Back to Journals » Clinical Ophthalmology » Volume 16
Effect of Postoperative Ocular Residual Astigmatism (ORA) on Treatment Outcome After Myopic Laser in situ Keratomileusis (LASIK)
Authors Nöthel J , Katz T, Druchkiv V, Frings A
Received 5 January 2022
Accepted for publication 8 April 2022
Published 23 June 2022 Volume 2022:16 Pages 2079—2092
DOI https://doi.org/10.2147/OPTH.S352410
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Johanna Nöthel,1,* Toam Katz,1,* Vasyl Druchkiv,2 Andreas Frings3
1Department of Ophthalmology, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany; 2Department of Research & Development, Clínica Baviera, Valencia, Spain; 3Department of Ophthalmology, Heinrich-Heine-University Duesseldorf, Duesseldorf, North Rhine-Westphalia, Germany
*These authors contributed equally to this work
Correspondence: Johanna Nöthel, Email [email protected]
Purpose: To analyze the impact of postoperative ocular residual astigmatism (ORA) on refraction, visual acuity and subjective satisfaction after myopic laser-in-situ-keratomileusis (LASIK) by a comprehensive analysis, which includes clinically relevant data and patient-reported outcomes.
Material and Methods: To evaluate the influence of ORA, comparison groups were built following Archer et al. Myopic patients were subdivided by the fraction ORA/MRC (matched and not matched for MRC) (MRC = manifest refractive cylinder), ORA magnitude and CA magnitude in high ORA eyes (CA = corneal astigmatism). Refractive and visual data were analyzed via retrospective cross-sectional analysis for multiple parameters. The subjective satisfaction was analyzed retrospectively 3– 4 years after having LASIK via patient reported outcome analysis.
Results: Refractive outcome: Only when grouped by ORA magnitude only, high ORA eyes resulted in approximately twice as cylinder magnitude compared to eyes with preoperative lower ORA. Furthermore, there appeared to be no statistically significant differences in any case. Visual outcome: There appeared to be no statistically significant differences for visual acuity parameters (safety index, efficacy index). Patient reported outcome: When grouped by the rate of ORA/MRC not matching for MRC, there were statistically significant differences in the subjective satisfaction (p = 0.006) and the postoperative side effects (p = 0.001, p = 0.01, p = 0.006), those differences appeared less strong when matched for MRC treated and result better for a higher ratio of ORA/MRC.
Conclusion: Patients with postoperatively high ORA report on higher satisfaction with treatment results than patients with postoperatively low ORA. This did not correlate with differences in the refractive nor visual outcome. As a matter of fact, there is a discrepancy between the objective analysis results and the subjective satisfaction of patients.
Keywords: ocular residual astigmatism, myopic LASIK, refractive outcome, visual outcome, patient reported outcome
Introduction
Worldwide, there is a prevalence of at least 40% of astigmatic patients. Weighing up the treatment options, the analysis of patient satisfaction has found refractive surgery to score significantly better than conventional methods (contact lenses, glasses).2 In this context, laser-in-situ-keratomileusis (LASIK) has established itself for treating ametropia with frequently performed procedures over the last decades.3 Although there exist alternative options like small incision lenticule extraction (SMILE),4 with a similar refractive outcome5 also in the long term,6 LASIK still remains an often-used reliable option.7 Particularly focusing on the treatment outcome of myopic astigmatism patients, LASIK was comparatively to SMILE investigated to be superior to the control of astigmatism and HOAs (high-order aberrations).7
Until now, there exist plenty of studies examining negative predictors for less sufficient refractive outcome or parameters associated with dissatisfaction of patients.8–10 This verifies that the treatment of myopia by LASIK is still to be a topic of broad interest in refractive surgery.
Preceding studies have proven that an arising correlation between astigmatism not only from the refractive cylinder at the corneal plane but also from the ocular residual astigmatism is evidenced to be a negative predictor when treating astigmatism with LASIK.10 Ocular residual astigmatism (ORA) is defined as the vectorial difference between the corneal topographic astigmatism and the refractive cylinder at the corneal plane.11 ORA mainly results from the posterior corneal surface and the crystalline lens.12 Many cases show that the discrepancy between ORA and the refractive cylinder can be a pitfall for the planning as treatment models are based either on topographic astigmatism or subjective preoperative cylinder.10,13,14
High amounts of preoperative ORA have been shown to be a potentially limiting factor for the predictability of refractive correction with an excimer laser.15 Because of this, preoperative high ORA is a negative predictor of refractive results one must consider.
If you go one step farther, there exists no study by now, which examines postoperative ORA in the context of objective (refractive and visual) outcomes after myopic astigmatic LASIK. Neither there exists an analysis, which examines postoperative ORA in connection with the subjective satisfaction of patients. In the authors’ opinion, it is consequent to fill all the missing aspects of the analysis of ORA, also from the postoperative perspective. At this point in time, it cannot be answered, if postoperative ORA might be correlated to subjective dissatisfaction, or postoperative side effects, for example. If there arise connections between those parameters, they could result in focusing on other priorities when planning a refractive procedure. For example, one could focus more on treating ocular residual astigmatism.
So, the aim of the current study was to evaluate if differences in the postoperative magnitude of ORA correlate with differences in the outcome after myopic astigmatic LASIK. Therefore, we created a comprehensive analysis, which considered refractive outcome, visual outcome and subjective patient reported outcome. By this, we included not only the physician’s basis (refractive and visual parameters) for decision making, but also the patient’s point of view into our wide investigation.
Patients and Methods
This retrospective analysis comprised consecutive myopic patients who had LASIK between 2011 and 2012 at a chain of private refractive surgery centers in Germany. All data were based on the Hamburg refractive Data Base (data retrieved from Care Vision Germany GmbH). Informed consent and permission to use their data for analyses and publication were obtained for each patient. Inclusion criteria were medically suitable for LASIK; no previous ocular, eyelid or orbital surgery; no visually significant cataract; myopic manifest sphere treated between 0.25 and 5D and refractive cylinder up to 4.00 D; corrected distance visual acuity (CDVA) not worse than 20/25; age younger than 55 years (to minimize the influence of early cataract); and a minimum follow-up of 3 months after LASIK. A full ophthalmologic examination was performed by an in-house optometrist as per protocol.
For all eyes, the targeted treatment result was to achieve emmetropia, within a preferably good predictability (SE = (sphere)+1/2 (cylinder)).
Patients were selected following an earlier study examining the effects of demographic and ocular parameters in myopic laser in situ keratomileusis on ocular residual astigmatism.10 Although the aim of this study is different, our determination of ORA occurred similarly and was used to examine other correlations afterwards. For one eye of each patient, magnitude and orientation of ORA were determined by using double-angle-vector analysis16 (10) which defines the vector difference between the preoperative refractive astigmatism (R), corneal plane and the topographic (simulated keratometry [R]) astigmatism.
Following Kugler et al,15 the R value was obtained from the manifest refraction, and the simulated K (keratometry) value was calculated from the manifest refraction and the simulated K value was calculated from the corneal topography17 based on the difference between the steepest meridian and the flattest meridian oriented 90 degrees from each other. Ocular residual astigmatism is the amount of the vector difference between R and simulated K (ie, R _ K) with its orientation directed to the refractive astigmatism value of the cornea and was calculated using a previously described formula.
We evaluated the effect of postoperative ORA on refractive, visual and PRO outcome by building comparison groups, which took the postoperative amount of ORA into account.
Considering previous results and methods, lower preoperative ORA was found to result better when treating corneal astigmatism with LASIK.15,18 Taking a closer look at the methods of those studies, the results are not related to the absolute amount of ORA only, but to the ratio of preoperative ORA to preoperative MRC, which was then described by the fraction of ORA/MRC. This fraction provides information about where the astigmatism mainly arises from. According to Qian et al,18 the outcome after refractive surgery is less effective, when the astigmatism arises mainly from the ocular residual astigmatism and not the manifest refractive cylinder.
This applies if the fraction of ORA/MRC is 1.1 or more. If the fraction of ORA/MRC is 1 or less, the amount of ORA is smaller than the MRC or equals it. Therefore, we included the ratio of ORA/MRC into our grouping methods, as it would not be sufficient to consider the absolute amount of ORA only, without considering the manifest refractive cylinder.
But there is more one must take into consideration. In a following study, Archer et al1 stated that the definition of low and high ORA only by the ratio of ORA/MRC could wrongly include eyes with high ORA and even higher MRC in the low ORA group and vice versa. By including the matching for the MRC into their method of grouping, those eyes could be excluded. That means that wrongly high ORA eyes were excluded from the low ORA group and vice versa. Therefore, the high ORA group was biased toward eyes with low refractive astigmatism and vice versa for the low ORA group. Additionally, the groups were also subdivided by the amount of ORA only and the magnitude of the corneal astigmatism.
At this point, the method of grouping is completely based on the method of Archer et al, apart from our focus on postoperative ORA instead of preoperative ORA. The criterion for the grouping is shown in Figure 1.
We created 4 superordinate groups (stage 1–4), which each represent the main parameter the group was defined by and tested for. Each superordinate group is divided up into subgroups.
In stage 1 the grouping is based on the ratio of postoperative ORA/MRC only. For each eye in the high ORA group from stage 1, an eye was found in the low ORA group from stage 1 that was matched for manifest sphere within G0.25D and for MRC with G0.25 D. Depending on the ratio of ORA/MRC, the superordinate group is divided into 2 subgroups. The ratio of ORA/MRC divides them into a low ORA group (a) and a high ORA group (b).
The definition of stage 2 principally equals the definition of stage 1 but includes that all eyes were matched for the MRC treated. That means that wrongly high ORA eyes were excluded from the low ORA group and vice versa. Therefore, the high ORA group was biased toward eyes with low refractive astigmatism and vice versa for the low ORA group. This was necessary, as the low ORA group also included eyes with a high ORA magnitude if the MRC was high, and for the same reasons, the high ORA group included eyes with a low ORA magnitude.
In stage 3 groups were selected based on the ORA magnitude alone. Eyes with an ORA magnitude less than 0.50D were included in the low ORA group, and eyes with an ORA magnitude of 1.25D or more were included in the high ORA group. The remaining eyes with an ORA magnitude of 0.5D more than and less than 1.25D were included in a third group representing mid-ORA values.
As in stage 2, the groups were then reduced to be matched for manifest sphere and astigmatism treated; for each eye in the high ORA group, an eye was found in both the low ORA and the mid-ORA groups that matched for the manifest sphere within G0.25D and the MRC with G0.25D.
In stage 4, the high ORA group generated in stage 3 was further subdivided into 2 groups based on the magnitude of corneal astigmatism. Eyes with corneal astigmatism of 0.75D or more were included in the high ORA/high corneal astigmatism group, and eyes with corneal astigmatism less than 0.75D were included in the high ORA/low corneal astigmatism group.
Patient Reported Outcome
To examine the patient-reported outcome and satisfaction, a previous questionnaire was used for this study (Table 1). All questions had predetermined possible answers framed in a number enabling the score to better compare and analyze the differences between the comparison groups. A lower score, for example, results from lower symptoms or higher satisfaction..
 |
Table 1 Pre-Operative and Postoperative Questions Used in Regression Analysis |
The questions (Nr.1–10) followed a published set-up, which was derived from the Joint LASIK study Task Force and used in a preceding study.19 Patients were asked to assess the pre- and postoperative side effects (starburst, glare, halo, double vision and ghost images, dry eyes).
All patients were interviewed by telephone retrospectively 3–4 years after they had LASIK by one of the authors.
Statistical Analysis
All data were summarized via Excel 2010 Software (Microsoft Corp.) and statistically analyzed via Stata. A level of significance 5% was determined and performed with Mann–Whitney-test or independent t-test.
For all subgroups, established refractive and visual parameters were statistically analyzed. In the analysis, the targeted goals of the refractive procedure are displayed by the postoperative manifest sphere and cylinder, the predictability by the postoperative SE and the safety and the efficacy by the safety index (SI) and efficacy index (EI).
The data analysis outcome for each subgroup was tested for statistical significance within each superordinate group (stage). By this, we could examine differences in the outcome depending on the definition of the superordinate groups.
As an example, the postoperative data of 780 consecutive eyes (780 patients; 34 ± 9 years) treated with myopic wavefront-optimized LASIK is summarized in Tables 2 and 4 (PRO & refractive outcome). Tables 2 and 4 evaluate stage 1 data according to our analysis protocol (Stage 1: Grouped by ORA/MRC, not matched for MRC). If statistically significant differences occur, we can relate them to a subgroup with lower or higher ORA (defined by the ratio of ORA/MRC).
 |
Table 2 Postoperative PRO Outcome Between 2 Groups: Grouped by Postoperative Ratio of ORA/MRC (Stage 1) |
 |
Table 3 Postoperative PRO Outcome Between 2 Groups: Grouped by Postoperative Ratio of ORA/MRC, Matched for MRC (Stage 2) |
 |
Table 4 Postoperative Refractive Outcome Between 2 Groups: Grouped by Postoperative Ratio of ORA/MRC (Stage 1) |
The complete statistical analysis of the refractive and visual outcome is attached (Supplementary Data 1–4).
Additionally, for the statistical analysis, we attached Snellen charts for the SI & EI if relevant for our results (Figures 2 and 3).
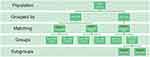 |
Figure 1 According to our purpose to examine the effect of postoperative ORA we based our grouping method on postoperative ORA. Reproduced with permission from Archer TJ, Reinstein DZ, Pinero DP, Gobbe M, Carp GI. Comparison of the predictability of refractive cylinder correction by laser in situ keratomileusis in eyes with low or high ocular residual astigmatism. J Cataract Refract Surg. 2015;41(7):1383–1392. https://www.sciencedirect.com/science/article/abs/pii/S088633501500838X Abbreviation: CA, corneal astigmatism; D, diopter; MRC, manifest refractive cylinder; N, number; ORA, ocular residual astigmatism. |
 |
Figure 2 Snellen diagram of the safety index (SI) of stage 1: postoperatively CDVA/ preoperatively CDVA. Abbreviations: CDVA, corrected distance visual acuity. |
For all subgroups, the PRO was statistically analyzed as well; for each question, the answers of each subgroup in each superordinate group (stage) were tested for statistically significant differences. The complete data analysis of the patient reported outcome you can find in the Supplementary Data 5–10 and in the Tables 2 and 3.
Below we focused on relevant data for answering our hypothesis only, mainly if statistically significant differences appeared.
Results
Patient Reported Outcome
Preoperative differences in subjective questionnaire scores between low and high ORA groups did not reach statistical significance throughout all stages (Supplementary Data 5–8).
According to our data analysis, differences in patient reported outcomes only appeared postoperatively and for patients when grouped by the ratio of ORA/MRC (Tables 2 and 3). There did not occur statistical differences when grouped by ORA magnitude only (stage 3) and when grouped by the amount of corneal astigmatism (stage 4) (Supplementary Data 9 and 10).
The statistical differences are stronger, if the included eyes were not matched for the MRC treated (stage 1).
For stage 1 (Table 2), those differences appear for starburst (p = 0.001), glare (p = 0.011), double vision and ghost images (p = 0.006) and satisfaction with vision (0.006). Relating these differences to the scale of symptoms, subgroup a has higher scores than subgroup b in all the above-mentioned side effects. This means that the patients of subgroup a experience more side effects or suffer more from them. Relating to our method of grouping, this group has a ratio of ORA/MRC ≤ 1, what corresponds to a refractive astigmatism mainly arising from the anterior corneal surface and not from ORA.
For stage 2 (Table 3), those differences only appear for starburst (p = 0.026). Relating to the scale of symptoms, subgroup a evaluated the side effects to be stronger than subgroup b. Relating to our method of grouping, this group has also a ratio of ORA/MRC ≤ 1, but in this group, the eyes were matched for the MRC treated.
Refractive Outcome
Throughout all stages, the refractive results, if evaluated based on the statistics of the subgroups, go in accordance with the treatment paradigm to achieve emmetropia. There did not appear to be statistically significant differences in the postoperative SE at any stage (Supplementary Data 1–4). The Mean SE was mainly very close to 0.00 D for all subgroups (Supplementary Data 1–4). If slight differences appeared, they did not result in statistically significant differences.
Although there were statistically significant differences in the PRO outcome of stage 1 and 2, those differences did not occur in the statistical evaluation of the refractive outcome (Tables 4 and 5).
 |
Table 5 Postoperative Refractive Outcome Between 2 Groups: Grouped by Postoperative Ratio of ORA/MRC. Matched for MRC (Stage 2) |
Only when grouped by ORA magnitude in stage 3 (Table 6), there appeared statistically significant differences in the postoperative refractive outcome for the manifest refractive cylinder (p = 0.002). High ORA eyes resulted in approximately twice as cylinder magnitude compared to eyes with preoperative lower ORA. Preoperatively, there appeared no statistically significant difference for the cylinder (p = 1) (Supplementary Data 1–4).
 |
Table 6 Postoperative Refractive Outcome Between 3 Groups: Grouped by Postoperative ORA Magnitude, Matched for MRC (Stage 3) |
Visual Outcome
There were no statistically significant differences in visual acuity (Tables 7 and 8) between the subgroups at all stages.
 |
Table 7 Postoperative Safety Index (SI) of All Stages According to Our Method of Grouping |
 |
Table 8 Postoperative Efficacy Index (EI) of All Stages According to Our Method of Grouping |
Discussion
In our study, having less satisfied patients correlates with a ratio of ORA/MRC ≤ 1 (stage 1 group a) only. Considering our method of grouping, this result is related to eyes in which the proportion of ORA on the refractive cylinder is comparatively smaller. Additionally, there is a correlation with experiencing more side effects (starburst, glare, double-vision, and ghost images).
Interestingly, all other “standard” refractive parameters and visual acuity parameters (Tables 7 and 8) were adequate and did not differ within the subgroups of stage 1, at least for the statistical mean of the refractive and visual outcome (Supplementary Data 1–4).
Taking a closer look at the statistical evaluation of the just mentioned subgroup (Table 4), one can see that the postoperative SE has 0.05D in the mean ± 0.43, which is an excellent result on average, but there appear outliers in the minimum cylinder (−2.00D) and sphere (−1.50) postoperatively. For the postoperative cylinder, the Q25 (−0.25D) and the Q75 (0.00D) were adequate, as well as for the postoperative sphere Q25 (0.00D) Q75 (0.50).
Additionally, the safety index, especially in this subgroup, showed no damage concerning the loss of Snellen lines in postoperative CDVA, comparatively to the preoperative CDVA (Figures 2 and 3). Due to our inclusion criteria for the patients, a loss of 1 line of postoperative CDVA results in a minimum postoperative CDVA of 20/30. The Snellen graph, which represents the efficacy index of this subgroup 1a (Figure 3), shows in 9% of cases that the postoperative UDVA could not equal the preoperative CDVA with the extension of 1 line less or more. This indicates that, for 9% of cases in subgroup 1a, the targeted emmetropia was not reached for the postoperative UDVA. Interestingly, this appears for subgroup b to the same extent, though their PRO outcome is comparatively better. Another possible explanation could be that the patients of stage 1 and 2, who participated in PRO are not a good sample for the number of patients, eyes, which were included in the refractive and visual data analysis, as our PRO outcome only included one third of all patients (278/780).
However, in the authors’ opinion, it would be odd, if the statistical limitations would cause this manifestly differences in the PRO outcome. Presumably, the dissatisfaction of the patients in subgroup a of stage 1 is most likely related to the experienced side effects, rather than to single outliers in the statistically analyzed refractive and visual outcome of this comparison group (N = 609).
In stage 2, there appeared to be less statistically significant differences than in stage 1. In this superordinate group, the patients were not only subdivided by the ratio of postoperative ORA/MRC but also matched for the MRC treated. That means that wrongly included high ORA eyes in the low ORA group and vice versa were excluded, which might be the reason for less different results within the subgroups. This is a limitation to the results of stage 1.
How can we classify this outcome in a larger context? Basically, having a correlation between experiencing side effects or HOAs (high-order aberrations) fits well with the results of other studies, which examined them to be reasons for dissatisfaction after refractive surgery and LASIK.3,8
What can we say about the correlation between ORA and side effects or HOAs?
In a preceding study such a correlation could not be verified so far by Mohammadpour et al 2016.20 In their study, no significant correlation was found between ORA and HOAs, but their grouping was based on the amount of refractive astigmatism only, plus their results based on preoperative refractive data.
At this point, our study was more extensive, as we built our comparison groups on multiple parameters (Figure 1), which investigated the ratio of postoperative ORA/MRC to correlate with side effects and dissatisfaction.
How relevant and clinically noticeable are our results for patients?
All comparison groups of stage 1–2 report a minimum mean satisfaction rate of at least 1–2 (Tables 2 and 3). Assuming the meaning of a scoring from 1 to 2, the mean is very satisfied with vision (1) or satisfied with vision (2) (Table 1). The mean satisfaction rate of group A (Mean = 1.58 ± 0.87) is statistically worse than that of group B (Mean = 1.35 ± 0.7). Clinically, these statistical differences result in a tendency for patients to be either highly satisfied or just satisfied. Furthermore, there are statistically differences in postoperative side effects in stage 1.
As a clinical consequence, HOAs should be examined even more precisely in correlation to the postoperative ratio of ORA/MRC, because of the relevance for the patients. Moreover, physicians should rethink which patients benefit the most from myopic LASIK. According to our data, considering the best clinical requirements (low ORA eyes) for refractive outcome could exclude patients with higher risk factors (like high ORA) from gaining more quality of life by undergoing refractive surgery, as their satisfaction not only correlates with the most predictable and efficient refractive outcome, as our results show.
Limitations to Our Study
Until now, there exist many reviews analyzing the quality of questionnaires in PRO for refractive surgery.21–23 In an extensive analysis by Kandel et al,22 for example, actual questionnaires were rated based on content development, psychometric properties, validity and reliability. However, we chose a preceding questionnaire,19 which matched our hypothesis and had a practicable scope for our wide comprehensive analysis, which did not focus on patient reported satisfaction only.
Having the quality criteria defined by actual reviews in mind, our study has some pros and cons.
Looking at our instrument precisely, it questioned the following aspects: starburst, glare, double vision, ghost images, and satisfaction pre- and postoperatively in a long-term follow-up.
These are aspects found to be highly relevant for dissatisfaction in our target population.3,8
Regarding one main criteria of items in PRO, it is crucial for the instrument to meet content validity21–23 for the target population and the study hypothesis. In our case, the long-term follow-up makes these criteria even stronger, because the impact of short-term postoperative side effects related to the corneal healing process are excluded.
On top of that, as long-term outcome report, the satisfaction of the patients remains high,24 even after 5 years postoperatively and predictors for dissatisfaction such as visual phenomena and uncorrected vision still remain.24
On the other hand, there are some limitations.
Firstly, our comparison groups were interviewed by one of the authors by telephone, which is less neutral than an examination without personal involvement. Having achieved at least a response from one-third of all patients in stage 1, the result is not satisfactory, but similar compared to other studies (287/780).19 The reason for that can be that we performed our PRO 5 years postoperatively and the contact details were no longer up-to-date.
Secondly, in comparison to highly rated questionnaires in refractive surgery, our instrument is not adequate, as many criteria are not fulfilled. The QoV questionnaire (Quality of Vision) which was rated to be the best existing in refractive surgery21 for example included more items (30), more symptoms (10), Rasch-Analysis and many more.
However, as a conclusion to the appropriateness of our methods to answer our hypothesis, our questionnaire was sufficient to indicate a correlation of the ratio of ORA/MRC with more or less satisfied patients.
So, however, there are limitations, as the patient-reported outcome could have been way more large-scaled, one must keep in mind that our goal was to create a comprehensive study not only focusing on PRO, but which goes along with compromises in our case. In the end, it was sufficient to answer our hypothesis and give a reference point where to go in detail for future studies.
Conclusion
In conclusion, the results of the current study show that preop high ORA remained postop high(er) ORA compared to low ORA eyes. Postoperatively high ORA individuals reported lower scoring and thus better satisfaction with treatment results. Individuals from the postoperative high ORA group reported on statistically significant higher satisfaction, even though they still had a significant amount of ORA left in the optical system. In conclusion, though postoperative high ORA is associated with less predictable and efficient refractive results after myopic LASIK compared to low ORA eyes, patients with postoperative high ORA report on higher satisfaction with treatment results and experience more side effects.
Value Statement
What was known:
- Preoperatively high ORA is associated with less accurate visual outcome and less predictable refractive outcome after refractive surgery.
What this paper adds:
- High ORA eyes correlated with twice cylinder magnitude postoperatively.
- Statistically differences in the PRO outcome (satisfaction, side-effects) didn’t correlate with statistically differences in the refractive nor visual outcome; this is a discrepancy between the objective and subjective outcome.
- Patients with a lower fraction of post-OP ORA/MRC reported on lower post-OP satisfaction and indicated to experience more side effects (starburst, glare, double-vision and ghost images); when matched for the MRC treated, this appeared less strong.
- Although postoperative high ORA has proven to be negatively predicting the refractive outcome after myopic LASIK, there is a discrepancy in the subjective satisfaction of patients, which appears to be even better than in other groups (when grouped by ORA/MRC).
Abbreviations
CDVA, corrected distance visual acuity; EI, efficacy index; HOA, high-order aberration; K cyl, corneal cylinder; K mean, mean keratometry; MRC, manifest refractive cylinder; ORA, ocular residual astigmatism; PRO, patient reported outcome; SE, spherical equivalent; SI, safety index; UDVA, uncorrected distance visual acuity.
Ethics Approval
This study was approved by the Institutional Review Board of the University Medical Centre Hamburg-Eppendorf. All data were based on the Hamburg refractive Data Base (data retrieved from Care Vision Germany GmbH). Informed consent and permission to use their data for analyses and publication were obtained for each patient. The research was performed in accordance with the guidelines of the Declaration of Helsinki.
Acknowledgment
This work was performed to fulfill the criteria of a Medical Thesis at University Hamburg, Faculty of Medicine, Hamburg, Germany.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Archer TJ, Reinstein DZ, Pinero DP, Gobbe M, Carp GI. Comparison of the predictability of refractive cylinder correction by laser in situ keratomileusis in eyes with low or high ocular residual astigmatism. J Cataract Refract Surg. 2015;41(7):1383–1392. doi:10.1016/j.jcrs.2014.10.046
2. Pesudovs K, Garamendi E, Elliott DB. A quality of life comparison of people wearing spectacles or contact lenses or having undergone refractive surgery. J Refract Surg. 2006;22(1):19–27. doi:10.3928/1081-597X-20060101-07
3. Solomon KD, Fernandez de Castro LE, Sandoval HP, et al. LASIK world literature review: quality of life and patient satisfaction. Ophthalmology. 2009;116(4):691–701. doi:10.1016/j.ophtha.2008.12.037
4. Lau YT, Shih KC, Tse RH, Chan TC, Jhanji V. Comparison of visual, refractive and ocular surface outcomes between small incision lenticule extraction and laser-assisted in situ keratomileusis for myopia and myopic astigmatism. Ophthalmol Ther. 2019;8(3):373–386. doi:10.1007/s40123-019-0202-x
5. Han T, Shang J, Zhou X, Xu Y, Ang M, Zhou X. Refractive outcomes comparing small-incision lenticule extraction and femtosecond laser-assisted laser in situ keratomileusis for high myopia. J Cataract Refract Surg. 2020;46(3):419–427. doi:10.1097/j.jcrs.0000000000000075
6. Tulu Aygun B, Cankaya KI, Agca A, et al. Five-year outcomes of small-incision lenticule extraction vs femtosecond laser-assisted laser in situ keratomileusis: a contralateral eye study. J Cataract Refract Surg. 2020;46(3):403–409. doi:10.1097/j.jcrs.0000000000000067
7. Bohac M, Koncarevic M, Dukic A, et al. Unwanted astigmatism and high-order aberrations one year after excimer and femtosecond corneal surgery. Optom Vis Sci. 2018;95(11):1064–1076. doi:10.1097/OPX.0000000000001298
8. Bailey MD, Mitchell GL, Dhaliwal DK, Boxer Wachler BS, Zadnik K. Patient satisfaction and visual symptoms after laser in situ keratomileusis. Ophthalmology. 2003;110(7):1371–1378. doi:10.1016/S0161-6420(03)00455-X
9. Li SM, Kang MT, Wang NL, Abariga SA. Wavefront excimer laser refractive surgery for adults with refractive errors. Cochrane Database Syst Rev. 2020;12:CD012687. doi:10.1002/14651858.CD012687.pub2
10. Frings A, Katz T, Richard G, Druchkiv V, Linke SJ. Efficacy and predictability of laser in situ keratomileusis for low astigmatism of 0.75 diopter or less. J Cataract Refract Surg. 2013;39(3):366–377. doi:10.1016/j.jcrs.2012.09.024
11. Alpins NA. New method of targeting vectors to treat astigmatism. J Cataract Refract Surg. 1997;23(1):65–75. doi:10.1016/s0886-3350(97)80153-8
12. Tejedor J, Guirao A. Agreement between refractive and corneal astigmatism in pseudophakic eyes. Cornea. 2013;32(6):783–790. doi:10.1097/ICO.0b013e31826dd44b
13. Alpins N, Ong JK, Stamatelatos G. New method of quantifying corneal topographic astigmatism that corresponds with manifest refractive cylinder. J Cataract Refract Surg. 2012;38(11):1978–1988. doi:10.1016/j.jcrs.2012.07.026
14. Alpins N, Stamatelatos G. Customized photoastigmatic refractive keratectomy using combined topographic and refractive data for myopia and astigmatism in eyes with forme fruste and mild keratoconus. J Cataract Refract Surg. 2007;33(4):591–602. doi:10.1016/j.jcrs.2006.12.014
15. Kugler L, Cohen I, Haddad W, Wang MX. Efficacy of laser in situ keratomileusis in correcting anterior and non-anterior corneal astigmatism: comparative study. J Cataract Refract Surg. 2010;36(10):1745–1752. doi:10.1016/j.jcrs.2010.05.014
16. Alpins NA. A new method of analyzing vectors for changes in astigmatism. J Cataract Refract Surg. 1993;19(4):524–533. doi:10.1016/s0886-3350(13)80617-7
17. Pesudovs K. Orbscan mapping in Ehlers-Danlos syndrome. J Cataract Refract Surg. 2004;30(8):1795–1798. doi:10.1016/j.jcrs.2004.05.002
18. Qian YS, Huang J, Liu R, et al. Influence of internal optical astigmatism on the correction of myopic astigmatism by LASIK. J Refract Surg. 2011;27(12):863–868. doi:10.3928/1081597X-20110629-01
19. Schallhorn SC, Venter JA, Hannan SJ, Hettinger KA, Teenan D. Effect of postoperative keratometry on quality of vision in the postoperative period after myopic wavefront-guided laser in situ keratomileusis. J Cataract Refract Surg. 2015;41(12):2715–2723. doi:10.1016/j.jcrs.2015.06.034
20. Mohammadpour M, Heidari Z, Mohammad-Rabei H, et al. Correlation of higher order aberrations and components of astigmatism in myopic refractive surgery candidates. J Curr Ophthalmol. 2016;28(3):112–116. doi:10.1016/j.joco.2016.04.007
21. Khadka J, McAlinden C, Pesudovs K. Quality assessment of ophthalmic questionnaires: review and recommendations. Optom Vis Sci. 2013;90(8):720–744. doi:10.1097/OPX.0000000000000001
22. Kandel H, Khadka J, Lundstrom M, Goggin M, Pesudovs K. Questionnaires for measuring refractive surgery outcomes. J Refract Surg. 2017;33(6):416–424. doi:10.3928/1081597X-20170310-01
23. Pesudovs K, Burr JM, Harley C, Elliott DB. The development, assessment, and selection of questionnaires. Optom Vis Sci. 2007;84(8):663–674. doi:10.1097/OPX.0b013e318141fe75
24. Schallhorn SC, Venter JA, Teenan D, et al. Patient-reported outcomes 5 years after laser in situ keratomileusis. J Cataract Refract Surg. 2016;42(6):879–889. doi:10.1016/j.jcrs.2016.03.032
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

