Back to Journals » Drug Design, Development and Therapy » Volume 17
Effect of Remimazolam on Postoperative Delirium in Older Adult Patients Undergoing Orthopedic Surgery: A Prospective Randomized Controlled Clinical Trial
Authors Yang JJ, Lei L, Qiu D, Chen S, Xing LK, Zhao JW, Mao YY, Yang JJ
Received 8 October 2022
Accepted for publication 11 January 2023
Published 20 January 2023 Volume 2023:17 Pages 143—153
DOI https://doi.org/10.2147/DDDT.S392569
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Jin-Jin Yang,1,2 Lei Lei,1,2 Di Qiu,1 Sai Chen,1 Li-Ka Xing,1 Jing-Wei Zhao,1 Yuan-Yuan Mao,1,2 Jian-Jun Yang1,2
1Department of Anesthesiology, Pain and Perioperative Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China; 2Henan Province International Joint Laboratory of Pain, Cognition and Emotion, Zhengzhou, People’s Republic of China
Correspondence: Jian-Jun Yang, Department of Anesthesiology, Pain and Perioperative Medicine, The First Affiliated Hospital of Zhengzhou University, No. 1 East Jianshe Road, Zhengzhou, People’s Republic of China, Tel +8613783537619, Email [email protected]
Background: Postoperative delirium is common in older adult patients and associated with a poor prognosis. The use of benzodiazepine was identified as an independent risk factor for delirium, but there is no randomized controlled trial regarding the relationship between remimazolam, a new ultra-short acting benzodiazepine, and postoperative delirium. We designed a randomized controlled trial to evaluate if remimazolam increases the incidence of postoperative delirium compared with propofol in older adult patients undergoing orthopedic surgery with general anesthesia.
Patients and Methods: We enrolled 320 patients aged more than 60 with American Society of Anesthesiologists physical status I–III who underwent orthopedic surgery. Patients were randomized to two groups to receive intraoperative remimazolam or propofol, respectively. Our primary outcome was the incidence of delirium within 3 days after surgery. Secondary outcome was emergence quality including the incidence of emergence agitation, extubation time, and length of post-anesthesia care unit (PACU) stay. Adverse events were also recorded.
Results: The incidence of postoperative delirium was 15.6% in the remimazolam group and 12.4% in the propofol group (Risk ratio, 1.26; 95% CI, 0.72 to 2.21; Risk difference, 3.2%; 95% CI, − 4.7% to 11.2%; P = 0.42). No significant differences were observed for time of delirium onset, duration of delirium, and delirium subtype between the two groups. Patients in remimazolam group had a lower incidence of hypotension after induction and consumed less vasoactive drugs intraoperatively, but had a longer postoperative extubation time and PACU stay.
Conclusion: General anesthesia with remimazolam was not associated with an increased incidence of postoperative delirium compared with propofol in older adult patients undergoing orthopedic surgery.
Keywords: benzodiazepines, delirium, older adults, orthopedic surgery, propofol, remimazolam
Introduction
Postoperative delirium (POD) is a common complication in older adult patients after anesthesia and surgery, with an estimated incidence of 10–50% depending on the type of surgery.1,2 Patients with POD have an increased risk of postoperative complications and mortality.3,4 The causes of POD are multifactorial, including predisposing and precipitating factors. Among them, perioperative medication, especially benzodiazepines, had gained attention as a critical and potentially intervenable factor of POD.5,6
Remimazolam, a new ultra-short acting benzodiazepine, was recently approved for procedural sedation and general anesthesia.7–9 Its metabolism is mainly induced by tissue esterase, independent of liver and kidney function, and its metabolites are inactive.10,11 Besides, incidences of injection pain and cardiovascular and respiratory depression were less with remimazolam than propofol. Based on these characteristics, remimazolam appears to be an ideal intravenous anesthetic for the older surgical patients. However, previous studies showed that benzodiazepines can increase the risk of POD.12–14 Guidelines from the American Geriatrics Society15 and other organizations16,17 also recommend minimization of benzodiazepines exposure for older surgical patients. As a new benzodiazepine, there are only two newly published observational studies regarding the association of remimazolam with POD.18,19 Therefore, whether remimazolam could increase the incidence of POD in older adult patients remains unclear.
Thus, we designed a randomized controlled trial (RCT) to assess the effect of intraoperative use of remimazolam versus propofol on POD in older adult patients undergoing orthopedic surgery.
Methods
Study Design and Ethics
The study was a single-center, patient- and evaluator-blinded RCT, which was approved by the Ethics Review Committee (2021-KY-0843-002) of the First Affiliated Hospital of Zhengzhou University in China. The trial was registered at Chinese ClinicalTrials.gov on August 26, 2021 (ChiCTR2100050372). This study complied with the 1964 Helsinki declaration and its later amendments. Written informed consent was obtained from all participants.
Participants
This study enrolled a group of patients who underwent orthopedic surgery at the First Affiliated Hospital of Zhengzhou University from November 2021 to June 2022. Inclusion criteria were: 1) age at least 60 years; 2) planned general anesthesia for the proposed orthopedic surgery; 3) American Society of Anesthesiologists physical status (ASA) class I–III; and 4) provision of ethical, signed informed consent. Patients were enrolled if they met all of the inclusion criteria. Exclusion criteria were: 1) emergency surgery; 2) inability to communicate verbally due to deafness and muteness; 3) preoperative delirium or dementia; 4) other conditions, including intolerance or allergy to benzodiazepines, myasthenia gravis, schizophrenia, and severe depressive states. Preoperative cognitive function was screened by the 30-point Mini-Mental State Examination (MMSE). We used the scoring and adjusted for the patient’s educational level as a proxy for preoperative dementia (<= 24 with educational level of secondary education, or MMSE <= 20 with educational level of primary education, or MMSE <= 17 with educational level of illiteracy).20 Delirium was assessed by the Confusion Assessment Method (CAM).21 The CAM is a standardized tool used for the identification of delirium through a diagnostic algorithm based on four cardinal features of delirium: acute onset and fluctuating course, inattention, disorganized thinking, and altered level of consciousness.
Randomization and Blinding
A computer-generated simple randomization list with 1:1 allocation was created by a researcher who was not involved in the trial process, follow-up, and analysis before the study. For allocation concealment, assignments were placed in sealed opaque envelopes, which were sequentially handed to clinicians after randomization, before entering the operating room. Participating anesthesiologists were aware of the patient group allocation because of the administration of anesthetics, but they did not participate in follow-up assessments. Follow-up investigators were blinded to the intervention.
Trial Procedure
Premedication was not permitted in either group. Standard anesthesia care was used, including routine monitoring of the patient’s electrocardiogram, blood pressure, and oxygen saturation. The depth of anesthesia was monitored with the bispectral index (BIS) during the surgery.
In patients assigned to the remimazolam group (R group), anesthesia was induced with remimazolam (0.2–0.3 mg kg−1) and alfentanil (0.04–0.06 mg kg−1) for amnesia and analgesia. Anesthesia was maintained with inhaled desflurane 0.3 age-adjusted minimum alveolar concentration (MAC) combined with intravenous infusion of remimazolam, and the BIS value was maintained between 40 and 60 by adjusting the dose of remimazolam.
In patients assigned to the propofol group (P group), anesthesia was induced with propofol (1.0–1.5 mg kg−1) and alfentanil (0.04–0.06 mg kg−1) for amnesia and analgesia. Anesthesia was maintained with inhaled desflurane 0.3 age-adjusted MAC combined with intravenous infusion of propofol, and the BIS value was maintained between 40 and 60 by adjusting the dose of propofol.
Remifentanil (0.1–0.2 μg.kg−1.min−1) was used for intraoperative analgesia in both groups. Rocuronium was administered for muscle relaxation. The lungs were mechanically ventilated with 60% oxygen in the air to maintain the end-tidal pressure of carbon dioxide at 35–40 mmHg. Blood pressure was maintained within 20% of baseline during the surgery. Anesthesiologists selected appropriate vasoactive drugs (ephedrine and/or norepinephrine) according to the patient’s condition, and recorded the consumption of the vasoactive drugs, which was eventually converted to norepinephrine equivalents (1 mg ephedrine is equivalent to 1 µg norepinephrine).
All medications were stopped at the end of the surgery, and patients were transferred to the post-anesthesia care unit (PACU) for recovery. Patient-controlled intravenous analgesia was provided for postoperative analgesia, which was established with hydromorphone 0.2 mg/kg plus palonosetron 0.5 mg. Clinicians avoided giving midazolam, ketamine, dexmedetomidine, and penehyclidine hydrochloride during general anesthesia. Follow-up assessments were performed postoperatively. Baseline and perioperative data and adverse events were also recorded.
Outcome Measures
The primary outcome was the incidence of delirium during the first three postoperative days. The delirium assessment was performed using CAM twice daily between 8:00–10:00 and 16:00–20:00 by the same research member who was trained before the study and not involved in the clinical care of the patients. Patients with POD were further classified into three subtypes according to the consciousness levels evaluated by the Richmond Agitation Sedation Scale (RASS) immediately before assessing delirium: Hyperactive delirium was defined when RASS was consistently positive (+1 to +4); hypoactive delirium was defined when RASS was consistently neutral or negative (–3 to 0); and mixed delirium was defined when both hypoactive and hyperactive delirium episodes were presented during the observation period. The time of onset and the duration of delirium were also recorded as days of POD per patient. Secondary outcome was emergency quality assessed by three aspects (the incidence of emergence agitation, extubation time and length of PACU stay). Emergence agitation is a pathological state characterized by psychomotor agitation, hyperactivity, and perceptual disturbance that occurs in the early stages of general anesthesia awakening, and was assessed by the Riker Sedation-Agitation Scale.22
For adverse events, the predefined criteria were intraoperative awareness, PACU hypoxia, postoperative nausea and vomiting, and non-planned transfer to the intensive care unit (ICU). Intraoperative awareness was assessed 24–48 h postoperatively. If the patients were in a delirium state at that time, intraoperative awareness would be evaluated before the patients were discharged from the hospital. Oxygen saturation <90% in the PACU is defined as PACU hypoxia. Blood pressure was recorded within 5 minutes after induction of anesthesia, and systolic blood pressure below 90 mmHg was defined as post-induction hypotension. A numeric rating scale (0, no pain—10, worst pain) was also used for pain measurement during the three postoperative days.
Sample Size Calculation
Based on the prevalence of POD in older adult patients undergoing orthopedic surgery reported to be 20%-30%,23,24 we assumed an incidence of POD of 25% in the propofol group. We estimated that a sample size of 300 participants (150 per group), would provide 80% power for detecting a 15% increase in POD prevalence, with a 2-sided significance level of 0.05. We, therefore, planned to enroll 360 patients with the expectation that 20% would drop out.
Statistical Analysis
Continuous variables were presented as mean (standard deviation) or median (interquartile range), depending on their distributions. Groups were compared with the t-test if normally distributed and the Mann–Whitney U-test if not. Categorical data were presented as frequencies and percentages and analyzed using 2-tailed χ2 tests or the Fisher exact test. For the primary outcome of delirium, the two groups were compared with the χ2 test for differences in probabilities of a 2×2 contingency table. Risk ratio (RR), risk difference (RD) and difference were reported with 95% confidence intervals (CIs). All statistical tests were 2-sided and a value of P < 0.05 was considered to be statistically significant. Statistical analyses were performed using SPSS version 24 (IBM, Armonk, NY, USA).
Results
Between November 2021 and June 2022, a total of 965 patients were assessed for eligibility, and 645 were excluded before randomization. Overall, 320 patients were randomly allocated: 158 in the remimazolam group and 162 in the propofol group. Among these 320 patients, 20 participants were dropped from the analysis: one participant was discontinued because of suspected remimazolam allergy; surgery was cancelled for nine participants, the anesthesia method was changed to intraspinal anesthesia for five participants, and five participants withdrew consent. Thus, 300 participants were finally analyzed in this study. Detailed participant information is shown in Figure 1.
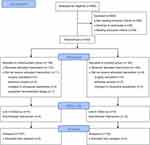 |
Figure 1 CONSORT 2010 flow diagram. Adapted from Schulz K F, Altman D G, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials BMJ. 2010;340: c332. Open Access.44 |
Baseline and Perioperative Characteristics
A total of 300 participants (68.5 ± 4.5 years old, 39.0% male) were included in the final data analysis. Overall demographic and baseline variables of patients (Table 1) and the majority of intra- and postoperative characteristics (Table 2) were well balanced between the two groups. Patients in the propofol group required more doses of the intraoperative vasoactive drug than those in the remimazolam group (P = 0.003) (Table 2).
 |
Table 1 Demographic and Baseline Variables |
 |
Table 2 Intra- and Postoperative Characteristics |
Primary Outcome
The overall incidence of POD among all study participants was 14.0%, with 23 of 147 (15.6%) remimazolam patients and 19 of 153 (12.4%) propofol patients experiencing POD (RR, 1.26; 95% CI, 0.72 to 2.21; RD, 3.2%; 95% CI, −4.7% to 11.2%; P = 0.42]) (Table 3). The onset time and duration of POD were similar between the two groups (P = 0.13, P = 0.77). The remimazolam and propofol groups were similar in the incidence of hyperactive delirium (47.8% vs 31.6%), hypoactive delirium (34.8% vs 47.4%), and mixed delirium (17.4% vs 21.1%), respectively.
 |
Table 3 Primary and Secondary Outcome |
Secondary Outcome
The patients with emergency agitation during the recovery period were 16 (10.9%) in the remimazolam group and 10 (6.6%) in the propofol group, but the difference was not statistically significant (RR, 1.35; 95% CI, 0.82 to 2.22; RD, 4.4%; 95% CI, −2.2% to 11.1%; P = 0.28). The extubation time and total PACU stay for the remimazolam group were higher compared with the propofol group, and the difference was statistically significant (P < 0.001, P<0.001) (Table 3).
Adverse Events
Perioperative adverse events are presented in Table 4. All patients in both groups had no intraoperative awareness. The proportion of hypotension after induction of anesthesia in the remimazolam group was 17.1%, which was lower than 43.0% in the propofol group, and the difference was statistically significant (RR, 0.58; 95% CI, 0.47–0.71; RD, −25.5%; 95% CI, −35% to −15.3%; P < 0.001). The patients with PACU hypoxia were 24 (16.4%) in the remimazolam group and 16 (10.7%) in the propofol group. Postoperative nausea and vomiting occurred in 37 (29.1%) remimazolam patients and 31 (22.7%) propofol patients, but the difference was not statistically significant. In addition, a total of five patients had an unplanned transfer to the ICU. Of these five patients, one in the remimazolam group was transferred to the ICU because of high intraoperative blood loss. The other four transfer patients were in the propofol group, three of whom were transferred to the respiratory ICU because of poor oxygenation, dyspnea, and wheezing (on the postoperative afternoon, postoperative day 1 evening, and postoperative day 3 evening, respectively). The remaining patient in the propofol was transferred to the ICU for cervical spine trauma.
 |
Table 4 Perioperative Adverse Events. n (%) |
Discussion
In this prospective RCT, our results showed that intraoperative remimazolam administration was not associated with an increased incidence of POD in the first 3 days after surgery compared with propofol. Furthermore, patients treated with remimazolam had a lower incidence of hypotension after induction and less dose of the intraoperative vasoactive drug, but a longer postoperative extubation time and PACU stay.
Previous studies showed that patients who were sedated with benzodiazepines in the ICU had a higher incidence of delirium than those with non-benzodiazepines (dexmedetomidine, propofol).25–27 Here, we revealed a novel and unexpected finding about the new benzodiazepine remimazolam. Our results showed no statistical difference in the incidence of POD in patients using remimazolam compared with propofol (15.6% vs 12.4%), which might be attributed to its unique metabolic characteristics. Accumulated evidence has demonstrated that the effects of benzodiazepines with different half-life on delirium appear to be different. Marcantonio et al found that patients who applied with long-acting benzodiazepine drugs (chlordiazepoxide, diazepam, and flurazepam) had a higher incidence of POD than those with short-acting benzodiazepine drugs (oxazepam, lorazepam, triazolam, midazolam, and temazepam).28 In addition, recent studies even indicated short-acting benzodiazepines were associated with decreased incidence of POD,13,29 which supported the findings of our study. As a novel ultra-short acting drug, remimazolam has a constant context-sensitive half-time and a rapid elimination half-life,30 and these unique pharmacokinetic properties greatly reduce the adverse effects of anesthetic drug accumulation on postoperative patients. Notably, a newly prospective observational study using propensity score matching also demonstrated no difference of incidence of POD in older adults undergoing elective cardiovascular surgery using remimazolam or not, which is consistent with our results.18 Another newly published study with 98 cases from Kaneko, Shohei et al19 showed a lower incidence of POD with remimazolam (with the antagonist flumazenil at the end of surgery) than propofol (without flumazenil) for patients undergoing transfemoral transcatheter aortic valve implantation under general anesthesia, However, this is a retrospective study with small sample size.
Previous studies on benzodiazepines and delirium except for the comparison with other sedative drugs in ICU, were mostly observational.31–33 Although many observational studies have concluded that perioperative benzodiazepines were associated with postoperative delirium, these studies have failed to clearly describe the drug class, purpose, duration, and timing, raising concerns about the potential confounders. It should be emphasized that benzodiazepines are widely used for chronic anxiety, insomnia, and sleep disorders, these patients themselves might be more susceptible to POD.34 Importantly, benzodiazepines can be used to treat alcohol or sedative drug withdrawal delirium, agitation symptoms of delirium, and early onset symptoms of delirium such as sleep disorder,35 raising questions about the impact of reverse causation bias. Our study was a well-designed randomized clinical trial. The baseline characteristics between the two groups were balanced, and the purpose of drug use were also consistent, which effectively avoids bias and has a reference value for clinical practice. In addition, a published protocol of a RCT aimed to investigate the effects of postoperative sedation with remimazolam versus propofol on POD after cardiac surgery, and we look forward to their results in the near future for a better understanding of the effects of remimazolam on delirium when used in different populations at different time.36
The total incidence of POD in our study was 14%, which was lower than in previous studies.23,24 Our lower incidence presumably reflects relatively low baseline risk. For example, we enrolled patients as young as 60 years old, whereas many delirium trials restrict enrolment to patients exceeding 65 or even 70 years old, and the majority of patients in our study cohort were ASA grades I–II. We also have taken a series of measures including intraoperative use of BIS monitoring for anesthesia depth, strict control of blood pressure, and avoidance of anticholinergic drugs in the current study, which might reduce the incidence of delirium to some extent.37–39
Our results showed that, compared with propofol, remimazolam had a lower incidence of hypotension and less dose of the intraoperative vasoactive drug, which was consistent with previous reports.40 Notably, hypotension is often associated with poor outcomes, especially in older adults with multiple comorbidities, which may contribute to perioperative cognitive decline.41 The stable hemodynamic properties of remimazolam may reduce the possibility of POD.
The extubation time of remimazolam in our study was longer, which was consistent with the previous study.42 However, we did not use flumazenil, a competitive antagonist of the benzodiazepine receptor. It was demonstrated that flumazenil can quickly reverse the sedative effect of remimazolam, shorten the median time to full alertness, and effectively reverse psychomotor and cardiovascular dysfunction.43 The use of flumazenil may offer the opportunity to even surpass the recovery speed of propofol, which requires further comparison. Although the metabolism of remimazolam was considered to be independent of liver and kidney function and does not produce accumulation, the latest research indicated that metabolism may slow down in people with severe liver dysfunction. This may suggest that we should reduce the dose of remimazolam or discontinue it early in special individuals.
Regarding adverse events, the current study did not find differences between the two groups in postoperative nausea, vomiting, and PACU hypoxia. A recent study suggested that remimazolam decreased the incidence of early postoperative nausea and vomiting compared with desflurane after laparoscopic gynecological surgery.45 But their sample size was relatively small. Moreover, the control group in their study was desflurane. Further research is needed to compare the incidence of postoperative nausea and vomiting with other intravenous anesthetics such as propofol among patients receiving remimazolam.
Nevertheless, this study has several limitations. First, as a single-center study only including patients undergoing orthopedic surgery, the generalizability of the results may be limited. Large multicenter studies involving patients undergoing different types of surgery are warranted in the future. Second, this study excluded patients with preoperative delirium and dementia, and the effect of remimazolam on these patients must be further explored. Third, we used BIS to monitor anesthesia depth during surgery, but the reliability of BIS for monitoring the depth of anesthesia with remimazolam is unclear. Despite this, intraoperative BIS values were maintained 40–60 and no patients reported intraoperative awareness. Fourth, we assessed the POD twice a day for three days, given the fluctuating nature of delirium, we might still miss some patients with nighttime delirium and those who started after three days. Fifth, considering the lower dropout rate than anticipated, we performed preliminary statistical analyses in advance when the minimum number of completed subjects reached 300. And the study was not powered to detect a difference between groups. Then we continued post hoc analysis, which indicated that a total of 3686 patients are needed to achieve adequate power with the current incidence. Finally, this study only conducted short-term follow-up for POD, and a long-term follow-up is required for other effects associated with benzodiazepine, such as cognitive trajectories, falls, and quality of life.
Conclusions
In summary, our study shows that general anesthesia with remimazolam was not associated with an increased incidence of POD compared with propofol in older adult patients undergoing orthopedic surgery. However, large multicenter studies involving patients undergoing different types of surgery are needed to verify the conclusions.
Data Sharing Statement
All data generated or analyzed during this study were included in the published article. Further inquiries about the datasets can be directed to the corresponding author on reasonable request.
Acknowledgments
The authors would like to acknowledge all of the staff who assisted this RCT.
Funding
This work was supported by the National Natural Science Foundation of China (82171189) and the Natural Science Foundation of Henan Province (212300410239).
Disclosure
The authors have no competing interests in this work.
References
1. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi:10.1016/S0140-6736(13)60688-1
2. Mattison MLP. Delirium. Ann Intern Med. 2020;173(7):ITC49–ITC64.3. doi:10.7326/AITC202010060
3. Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–39. doi:10.1056/NEJMoa1112923
4. Goldberg TE, Chen C, Wang Y, et al. Association of delirium with long-term cognitive decline: a meta-analysis. JAMA Neurol. 2020;77(11):1373–1381. doi:10.1001/jamaneurol.2020.2273
5. Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020;125(4):492–504. doi:10.1016/j.bja.2020.06.063
6. Duprey MS, Devlin JW, Griffith JL, et al. Association between perioperative medication use and postoperative delirium and cognition in older adults undergoing elective noncardiac surgery. Anesth Analg. 2022;134(6):1154–1163. doi:10.1213/ANE.0000000000005959
7. Masui K. Remimazolam besilate, a benzodiazepine, has been approved for general anesthesia. J Anesth. 2020;34(4):479–482. doi:10.1007/s00540-020-02755-1
8. Lohmer LL, Schippers F, Petersen KU, Stoehr T, Schmith VD. Time-to-event modeling for remimazolam for the indication of induction and maintenance of general anesthesia. J Clin Pharmacol. 2020;60(4):505–514. doi:10.1002/jcph.1552
9. Keam SJ. Remimazolam: first Approval. Drugs. 2020;80(6):625–633. doi:10.1007/s40265-020-01299-8
10. Sneyd JR, Gambus PL, Rigby-Jones AE. Current status of perioperative hypnotics, role of benzodiazepines, and the case for remimazolam: a narrative review. Br J Anaesth. 2021;127(1):41–55. doi:10.1016/j.bja.2021.03.028
11. Zhou J, Leonowens C, Ivaturi VD, et al. Population pharmacokinetic/pharmacodynamic modeling for remimazolam in the induction and maintenance of general anesthesia in healthy subjects and in surgical subjects. J Clin Anesth. 2020;66:109899. doi:10.1016/j.jclinane.2020.109899
12. Kudoh A, Takase H, Takahira Y, Takazawa T. Postoperative confusion increases in elderly long-term benzodiazepine users. Anesth Analg. 2004;99(6):1674–1678. doi:10.1213/01.ANE.0000136845.24802.19
13. Memtsoudis S, Cozowicz C, Zubizarreta N, et al. Risk factors for postoperative delirium in patients undergoing lower extremity joint arthroplasty: a retrospective population-based cohort study. Reg Anesth Pain Med. 2019;44:934–943. doi:10.1136/rapm-2019-100700
14. Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653. doi:10.1001/jama.298.22.2644
15. By the American Geriatrics Society Beers Criteria Update Expert P. American geriatrics society 2019 updated AGS beers criteria(R) for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–694. doi:10.1111/jgs.15767
16. Hughes CG, Boncyk CS, Culley DJ, et al. American society for enhanced recovery and perioperative quality initiative joint consensus statement on postoperative delirium prevention. Anesth Analg. 2020;130(6):1572–1590. doi:10.1213/ANE.0000000000004641
17. Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34(4):192–214. doi:10.1097/EJA.0000000000000594
18. Aoki Y, Kurita T, Nakajima M, et al. Association between remimazolam and postoperative delirium in older adults undergoing elective cardiovascular surgery: a prospective cohort study. J Anesth. 2022;2022:1.
19. Kaneko S, Morimoto T, Ichinomiya T, Murata H, Yoshitomi O, Hara T. Effect of remimazolam on the incidence of delirium after transcatheter aortic valve implantation under general anesthesia: a retrospective exploratory study. J Anesth. 2022. doi:10.1007/s00540-022-03148-2
20. Li H, Jia J, Mini-Mental State YZ. Examination in elderly Chinese: a population-based normative study. J Alzheimers Dis. 2016;53(2):487–496. doi:10.3233/JAD-160119
21. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi:10.7326/0003-4819-113-12-941
22. Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med US. 1999;27:1325–1329. doi:10.1097/00003246-199907000-00022
23. Mei X, Zheng HL, Li C, et al. The effects of propofol and sevoflurane on postoperative delirium in older patients: a randomized clinical trial study. J Alzheimers Dis. 2020;76(4):1627–1636. doi:10.3233/JAD-200322
24. Hong N, Park JY. The motoric types of delirium and estimated blood loss during perioperative period in orthopedic elderly patients. Biomed Res Int. 2018;2018:9812041. doi:10.1155/2018/9812041
25. Pandharipande P, Shintani A, Peterson J. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:121. doi:10.1097/00000542-200601000-00005
26. Zaal IJ, Devlin JW, Hazelbag M, et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med. 2015;41(12):2130–2137. doi:10.1007/s00134-015-4063-z
27. Casault C, Soo A, Lee CH, et al. Sedation strategy and ICU delirium: a multicentre, population-based propensity score-matched cohort study. BMJ Open. 2021;11(7):e045087. doi:10.1136/bmjopen-2020-045087
28. Marcantonio ER, Juarez G, Goldman L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272(19):1518–1522. doi:10.1001/jama.1994.03520190064036
29. Poeran J, Cozowicz C, Zubizarreta N, et al. Modifiable factors associated with postoperative delirium after Hip fracture repair: an age-stratified retrospective cohort study. Eur J Anaesthesiol. 2020;37(8):649–658. doi:10.1097/EJA.0000000000001197
30. Sheng XY, Liang Y, Yang XY, et al. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol. 2020;76(3):383–391. doi:10.1007/s00228-019-02800-3
31. Wang J, Li Z, Yu Y, Li B, Shao G, Wang Q. Risk factors contributing to postoperative delirium in geriatric patients post orthopedic surgery. Asia Pac Psychiatry. 2015;7(4):375–382. doi:10.1111/appy.12193
32. Park SA, Tomimaru Y, Shibata A, Miyagawa S, Noguchi K, Dono K. Incidence and risk factors for postoperative delirium in patients after hepatectomy. World J Surg. 2017;41(11):2847–2853. doi:10.1007/s00268-017-4079-3
33. Tomimaru Y, Park SA, Shibata A, et al. Predictive Factors of Postoperative Delirium in Patients After Pancreaticoduodenectomy. J Gastrointest Surg. 2020;24(4):849–854. doi:10.1007/s11605-019-04212-1
34. Gerlach LB, Wiechers IR, Maust DT. prescription benzodiazepine use among older adults: a critical review. Harv Rev Psychiatry. 2018;26(5):264–273. doi:10.1097/HRP.0000000000000190
35. Attard A, Ranjith G, Taylor D. Delirium and its treatment. CNS Drugs. 2008;22(8):631–644. doi:10.2165/00023210-200822080-00002
36. Yang M, Liu X, Yang D, et al. Effect of remimazolam besylate compared with propofol on the incidence of delirium after cardiac surgery: study protocol for a randomized trial. Trials. 2021;22(1):717. doi:10.1186/s13063-021-05691-x
37. Evered LA, Chan MTV, Han R, et al. Anaesthetic depth and delirium after major surgery: a randomised clinical trial. Br J Anaesth. 2021;127(5):704–712. doi:10.1016/j.bja.2021.07.021
38. Wachtendorf LJ, Azimaraghi O, Santer P, et al. Association between intraoperative arterial hypotension and postoperative delirium after noncardiac surgery: a retrospective multicenter cohort study. Anesth Analg. 2022;134(4):822–833. doi:10.1213/ANE.0000000000005739
39. Mueller A, Spies CD, Eckardt R, et al. Anticholinergic burden of long-term medication is an independent risk factor for the development of postoperative delirium: a clinical trial. J Clin Anesth. 2020;61:109632. doi:10.1016/j.jclinane.2019.109632
40. Mao Y, Guo J, Yuan J, Zhao E, Yang J. Quality of recovery after general anesthesia with remimazolam in patients’ undergoing urologic surgery: a randomized controlled trial comparing remimazolam with propofol. Drug Des Devel Ther. 2022;16:1199–1209. doi:10.2147/DDDT.S359496
41. Hu AM, Qiu Y, Zhang P, et al. Higher versus lower mean arterial pressure target management in older patients having non-cardiothoracic surgery: a prospective randomized controlled trial. J Clin Anesth. 2021;69:110150. doi:10.1016/j.jclinane.2020.110150
42. Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34(4):543–553. doi:10.1007/s00540-020-02788-6
43. Chen X, Sang N, Song K, et al. Psychomotor recovery following remimazolam-induced sedation and the effectiveness of flumazenil as an antidote. Clin Ther. 2020;42(4):614–624. doi:10.1016/j.clinthera.2020.02.006
44. Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi:10.1136/bmj.c332
45. Hari Y, Satomi S, Murakami C, et al. Remimazolam decreased the incidence of early postoperative nausea and vomiting compared to desflurane after laparoscopic gynecological surgery. J Anesth. 2022;36(2):265–269. doi:10.1007/s00540-022-03041-y
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
