofenac sodium) to maintain VAS <4, preventing the pain from affecting breathing, sleep, or normal life. Remedial analgesia scheme in the ward: if VAS ≥4, a 75 mg diclofenac sodium hydrochloride sustained-release tablet would be administered, and again within clinical dose if necessary.
Outcome Assessments, Collection of Blood Samples and Testing
The QoR-40 scale (the simplified Chinese version by Myles et al.)2 was used to record and evaluate postoperative recovery quality by the same trained anesthesiologist at preoperative visits and postoperative follow-ups. Global QoR-40 score ranges from 40 to 200 points (best quality). It includes five clinically relevant dimensions: physical comfort (12 items), emotional state (9 items), physical independence (5 items), psychological support (7 items), and pain (7 items), each of which rates from 1 to 5 points. Pain intensity was evaluated with the VAS scores (0 = no pain, 10 = unbearable pain) at six time points, namely at postoperative hour (POH) 1, 2, 6, 12, 24 and 48. Patients were assessed for PONV and consumption of diclofenac in the ward within POH 24. Further assessments included consumption of anesthetics and vasoactive drugs, and incidences of adverse events during surgery and extubation. Data on medical history, surgery, the time to first defecation after surgery, and length of hospital stay were extracted from the medical records.
Blood samples were collected from radial artery before anesthesia induction (T0) and at the end of surgery (T4), subsequently stored at 4 °C in refrigerator. These samples were centrifuged for 10 min at 3000 rpm to separate serum, which would be stored at −80 °C for subsequent testing. Plasma concentrations of S100B, NSE, IL-6 and TNF-α were tested by an enzyme-linked immunosorbent assay (ELISA), using commercially available kits (Shanghai Lanpai Biotechnology Co., Ltd, Shanghai, China).
Outcomes
Primary outcomes were QoR-40 scores on POD 1 and POD 2. Secondary outcomes were as follows: QoR-40 scores on preoperative (Pre) day and POD 7; concentrations of pro-inflammatory factors (IL-6, TNF-α) and brain injury-related factors (S100B, NSE) assessed preoperatively (T0) and at the end of surgery (T4); mean artery pressure (MAP) and HR were recorded before anesthesia induction (T0), immediately after intubation (T1), scalp incision (T2), dura mater incision (T3), end of surgery (T4), and extubation (T5); intraoperative consumption of sufentanil, remifentanil and propofol; incidences of coughing and agitation during extubation; neutrophil to lymphocyte ratio (NLR); incidence of 24 h PONV; time to first defecation; postoperative hospital stay; and pain intensity.15,16 VAS and Athens Insomnia Scale (AIS) were used to evaluate pain intensity and sleep quality, respectively. Consumption of diclofenac in the ward and perioperative adverse events were also recorded.
Statistical Analysis
The sample size was calculated with PASS (version 15, NCSS, LLC, Kaysville, UT, USA), based on primary outcome measures, i.e. QoR-40 scores. The clinical recovery quality improvement was defined as a jump of at least 10 points in the QoR-40 score on the first 7 days after surgery.17 Based on results by Lee et al.,18 average QoR-40 scores on POD 1 between two groups had a difference of 13. The estimated sample size was 28 patients per group, with a power of 80% and an α risk of 0.05. To compensate for possible loss of follow-up or rejection, a 10% drop-out would be considered. Finally, 64 patients were included in our study.
Shapiro–Wilk test was performed for normality test, and Levene’s test was used for homogeneity of variance test. Normally distributed data were analysed by Student’s t-test, presented as mean and standard deviation (SD). Non-normally distributed data were compared by Mann–Whitney U-test, presented as median and inter-quartile range (IQR). Incidences of coughing and agitation during extubation and PONV were compared by Fisher’s exact test or chi-square test, displayed as number and percentage. Repeated measurement analyses of variance (ANOVA) were performed to compare hemodynamic parameters at different time points and QoR-40 scores on different days. Variables such as age, weight, height, consumption of propofol and remifentanil, duration of surgery and anesthesia, pulmonary infection, the VAS score, usage of colloidal solution and crystalloid solution, urine and bleeding volume, AIS, and NLR were included in the univariate regression analysis. Factors with P <0.2, the univariate linear regression analysis identified, and other variables that may be statistically significant, such as consumption of propofol and remifentanil, were included in the subsequent multivariate linear regression analysis. All statistical analyses were performed using IBM SPSS Statistics (Version 26, IBM SPSS, Chicago, IL, USA) and GraphPad Prism (Version 9, GraphPad Software, La Jolla, CA, USA).
Results
Patient Characteristics
During the period of recruitment, a total of 84 patients who were scheduled for supratentorial tumor resection under elective general anesthesia were screened from the Affiliated Hospital of Xuzhou Medical University. Fourteen participants were subsequently excluded for not satisfying the inclusion criteria or refusing to participate. Thus, 70 individuals were ultimately randomized into Group L or Group C. After randomization, 3 patients were excluded: 2 for cancellation of the operation due to systolic blood pressure exceeding 200 mmHg, and 1 for cancellation of the operation due to severely difficult airway. Seven patients were excluded after follow-up: 3 for transferring to ICU after surgery, and 4 for postoperative pathology showing non-tumor. Sixty patients finally completed our study (Figure 1): 30 patients were allocated to the lidocaine group, and 30 patients were allocated to the normal saline group. Eligible patients were enrolled consecutively from 1 November 2020 to 30 June 2021, and there were no significant differences in baseline characteristics between two groups (Table 1).
 |
Table 1 Patient Characteristics and Surgical Data |
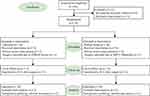 |
Figure 1 Flow diagram of the study. |
Study Endpoints
Global QoR-40 scores and their 5 dimensions on Pre were comparable in the two groups (Figure 2). Compared with Pre, global QoR-40 scores, as well as physical comfort scores, emotional state scores, physical independence scores, and pain scores had decreased (P <0.05) on POD 1 and POD 2; psychological support scores, on POD 1 in group C and on POD 2 in group L, had decreased (P <0.05); global QoR-40 scores, physical comfort scores, and physical independence scores on POD 7, as well as pain scores in group C, had also significantly declined; while psychological support scores had significantly increased (P <0.05).
S100B, NSE, IL-6, and TNF-α in plasma at T4 were higher than at T0 (P <0.05) (Table 2), and lower in group C than in group L (P <0.05). Consumption of propofol and remifentanil between two groups were statistically significantly different (P <0.05).
 |
Table 2 Plasma Concentrations of S100B, NSE, IL-6 and TNF-α |
In group L, HR was slower at T4, and MAP was higher at T1 and lower at T5 than group C (P <0.05) (Figure 3). Incidences of intraoperative hypertension and hypotension, postoperative coughing and mania, the PONV, postoperative hospital stay, usage of vasopressor drugs and diclofenac in group L were lower than in group C (P <0.05) (Table 3). In group C, the VAS scores at POH 1, 2, 6, 12 and 24 after surgery were significantly lower than in group L (P <0.05). No significant differences were identified with regard to the consumption of sufentanil, the usage of antihypertension drugs, NLR, time to first defecation, and the VAS scores at POH 48 between the two groups. None experienced persistent arrhythmia, convulsions, or other adverse events originating from lidocaine during our study period.
 |
Table 3 Perioperative Parameters Between the Two Groups |
The results of univariate linear regression analysis and multivariate linear regression analysis of global recovery quality on POD 1 are shown in Table 1A and 1B, in the Supplementary 1. Usage of intraoperative fluids had no difference significantly between two groups (Table 2A, in the Supplementary 2). Incidences and grades of coughing and sedation were lower in group L than in group C (Table 3A, in the Supplementary 3).
Discussion
The results of our study showed that patients with systemic infusion of lidocaine during supratentorial tumor resection had higher postoperative recovery quality than those in the normal saline group. In view of the lower consumption of opioids, lighter intensity of pain, less incidence of PONV in the lidocaine group, as well as lower plasma levels of pro-inflammatory (IL-6, TNF-α) and brain injury-related (S100B, NSE) factors, we could draw conclusions that inflammation-alleviating and analgesic properties of lidocaine participated in the improvement of postoperative recovery quality, as well as its brain-injury alleviation effect.
The World Health Organization (WHO) defined health as: a state of perfect physical, mental and social activities. In the 21st century, along with the social structure of population aging, the ability to manage one’s own discomfort and health conditions has been increasingly valued, i.e. to respond to various environmental events.8,19 In our study, scores of physical comfort, emotional state and pain in the lidocaine group were significantly improved on POD 1 and POD 2 compared with the matched group; and it was similar to the study by Kim et al.20 However, lidocaine did not significantly improve patients’ physical independence scores and psychological support scores. Frailty is a difficult problem that must be faced and dealt with in the process of postoperative recovery even affecting postoperative cognitive function.21,22 Donald et al. found that frailty, significantly related to the physical independence of elderly patients after surgery, delayed postoperative recovery process or even led to death.23 Due to wide ranges of age in our study, the benefit of lidocaine on frailty in elderly people remains to be further confirmed. Multivariate regression analysis showed that age was a significant risk of recovery quality after supratentorial tumor resection, which should also be paid more attention in the aging society. At the same time, it is necessary for clinicians to give more humanistic care, help to improve patients’ psychological quality and offer more social support, so as to enhance a sense of happiness and promote postoperative recovery.24
When put into clinical practice for the first time, lidocaine was known for its ability to block sodium ion channels, as a local anesthetic. An increasing number of studies proved usage of lidocaine during general anesthesia contributes to reducing consumption of propofol and remifentanil, which was also confirmed in our study.12,25,26 This phenomenon could be explained by the fact that lidocaine shares certain common pathways (such as blocking sodium ion channels) with propofol and remifentanil, in aspects of analgesic mechanisms.27 Given the complexity of pain mechanisms, these pathways remain to be explored with further randomized controlled trials (RCTs) and confirmed by more high-level fundamental experiments, with more anesthesiologists’ efforts. Corcoran et al. found that propofol alleviated the stress response from surgery by inhibiting the inflammatory reaction.28 An interesting phenomenon found in our study was that, with intraoperative infusion of lidocaine, the propofol consumption reduced while inflammatory factors’ levels significantly declined. Several possible interpretations involved were as follows: (1) Anesthetic mechanisms of lidocaine and propofol were not completely overlapping; (2) lidocaine had a protective effect on endothelial cells, but excessive infusion of propofol may destroy endothelial cells, which consequently weakened the anti-inflammatory effect of propofol by damaging the immune system; (3) lidocaine itself had an effect of inhibiting inflammatory response.10,25,29,30
Lidocaine promotes postoperative recovery by ameliorating analgesic effect.31,32 It was obvious that higher pain scores in our study significantly lowered postoperative recovery quality, especially POH 12 and POH 24; and this was consistent with the study by Licina et al.33 Pain not only causes short-term or long-term fluctuations in hemodynamics, but also delays brain function returning to normal levels, leading to postoperative cognitive dysfunction or abnormal mental activity; afterwards it slows down the postoperative recovery process.34,35 The former was revealed by lower fluctuations of intraoperative hemodynamic parameters (such as MAP and HR) and lower utilization of vasoactive drugs in our study; and the incidence and extent of agitation reduced significantly during extubation, suggesting that lidocaine played a positive role in postoperative brain function recovery. Furthermore, there was no significant difference in peripheral fluid infusion between groups in this study. Considering indicators closely related to tissue perfusion were not monitored, it remains to be further confirmed which strategy, in terms of maintaining stable blood pressure and maintaining adequate tissue perfusion, was more beneficial.
The surgery, as one stressor, will inevitably stimulate the neuroendocrine system and the inflammatory immune response, hindering postoperative recovery.36 However, controlling intraoperative stress responses helps to improve postoperative recovery quality.37 In this study, inflammatory and brain injury-related factors were significantly increased compared with those before anesthesia induction. However, lidocaine significantly reduced inflammatory factors levels in comparison to normal saline, providing the evidence that it mitigated the inflammatory response, and accelerated rather than completely reversed the brain damage that originated from anaesthetic and operative stresses. We could speculate that the inflammation alleviation effect of lidocaine is one of its routes to improve postoperative recovery quality and plays a positive role in brain protection. In addition, Tu et al. found that controlling levels of inflammatory factors could also contribute to cognitive function recovery, which further confirmed the benefit to brain of lidocaine.38
PONV is one of the complex and important problems in anesthesia practice. It is well-known that opioids, mostly belonging to μ-, δ- or κ-receptor agonists, can directly activate the emetic chemoreceptor trigger zone (CTZ) located in the medulla oblongata, evoking nausea and vomiting; and this effect is correlated with usage amount of opioids.39 Echevarria et al. found that, in children, there was no correlation between reduction of opioid consumption and PONV.40 Thus, it could be predicted that age was an important risk factor for PONV. In this study, lidocaine may accelerate gastrointestinal function recovery and reduce the incidence of PONV mainly through reducing consumption of opioid drugs and improving analgesic effect. In addition, in types of surgery such as neurosurgery, the stress response derived from anaesthetic and surgical operations, and pain events are also risk factors for PONV.41 It is still necessary and challenging for anesthesiologists to actively participate in multidisciplinary cooperation and promote completion of high-quality enhanced recovery after surgery.42
Intravenous lidocaine may cause various adverse effects, including tongue and lip numbness, metallic taste in the mouth, dizziness, convulsions, and arrhythmias. Exclusion criteria were strictly followed to ensure potentially at risk individuals were excluded as completely as possible. If the P-R interval prolonged, the QRS wave widened in ECG, or arrhythmia occurred during lidocaine infusion, the trial would be cancelled. The common intravenous lidocaine infusion regimens in clinical practice were 100 mg or 1.5–2.0 mg/kg initially, and 1–3 mg/kg/h subsequently.43 Carabalona et al. also found that the median serum concentration of lidocaine was 1.45 (0.98–1.88) µg/mL following a 1.5 mg/kg bolus and intravenous infusion at a rate of 2.0 mg/kg/h, which did not reach toxic concentrations (defined as >5 µg/mL).44 Therefore, the dose of lidocaine used in this study could be considered safe, in which the total dose of lidocaine per individual was normally distributed (529.3±145.8 mg), ranging from 253.3 mg to 893.5 mg. At the same time, we did not find any adverse effects attributing to lidocaine occurring in this study.
Our conclusions in this study were obtained in the context of certain limitations. Firstly, lidocaine concentration in plasma was not monitered. The dose of lidocaine administrated referred to previous studies.43,45,46 Although toxic effects of lidocaine are easily recognized in conscious subjects (in excess of 5 μg/mL in plasma), subjects in the status of general anesthesia need higher level of lidocaine up to 10 μg/mL in plasma when circulatory depression could be discovered. Luckily, none had yet to experience persistent arrhythmia, convulsions or other adverse events originating from lidocaine in our study. Secondly, the recovery quality in this study was only assessed within postoperative 7 days, and failed to be continued follow-up for longer periods of time. The effect of intravenous lidocaine on long-term recovery requires more clinical trials to be carried out. Thirdly, previous studies have explored different infusion durations and rates of lidocaine administration, mainly ranging from the end of the surgery to 48 h later and from 1.0 mg/kg/h to 3.0 mg/kg/h, respectively, but we only adopted one type of dose of lidocaine and infused intravenously lasting up to the end of surgery. Therefore, optimal dose of lidocaine on accelerating postoperative recovery quality remains to be further evaluated. Fourthly, several recent studies have reported the anti-tumor effect of lidocaine, whereas our study did not focus on prognoses such as progression-free survival, survival and mortality rates.47,48 Further studies will be needed to explore this benefit in patients after neurosurgery. Finally, elevated S100B, as a sign of central nervous system injury, could also occur in the absence of brain injury. Although there was no statistical difference in the distribution of surgery types or surgery duration between the two groups in our study, S100B in plasma could also be affected by multiple factors, such as the amount of exercise, degree of muscle injury, hemolysis, or even measurement methods.16 At the same time, lidocaine could reduce levels of inflammatory factors by alleviating pain, which would make the direct anti-inflammatory effect of lidocaine unclear. Therefore, further control of these variables or further studies to reduce the confounding bias will be necessary.
Conclusion
Systemic lidocaine notably improved postoperative early recovery quality after supratentorial tumor resection, with inflammation and pain alleviated, and perioperative opioid consumption reduced. It also had a positive brain injury alleviation effect in patients after neurosurgery. Considering there are few studies validating its brain protection effect, further studies confirming the brain-protection mechanism of lidocaine are warranted.
Data Sharing Statement
The individual participant’s data underlying the results reported in this article may be accessed with approval from the corresponding author 6 months after publication of this study. The study protocol, statistical analysis plan, and clinical study report will also be made available.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
All authors have no conflicts of interest to report.
References
1. Chen Y, Wang J, Liu S, et al. Development and validation of the Chinese version of the quality of recovery-40 questionnaire. Ther Clin Risk Manag. 2020;16:1165–1173. doi:10.2147/TCRM.S281572
2. Myles PS, Weitkamp B, Jones K, Melick J, Hensen S. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth. 2000;84(1):11–15. doi:10.1093/oxfordjournals.bja.a013366
3. Gornall BF, Myles PS, Smith CL, et al. Measurement of quality of recovery using the QoR-40: a quantitative systematic review. Br J Anaesth. 2013;111(2):161–169. doi:10.1093/bja/aet014
4. Leslie K, Troedel S, Irwin K, et al. Quality of recovery from anesthesia in neurosurgical patients. Anesthesiology. 2003;99(5):1158–1165. doi:10.1097/00000542-200311000-00024
5. Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet. 2019;393(10180):1558–1568. doi:10.1016/S0140-6736(19)30430-1
6. Lavand’homme P, Steyaert A. Opioid-free anesthesia opioid side effects: tolerance and hyperalgesia. Best Pract Res Clin Anaesthesiol. 2017;31(4):487–498. doi:10.1016/j.bpa.2017.05.003
7. Alexander JC, Patel B, Joshi GP. Perioperative use of opioids: current controversies and concerns. Best Pract Res Clin Anaesthesiol. 2019;33(3):341–351. doi:10.1016/j.bpa.2019.07.009
8. Nakhli MS, Kahloul M, Guizani T, Zedini C, Chaouch A, Naija W. Intravenous lidocaine as adjuvant to general anesthesia in renal surgery. Libyan J Med. 2018;13(1):1433418. doi:10.1080/19932820.2018.1433418
9. Sumathi PA, Shenoy T, Ambareesha M, Krishna HM. Controlled comparison between betamethasone gel and lidocaine jelly applied over tracheal tube to reduce postoperative sore throat, cough, and hoarseness of voice. Br J Anaesth. 2008;100(2):215–218. doi:10.1093/bja/aem341
10. Lv X, Li X, Guo K, et al. Effects of systemic lidocaine on postoperative recovery quality and immune function in patients undergoing laparoscopic radical gastrectomy. Drug Des Devel Ther. 2021;15:1861–1872. doi:10.2147/DDDT.S299486
11. Sun Y, Li T, Wang N, Yun Y, Gan TJ. Perioperative systemic lidocaine for postoperative analgesia and recovery after abdominal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 2012;55(11):1183–1194. doi:10.1097/DCR.0b013e318259bcd8
12. Hung KC, Chu CC, Hsing CH, et al. Association between perioperative intravenous lidocaine and subjective quality of recovery: a meta-analysis of randomized controlled trials. J Clin Anesth. 2021;75:110521. doi:10.1016/j.jclinane.2021.110521
13. Naito H, Takeda Y, Danura T, Kass IS, Morita K. Effect of lidocaine on dynamic changes in cortical reduced nicotinamide adenine dinucleotide fluorescence during transient focal cerebral ischemia in rats. Neuroscience. 2013;235:59–69. doi:10.1016/j.neuroscience.2013.01.010
14. Niiyama S, Tanaka E, Tsuji S, et al. Neuroprotective mechanisms of lidocaine against in vitro ischemic insult of the rat hippocampal CA1 pyramidal neurons. Neurosci Res. 2005;53(3):271–278. doi:10.1016/j.neures.2005.07.004
15. Buonora JE, Yarnell AM, Lazarus RC, et al. Multivariate analysis of traumatic brain injury: development of an assessment score. Front Neurol. 2015;6:68. doi:10.3389/fneur.2015.00068
16. Bloomfield SM, McKinney J, Smith L, Brisman J. Reliability of S100B in predicting severity of central nervous system injury. Neurocrit Care. 2007;6(2):121–138. doi:10.1007/s12028-007-0008-x
17. De Oliveira GS, Fitzgerald P, Streicher LF, Marcus RJ, McCarthy RJ. Systemic lidocaine to improve postoperative quality of recovery after ambulatory laparoscopic surgery. Anesth Analg. 2012;115(2):262–267. doi:10.1213/ANE.0b013e318257a380
18. Lee WK, Kim MS, Kang SW, Kim S, Lee JR. Type of anaesthesia and patient quality of recovery: a randomized trial comparing propofol-remifentanil total i.v. anaesthesia with desflurane anaesthesia. Br J Anaesth. 2015;114(4):663–668. doi:10.1093/bja/aeu405
19. Fallon CK, Karlawish J. Is the WHO definition of health aging well? Frameworks for “health” after three score and ten. Am J Public Health. 2019;109(8):1104–1106. doi:10.2105/AJPH.2019.305177
20. Kim MH, Kim MS, Lee JH, Kim ST, Lee JR. Intravenously administered lidocaine and magnesium during thyroid surgery in female patients for better quality of recovery after anesthesia. Anesth Analg. 2018;127(3):635–641. doi:10.1213/ANE.0000000000002797
21. Montgomery CL, Rolfson DB, Bagshaw SM. Frailty and the association between long-term recovery after Intensive care unit admission. Crit Care Clin. 2018;34(4):527–547. doi:10.1016/j.ccc.2018.06.007
22. Rothrock RJ, Steinberger JM, Badgery H, et al. Frailty status as a predictor of 3-month cognitive and functional recovery following spinal surgery: a prospective pilot study. Spine J. 2019;19(1):104–112. doi:10.1016/j.spinee.2018.05.026
23. Donald GW, Ghaffarian AA, Isaac F, et al. Preoperative frailty assessment predicts loss of Independence after vascular surgery. J Vasc Surg. 2018;68(5):1382–1389. doi:10.1016/j.jvs.2018.02.044
24. Archer S, Pinto A, Vuik S, et al. Surgery, complications, and quality of life: a longitudinal cohort study exploring the role of psychosocial factors. Ann Surg. 2019;270(1):95–101. doi:10.1097/SLA.0000000000002745
25. Forster C, Vanhaudenhuyse A, Gast P, et al. Intravenous infusion of lidocaine significantly reduces propofol dose for colonoscopy: a randomised placebo-controlled study. Br J Anaesth. 2018;121(5):1059–1064. doi:10.1016/j.bja.2018.06.019
26. Sloan TB, Mongan P, Lyda C, Koht A. Lidocaine infusion adjunct to total intravenous anesthesia reduces the total dose of propofol during intraoperative neurophysiological monitoring. J Clin Monit Comput. 2014;28(2):139–147. doi:10.1007/s10877-013-9506-x
27. Magori N, Fujita T, Mizuta K, Kumamoto E. Inhibition by general anesthetic propofol of compound action potentials in the frog sciatic nerve and its chemical structure. Naunyn Schmiedebergs Arch Pharmacol. 2019;392(3):359–369. doi:10.1007/s00210-018-01596-w
28. Corcoran TB, Engel A, Sakamoto H, O’Shea A, O’Callaghan-Enright S, Shorten GD. The effects of propofol on neutrophil function, lipid peroxidation and inflammatory response during elective coronary artery bypass grafting in patients with impaired ventricular function. Br J Anaesth. 2006;97(6):825–831. doi:10.1093/bja/ael270
29. de Klaver MJM, Buckingham MG, Rich GF. Lidocaine attenuates cytokine-induced cell injury in endothelial and vascular smooth muscle cells. Anesth Analg. 2003;97(2):465–470. doi:10.1213/01.ANE.0000073162.27208.E9
30. Lin MC, Lin CF, Li CF, Sun DP, Wang LY, Hsing CH. Anesthetic propofol overdose causes vascular hyperpermeability by reducing endothelial glycocalyx and ATP production. Int J Mol Sci. 2015;16(12):12092–12107. doi:10.3390/ijms160612092
31. Xu Y, Ye M, Hong Y, et al. Efficacy of perioperative continuous intravenous lidocaine infusion for 72 hours on postoperative pain and recovery in patients undergoing hepatectomy: study protocol for a prospective randomized controlled trial. J Pain Res. 2021;14:3665–3674. doi:10.2147/JPR.S341550
32. Rekatsina M, Theodosopoulou P, Staikou C. Effects of intravenous dexmedetomidine versus lidocaine on postoperative pain, analgesic consumption and functional recovery after abdominal gynecological surgery: a randomized placebo-controlled double blind study. Pain Physician. 2021;24(7):E997–E1006.
33. Licina A, Silvers A. Perioperative intravenous lidocaine infusion for post-operative analgesia in patients undergoing surgery of the spine systematic review and meta-analysis. Pain Med. 2022;23(1):45–56. doi:10.1093/pm/pnab210
34. Yan W, Mao H, Qiu P. Effects of different analgesia regimens on early post-operative cognitive dysfunction in elderly patients undergoing radical resection of cervical carcinoma. Exp Ther Med. 2019;18(2):1465–1469. doi:10.3892/etm.2019.7702
35. Du X, Song F, Zhang X, Ma S. Protective efficacy of combined use of parecoxib and dexmedetomidine on postoperative hyperalgesia and early cognitive dysfunction after laparoscopic cholecystectomy for elderly patients. Acta Cir Bras. 2019;34(9):e201900905. doi:10.1590/s0102-865020190090000005
36. Kehlet H. Enhanced postoperative recovery: good from afar, but far from good? Anaesthesia. 2020;75(Suppl 1):e54–e61. doi:10.1111/anae.14860
37. Li X, Xiang H, Zhang W, Peng C. The effects of remifentanil combined with propofol on the oxidative damage and the stress and inflammatory responses in cardiac surgery patients. Am J Transl Res. 2021;13(5):4796–4803.
38. Tu W, Yuan H, Zhang S, et al. Influence of anesthetic induction of propofol combined with esketamine on perioperative stress and inflammatory responses and postoperative cognition of elderly surgical patients. Am J Transl Res. 2021;13(3):1701–1709.
39. Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118(1):85–113. doi:10.1213/ANE.0000000000000002
40. Echevarria GC, Altermatt FR, Paredes S, et al. Intra-operative lidocaine in the prevention of vomiting after elective tonsillectomy in children: a randomised controlled trial. Eur J Anaesthesiol. 2018;35(5):343–348. doi:10.1097/EJA.0000000000000807
41. Grape S, Kirkham KR, Frauenknecht J, Albrecht E. Intra-operative analgesia with remifentanil vs. dexmedetomidine: a systematic review and meta-analysis with trial sequential analysis. Anaesthesia. 2019;74(6):793–800. doi:10.1111/anae.14657
42. Lu D, Wang Y, Zhao T, et al. Successful implementation of an enhanced recovery after surgery (ERAS) protocol reduces nausea and vomiting after infratentorial craniotomy for tumour resection: a randomized controlled trial. BMC Neurol. 2020;20(1):150. doi:10.1186/s12883-020-01699-z
43. Weibel S, Jelting Y, Pace NL, et al. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev. 2018;6:CD009642. doi:10.1002/14651858.CD009642.pub3
44. Carabalona JF, Delwarde B, Duclos A, et al. Serum concentrations of lidocaine during bariatric surgery. Anesth Analg. 2020;130(1):e5–e8. doi:10.1213/ANE.0000000000003905
45. Beaussier M, Delbos A, Maurice-Szamburski A, Ecoffey C, Mercadal L. Perioperative use of intravenous lidocaine. Drugs. 2018;78(12):1229–1246. doi:10.1007/s40265-018-0955-x
46. de Oliveira CMB, Coelho LMG, Valadao JA, et al. Assessment of the effect of perioperative venous lidocaine on the intensity of pain and IL-6 concentration after laparoscopic gastroplasty. Obes Surg. 2020;30(10):3912–3918. doi:10.1007/s11695-020-04748-1
47. Chamaraux-Tran TN, Piegeler T. The amide local anesthetic lidocaine in cancer surgery-potential antimetastatic effects and preservation of immune cell function? A narrative review. Front Med. 2017;4:235. doi:10.3389/fmed.2017.00235
48. Johnson MZ, Crowley PD, Foley AG, et al. Effect of perioperative lidocaine on metastasis after sevoflurane or ketamine-xylazine anaesthesia for breast tumour resection in a murine model. Br J Anaesth. 2018;121(1):76–85. doi:10.1016/j.bja.2017.12.043
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


