Back to Journals » International Journal of General Medicine » Volume 16
Effect of rScO2-Guided Blood Pressure Management on Postoperative Complications in Elderly Patients After Major Noncardiac Surgery: Protocol for a Randomized Controlled Trial
Authors Yang YF , Liu LL, Huang MJ, Ma ZM , Huo WW, Zhu YJ, Liu H , Peng K , Ji FH
Received 18 June 2023
Accepted for publication 18 August 2023
Published 25 August 2023 Volume 2023:16 Pages 3789—3796
DOI https://doi.org/10.2147/IJGM.S426245
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yuriy Sirenko
Yu-fan Yang,1,2,* Lin-Lin Liu,1,2,* Ming-jie Huang,1,2,* Zheng-min Ma,1,2 Wen-wen Huo,1,2 Ya-juan Zhu,1,2 Hong Liu,3 Ke Peng,1,2 Fu-Hai Ji1,2
1Department of Anesthesiology, First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, People’s Republic of China; 2Institute of Anesthesiology, Soochow University, Suzhou, Jiangsu, People’s Republic of China; 3Department of Anesthesiology and Pain Medicine, University of California Davis Health, Sacramento, CA, USA
*These authors contributed equally to this work
Correspondence: Ke Peng; Fu-Hai Ji, Department of Anesthesiology, First Affiliated Hospital of Soochow University, 188 Shizi Street, Suzhou, Jiangsu, 215006, People’s Republic of China, Email [email protected]; [email protected]
Background: Postoperative complications are common after major surgical procedures, leading to increased morbidity and mortality. Regional cerebral oxygen saturation (rScO2) reflects cerebral and global perfusion, and thus it can be used to guide hemodynamic management. We aim to explore the effect of rScO2-guided blood pressure management strategy on postoperative major complications in older adults who undergo major noncardiac surgery.
Methods: This randomized controlled clinical trial includes a total of 400 elderly patients receiving major noncardiac surgery and general anesthesia. Patients will be randomized (1:1) to one of two blood pressure management groups: a standard care group (targeting mean arterial pressure > 65 mmHg or within 20% of baseline value), and a rScO2-guided group (absolute value of rScO2 > 60% or decrease in rScO2 < 10% of baseline). The primary outcome is the composite outcome of major complications (including infectious, respiratory, neurologic, cardiovascular, renal, thromboembolic gastrointestinal, and surgical complications) and deaths within the first 7 days after surgery. Secondary outcomes include the individual components of the primary outcome by day 7 after surgery and 30-day mortality. Data will be analyzed in the modified intention-to-treat population.
Discussion: This study will provide evidence for improving postoperative outcomes using the rScO2-guided blood pressure management among older adults who undergo major noncardiac surgery.
Trial Registration: Chinese Clinical Trial Registry (Identifier: ChiCTR2200060816).
Plain Language Summary: This is a protocol for a prospective, randomized, controlled clinical trial to evaluate the use of intraoperative individualized regional cerebral oxygen saturation (rScO2) optimization for blood pressure management in older adults undergoing major noncardiac surgery.
The primary focus of this trial is the composite outcome of major complications (including infectious, respiratory, neurologic, cardiovascular, renal, thromboembolic gastrointestinal, and surgical complications) and deaths within the first 7 days after surgery. The secondary outcomes are the individual components of the primary outcome by day 7 after surgery and 30-day mortality.
The findings of this trial will provide clinical evidence for the rScO2-guided blood pressure management to improve postoperative outcomes in older patients who are scheduled for major noncardiac surgery.
Keywords: regional cerebral oxygen saturation, blood pressure management, postoperative major complications, major noncardiac surgery, elderly patients
Introduction
With the increase in life expectancy and improved medical technology, more elderly patients are receiving major surgical procedures that undoubtedly increase the risk of postoperative morbidity and mortality.1,2 However, postoperative complications are common, which lead to prolonged hospitalization, increased healthcare costs, compromised quality of life, and increased deaths after surgery.3,4 Perioperative hemodynamic management is crucial for patient outcomes after surgery. Previous studies have suggested that optimizing fluid therapy, increasing cardiac output and oxygen delivery, and appropriate blood pressure management may reduce postoperative complications.5–7
Regional cerebral oxygen saturation (rScO2) monitoring by non-invasive near-infrared spectroscopy has been used recently to illustrate the balance between oxygen demand and supply in brain. Low rScO2 values were associated with perioperative low hemoglobin concentration, cognitive dysfunction, prolonged recovery, and increased complications and deaths after cardiac and noncardiac surgery.8–11 With the index organ of brain, rScO2 has been considered to reflect global perfusion, and thus strategies that optimize rScO2 may improve perfusion in vital organs and patients’ clinical outcomes.12,13
In this context, we design this randomized clinical study to evaluate the use of rScO2-guided blood pressure management strategy through individualized rScO2 optimization among older adults who undergo major noncardiac surgery. We hypothesize that the rScO2-guided blood pressure management would reduce postoperative major complications in this patient population.
Methods
Study Design
This prospective randomized controlled trial is carried out at two medical centers of the First Affiliated Hospital of Soochow University, China. A total of 400 elderly patients undergoing major noncardiac surgery will be recruited. Figure 1 shows the study flowchart. We report this protocol according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guideline (Table S1).14
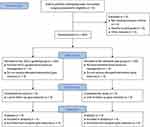 |
Figure 1 Study flowchart. Abbreviation: rScO2, regional cerebral oxygen saturation. |
Ethics and Dissemination
This trial was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (Approval No. 2022-044) on April 28, 2022, and then registered on the Chinese Clinical Trial Registry (http://www.chictr.org.cn, ChiCTR2200060816) on June 12, 2022. Due to the COVID-19 pandemic and shortage of staff, we only recruited 70 patients (17.5% of total) by the time of this submission. We expect to complete patient recruitment before December 31, 2024. All patients will provide their written informed consent. Patients can withdraw their consent at any time during the study. This study follows the Declaration of Helsinki. The study results will be published in a peer-reviewed journal.
Inclusion Criteria
- Older patients aged 60−90 years;
- ASA classification II or III;
- Undergoing major noncardiac surgery under general anesthesia.
Major noncardiac surgical procedures include abdominal, urologic, gynecologic, and orthopedic procedures, with an estimated surgical duration ≥2 hours and hospital stay ≥2 days.
Exclusion Criteria
- Emergent surgery;
- Use of spinal and/or epidural anesthesia only;
- Uncontrolled hypertension, which is defined as systolic blood pressure ≥180 mmHg or diastolic blood pressure ≥110 mmHg;
- Acute cardiovascular events including acute or decompensated heart failure, acute coronary syndrome;
- Acute phase of cerebrovascular disease or cerebrovascular accident within 1 month of onset;
- Liver function insufficiency, ie, Child-Pugh grade ≥B;
- Chronic renal disease with creatinine ≥200 μmol/L or end-stage renal disease which is defined as requirement for renal replacement therapy;
- Preoperative sepsis;
- Declined to provide consent.
Randomization and Blinding
An independent research personnel will use the online randomization service (www.91trial.com) to generate a random allocation list, in a 1:1 ratio with stratification according to surgical type (abdominal or nonabdominal surgery) and study center. Patients will be randomly allocated into either a standard care group or a rScO2-guided group. The details of randomization and allocation will be concealed by password protection. It is impossible to mask the group allocation to the anesthesiologists who provide anesthesia, due to obvious differences in the clinical practice of these strategies. All participants, surgeons, data collectors, outcome assessors, perioperative care staff, statisticians, and data monitoring committee will be unaware of the allocation details throughout the study.
Anesthesia
Baseline data such as mean arterial pressure (MAP) and heart rate (HR) are documented in surgical ward before surgery. In the operating room, the routine monitoring includes noninvasive cuff blood pressure, electrocardiography, and pulse oximetry. The radial artery is cannulated after local anesthesia for continuous monitoring of arterial blood pressure. The rScO2 monitoring (ELITE01-06-300, Foresight) will be applied on both sides of the forehead in all patients. Before anesthesia induction, the baseline rScO2 value is obtained when patients breathe room air in the supine position.
Anesthesia is induced using i.v. propofol 1.5–2 mg/kg or etomidate 0.2–0.3 mg/kg, sufentanil 0.2–0.4 μg/kg, and rocuronium 0.6 mg/kg. After endotracheal intubation, anesthesia is maintained with 1–3% sevoflurane or propofol infusion 50–150 μg/kg/min. Boluses of sufentanil and rocuronium are given if needed. The lungs are mechanically ventilated using tidal volume of 6–8 mL/kg (predicted body weight), fraction of inspired oxygen (FIO2) of 50%, and respiratory rate of 12–20 breaths/minute, positive end-expiratory pressure of 5 cm H2O to maintain end-tidal carbon dioxide between 35 and 45 mmHg. Lactated Ringer’s solution of 4 mL/kg/h is infused as basal fluid repletion, and boluses of 6% hydroxyethyl starch (130/0.4) 200 mL over 10 minutes will be given to treat hypovolemia. A warming blanket and/or a fluid warming device are used to maintain nasopharyngeal temperature at 36–37°C. Wound infiltration or nerve blocks can be used at the clinicians’ discretion.
Study Interventions
Table 1 presents the process of enrollment, intervention, and assessment conforming to the SPIRIT statement. For the standard care group, the target is MAP ≥65 mmHg or within ±20% of the baseline value. For the rScO2-guided group, the target is rScO2 ≥60% (the absolute value) or decrease in rScO2 <10% of baseline.15,16 For patients who do not reach the rScO2 target, those who have coexisting hypotension (MAP <65 mmHg or reduction of MAP >20% of baseline that persists for ≥1 min) will be treated with continuous infusion of norepinephrine (10 μg/mL); if patients already show hypertension (increasement of MAP >20% above baseline that persists for ≥1 min), the following interventions will be applied: increasing FIO2 to 100%, maintaining PaCO2 close to 40 mmHg, and erythrocytes transfusion when hematocrit <28%.12,17
 |
Table 1 Schedule of Enrollment, Intervention, and Assessment |
Outcome Measures
The primary outcome is the incidence of composite outcome of major complications and deaths within the first 7 days postoperatively. The secondary outcome measures are the individual components of the primary outcome by day 7 after surgery and 30-day mortality.
The definitions of the major complications are listed in Table S2, including infectious complications (systemic inflammatory response syndrome or sepsis), respiratory complications (hypoxemia, pneumonia, acute respiratory distress syndrome, or need for mechanical ventilation), neurologic complications (altered consciousness, postoperative delirium [POD], or stroke), cardiovascular complications (myocardial ischemia or infarction, acute heart failure, new-onset arrhythmia, or cardiac arrest), renal complications (acute kidney injury or need for renal replacement therapy), thromboembolic complications (deep venous thrombosis or pulmonary embolism), gastrointestinal complications (ileus, intestinal ischemia, acute mesenteric ischemia, or gastrointestinal bleeding), and surgical complications (anastomotic dehiscence or leak, surgical site infection, bleeding, or need for reoperation).
The perioperative data and exploratory outcomes include MAP values, left and right rScO2 values, low rScO2 events (rScO2 <80% of baseline), intraoperative volume of fluids, use of vasopressors, hemodynamic events including hypotension, hypertension, bradycardia (HR <45 beats/min for ≥1 min), and tachycardia (HR >100 beats/min for ≥1 min), postoperative nausea and vomiting, recovery time of bowel function, admission to intensive care unit, duration of intensive care unit stay, length of postoperative hospital stay. In addition, we will collect perioperative blood sample lab results (hemoglobin, hematocrit, red blood cell distribution width [RDW], white blood cells, platelets, prothrombin time, activated partial thromboplastin time, blood urea nitrogen, creatinine, lactate, etc.). Increased RDW has been shown to predict poor outcomes in patients with heart failure or coronary artery disease as well as in the general population.18
Data Collection
An independent investigator screens patients according to the eligibility criteria and collects the demographics and baseline characteristics. The outcome measurements and perioperative data are documented by independent research investigators via postoperative patient visits and reviewing electronic medical records. The 30-day mortality data are acquired by telephone calls. All data are recorded in the case report forms and registered in the electronic database. An independent data monitoring committee conducts an ongoing data review throughout this study. Patients’ confidentiality will be ensured and deidentified data will be analyzed.
Sample Size Estimation
The required sample size was estimated using the PASS software (version 21.0.3, NCSS, LCC, USA). According to the previous study, the incidence of composite outcome of major complications by day 7 after major noncardiac surgery was about 40% in elderly patients receiving a standard blood pressure management.7 We expect that the rScO2-guided blood pressure management strategy would reduce the incidence of composite outcome by 35% (ie, an absolute reduction of 14%). To detect such a difference at two-sided α = 0.05 and power = 80%, 176 patients in each group are needed. By considering that 10% of patients may drop out from this study, we plan to recruit 200 in each group (ie, a total of 400 patients).
Statistical Analysis
The normality of continuous data will be checked by the Shapiro–Wilk test. These data are presented as mean ± standard deviation and median with interquartile ranges and compared with the independent t-test, repeated measures analysis of variance, Mann–Whitney rank-sum test or Kruskal–Wallis test, as appropriate. Categorical data will be presented as numbers (percentages) and compared with the Chi-square test or Fisher’s exact test.
For the study outcomes, the between-group difference will be analyzed using the odds ratio with 95% confidence interval. For the primary outcome, a multivariate logistic regression will be used to adjust for age, surgical type, and study center. Subgroup analyses will be performed based on the stratification. Multiple comparisons of the secondary outcomes will be adjusted using the Benjamini–Hochberg procedure. Survival analysis and Kaplan–Meier curves will be plotted for 30-day mortality using the Cox proportional hazards model. For the exploratory outcomes, no statistical inference should be made.
Data will be analyzed in the modified intention-to-treat population, consisting of all patients after randomization with outcome data available. In addition, we will perform a sensitivity analysis in the per-protocol population, including patients who receive planned interventions without significant protocol violation. Imputation of missing data is not planned. An interim analysis after recruiting 50% of patients is planned, with the stopping rule of α = 0.00305 using the Lan–DeMets method. An independent statistician will analyze all data with the use of SPSS software (version 25.0; IBM SPSS, USA). A two-sided P <0.05 indicate a statistically significant difference.
Discussion
This randomized controlled clinical trial will enroll 400 elderly patients undergoing major noncardiac surgery to compare the rScO2-guided blood pressure management strategy with the standard care strategy. The primary focus is to assess the incidence of composite outcome of major complications by day 7 postoperatively. In addition, we investigate the mortality during the 30-day follow-up. This study will be conducted following the guideline of Consolidated Standards of Reporting Trials.19
Previous studies reported that intraoperative rScO2 reduction was significantly correlated with the development of postoperative cognitive decline, such as POD and postoperative cognitive dysfunction (POCD), in patients undergoing cardiac and noncardiac surgery.20–22 Moreover, a reduction in rScO2 has been used to predict postoperative neurological complications after various types of surgery (such as total hip arthroplasty and carotid endarterectomy).22,23
Interventional studies using rScO2-guilded anesthesia have yielded mixing results. Colak et al reported that maintaining rScO2 >50% (the absolute value) or >80% of baseline reduced cognitive impairment following coronary artery bypass surgery.24 Uysal et al maintained the intraoperative rScO2 value >60% to improve postoperative memory outcome in cardiac surgical patients.16 Recently, another study suggested that the rScO2-guilded management stabilized hemodynamics and reduced the POCD incidence in older patients with hypertension after shoulder arthroscopy.25 However, Lei et al showed that restoration of rScO2 to ≥75% of baseline did not reduce POD in elderly patients following cardiac surgery, while preoperative value of rScO2 ≤50% was associated with higher risk of POD.12 Similarly, a recent meta-analysis suggested that rScO2 monitoring helped to reduce POCD after cardiac and major noncardiac surgical procedures but may have no impact on the development of POD.26
For surgical patients, the primary aim of intraoperative hemodynamic management is to ensure adequate tissue oxygenation and organ perfusion. Mixed venous oxygen saturation monitoring reflects systemic oxygen balance, but this invasive technique cannot be routinely applied in daily practice. The rScO2 monitoring reflects acute hemodynamic fluctuations and tissue perfusion without time delay, which is more reliable than mixed venous oxygen saturation.27 When cardiac output decreases progressively, brain perfusion is preserved preferentially via cerebral autoregulation and blood flow redistribution.28 Low rScO2 reflects cerebral desaturation, providing the clinicians important information when anemia-related impaired oxygenation is still compensated or transfusion may be indicated, especially in patients at lower pretransfusion hemoglobin level.11 Hence, low rScO2 is accompanied by a decreased systemic perfusion, providing an early-warning indicator of regional and global oxygen desaturation. Specifically, low preoperative rScO2 values were associated with cardiopulmonary dysfunction and short- and long-term complications and deaths in patients following cardiac surgical procedures,13,29 and low intraoperative rScO2 was an independent predictor of acute kidney injury.30
To our knowledge, this is the first study to determine whether the rScO2-guided blood pressure management strategy, as compared to the standard, would reduce major postoperative complications in older adults following major noncardiac surgery. The limitations include the lack of longer-term outcomes and exclusion of patients with ASA classification IV. The results of this study would inform future investigations on the very elderly and vulnerable surgical patients.
In summary, the findings of this prospective, randomized, controlled clinical trial will provide clinical evidence for the rScO2-guided intraoperative blood pressure management strategy to improve postoperative outcomes in older patients who are scheduled for major noncardiac surgery.
Data Sharing Statement
Data will be available from the corresponding author on reasonable request.
Funding
This work will be supported by Suzhou Youth Science and Technology Project (KJXW2021008 to YFY), Jiangsu Medical Association Anesthesia Research Project (SYH-32021-0036 (2021031) to KP), Suzhou Medical Health Science and Technology Innovation Project (SKY2022136 to KP), Natural Science Foundation of Jiangsu Province (BK20200195 to WWH), National Natural Science Foundation of China (82072130 to FHJ), Six Talent Peaks Project in Jiangsu Province (WSN-022 to FHJ), Key Medical Research Projects in Jiangsu Province (ZD2022021 to FHJ), Suzhou Clinical Medical Center for Anesthesiology (Szlcyxzxj202102 to FHJ).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372(9633):139–144. doi:10.1016/S0140-6736(08)60878-8
2. Weiser TG, Haynes AB, Molina G, et al. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet. 2015;385(Suppl 2):S11. doi:10.1016/S0140-6736(15)60806-6
3. Staiger RD, Gerns E, Castrejon Subira M, Domenghino A, Puhan MA, Clavien PA. Can early postoperative complications predict high morbidity and decrease failure to rescue following major abdominal surgery? Ann Surg. 2020;272(5):834–839. doi:10.1097/SLA.0000000000004254
4. Pearse RM, Moreno RP, Bauer P, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380(9847):1059–1065. doi:10.1016/S0140-6736(12)61148-9
5. Aaen AA, Voldby AW, Storm N, et al. Goal-directed fluid therapy in emergency abdominal surgery: a randomised multicentre trial. Br J Anaesth. 2021;127(4):521–531. doi:10.1016/j.bja.2021.06.031
6. Brienza N, Biancofiore G, Cavaliere F, et al. Clinical guidelines for perioperative hemodynamic management of non cardiac surgical adult patients. Minerva Anestesiol. 2019;85(12):1315–1333. doi:10.23736/S0375-9393.19.13584-5
7. Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346–1357. doi:10.1001/jama.2017.14172
8. Li H, Fu Q, Wu Z, et al. Cerebral oxygen desaturation occurs frequently in patients with hypertension undergoing major abdominal surgery. J Clin Monit Comput. 2018;32(2):285–293. doi:10.1007/s10877-017-0024-0
9. Roberts ML, Lin HM, Tinuoye E, et al. The association of cerebral desaturation during one-lung ventilation and postoperative recovery: a Prospective Observational Cohort Study. J Cardiothorac Vasc Anesth. 2021;35(2):542–550. doi:10.1053/j.jvca.2020.07.065
10. Tian LJ, Yuan S, Zhou CH, Yan FX. The effect of intraoperative cerebral oximetry monitoring on postoperative cognitive dysfunction and ICU stay in adult patients undergoing cardiac surgery: an updated systematic review and meta-analysis. Front Cardiovasc Med. 2022;8:814313. doi:10.3389/fcvm.2021.814313
11. Delis A, Bautz D, Ehrentraut H, et al. Effects of different hemoglobin levels on near-infrared spectroscopy-derived cerebral oxygen saturation in elderly patients undergoing noncardiac surgery. Transfus Med Hemother. 2023;50:1–7. doi:10.1159/000528958
12. Lei L, Katznelson R, Fedorko L, et al. Cerebral oximetry and postoperative delirium after cardiac surgery: a randomised, controlled trial. Anaesthesia. 2017;72(12):1456–1466. doi:10.1111/anae.14056
13. Murkin JM. Cerebral oximetry: monitoring the brain as the index organ. Anesthesiology. 2011;114(1):12–13. doi:10.1097/ALN.0b013e3181fef5d2
14. Chan AW, Tetzlaff JM, Gotzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346(jan08 15):e7586. doi:10.1136/bmj.e7586
15. Li Y, Phan J, Mamoor A, Liu H. Hypotension prediction index together with cerebral oxygenation in guiding intraoperative hemodynamic management: a case report. J Biomed Res. 2022;36(1):63–67. doi:10.7555/JBR.36.20210164
16. Uysal S, Lin HM, Trinh M, Park CH, Reich DL. Optimizing cerebral oxygenation in cardiac surgery: a randomized controlled trial examining neurocognitive and perioperative outcomes. J Thorac Cardiovasc Surg. 2020;159(3):943–953 e943. doi:10.1016/j.jtcvs.2019.03.036
17. Soeding PF, Hoy S, Hoy G, Evans M, Royse CF. Effect of phenylephrine on the haemodynamic state and cerebral oxygen saturation during anaesthesia in the upright position. Br J Anaesth. 2013;111(2):229–234. doi:10.1093/bja/aet024
18. Van Craenenbroeck EM, Pelle AJ, Beckers PJ, et al. Red cell distribution width as a marker of impaired exercise tolerance in patients with chronic heart failure. Eur J Heart Fail. 2012;14(1):54–60. doi:10.1093/eurjhf/hfr136
19. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. doi:10.1016/j.ijsu.2011.10.001
20. Kim J, Shim JK, Song JW, Kim EK, Kwak YL. Postoperative cognitive dysfunction and the change of regional cerebral oxygen saturation in elderly patients undergoing spinal surgery. Anesth Analg. 2016;123(2):436–444. doi:10.1213/ANE.0000000000001352
21. Lim L, Nam K, Lee S, et al. The relationship between intraoperative cerebral oximetry and postoperative delirium in patients undergoing off-pump coronary artery bypass graft surgery: a retrospective study. BMC Anesthesiol. 2020;20(1):285. doi:10.1186/s12871-020-01180-x
22. Slater JP, Guarino T, Stack J, et al. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg. 2009;87(1):36–44; discussion 44–35. doi:10.1016/j.athoracsur.2008.08.070
23. Bissacco D, Attisani L, Settembrini AM, et al. Modifications in near infrared spectroscopy for cerebral monitoring during carotid endarterectomy in asymptomatic and symptomatic patients. Ann Vasc Surg. 2022;79:239–246. doi:10.1016/j.avsg.2021.06.047
24. Colak Z, Borojevic M, Bogovic A, Ivancan V, Biocina B, Majeric-Kogler V. Influence of intraoperative cerebral oximetry monitoring on neurocognitive function after coronary artery bypass surgery: a randomized, prospective study. Eur J Cardiothorac Surg. 2015;47(3):447–454. doi:10.1093/ejcts/ezu193
25. Jing Z, Wu D. Application of regional cerebral oxygen saturation monitoring with near-infrared spectroscopy in peri-anesthesia management of elderly hypertensive patients undergoing shoulder arthroscopic surgery. Am J Transl Res. 2021;13(5):5568–5574.
26. Ding L, Chen DX, Li Q. Effects of electroencephalography and regional cerebral oxygen saturation monitoring on perioperative neurocognitive disorders: a systematic review and meta-analysis. BMC Anesthesiol. 2020;20(1):254. doi:10.1186/s12871-020-01163-y
27. Moerman A, Vandenplas G, Bove T, Wouters PF, De Hert SG. Relation between mixed venous oxygen saturation and cerebral oxygen saturation measured by absolute and relative near-infrared spectroscopy during off-pump coronary artery bypass grafting. Br J Anaesth. 2013;110(2):258–265. doi:10.1093/bja/aes375
28. Moerman A, De Hert S. Cerebral oximetry: the standard monitor of the future? Curr Opin Anaesthesiol. 2015;28(6):703–709. doi:10.1097/ACO.0000000000000256
29. Heringlake M, Garbers C, Kabler JH, et al. Preoperative cerebral oxygen saturation and clinical outcomes in cardiac surgery. Anesthesiology. 2011;114(1):58–69. doi:10.1097/ALN.0b013e3181fef34e
30. Ko SH, Song JW, Shim JK, Soh S, Kwak YL. Low intraoperative cerebral oxygen saturation is associated with acute kidney injury after off-pump coronary artery bypass. J Clin Med. 2023;12(1). doi:10.3390/jcm12010359
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
