Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Effect of Metformin on Breast Density in Overweight/Obese Premenopausal Women
Authors Leng W, Pu D, Jiang J, Lei X, Wu Q , Chen B
Received 22 July 2021
Accepted for publication 23 October 2021
Published 3 November 2021 Volume 2021:14 Pages 4423—4432
DOI https://doi.org/10.2147/DMSO.S330625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Weiling Leng,1 Danlan Pu,2 Juan Jiang,2 Xiaotian Lei,1 Qinan Wu,3,* Bing Chen1,*
1Endocrinology Department, The First Affiliated Hospital of the Third Military Medical University (Army Medical University), Chongqing, People’s Republic of China; 2Endocrinology and Nephrology Department, Chongqing University Cancer Hospital and Chongqing Cancer Institute and Chongqing Cancer Hospital, Chongqing, People’s Republic of China; 3Endocrinology Department, Chongqing Medical University Affiliated Dazu Hospital, Dazu District People’s Hospital, Chongqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qinan Wu; Bing Chen Email [email protected]; [email protected]
Background: This study investigated the effects of metformin on breast density in overweight/obese premenopausal women.
Methods: Overweight/obese premenopausal women (n=120) were randomly assigned to the metformin or placebo group, and all women received lifestyle interventions. The outcomes included weight, BMI, FPG, FIN, glucose, HOMA-IR, LDL-C, HDL-C, TG, TC, SBP, DBP, FSH, E, AD, and the BIRADS grade, and the incidence of breast cancer was assessed by pathological biopsy and BIRADS grade greater than 4.
Results: In total, 120 overweight/obese women completed the 1-year trial. Seven patients had a BIRADS grade greater than 4, including 5 patients who were biopsy positive, in the control group, and 2 patients had a BIRADS grade greater than 4, including 1 patient who was biopsy positive, in the metformin group. Compared with those in the control group, the body weight, BMI, FIN, FPG, HOMA-IR, TC, BIRADS grade and positive pathological biopsy rate in the metformin group were significantly decreased (P< 0.05), while AD was significantly increased (P< 0.05). The correlation analysis indicated that the BIRADS grade was significantly correlated with weight, BMI, FPG, FIN, HOMA-IR, SBP, AD and the positive pathological biopsy rate, and the positive pathological biopsy rate was significantly correlated with weight, BMI, HOMA-IR, SBP, AD and BIRADS grade. The logistic regression analysis revealed that the BIRADS grade was significantly correlated with the positive pathological biopsy rate and AD and that the positive pathological biopsy rate was significantly correlated with the BIRADS grade.
Conclusion: As adjunctive therapy, the combination of lifestyle changes and metformin was found to be a safe strategy for improving related metabolic markers and increasing adiponectin. The BIRADS grade was significantly correlated with the positive pathological biopsy rate and AD, and the positive pathological biopsy rate was significantly correlated with the BIRADS grade.
Keywords: metformin, breast density, overweight/obese, adiponectin
Introduction
The latest global cancer statistics in 2020 estimated that there would be 19.29 million new cancer patients worldwide and 4.57 million confirmed cancer patients in China in 2020.1,2 Currently, the prevalence of cancer in China is among the highest worldwide, and China ranks first globally in breast cancer prevalence. Studies have indicated that the breast density is an important risk factor for breast cancer2 An elevated breast density and overweight/obesity are the two most prevalent risk factors for breast cancer.3 The breast density represents the relative amounts of dense (fibro glandular) and nondense (fatty) areas on a mammogram. Among the density measures, the percent density, ie, the ratio of dense to overall breast area, is the most strongly associated with risk.4 Breast density has become the most important risk prediction index for breast cancer.3 On mammography, dense breast tissue is usually defined as ≥75% of the total breast. In general, dense breasts are 4–6 times more likely to develop breast cancer than nondense breasts.5 Overweight/obesity increases the prevalence of breast cancer by 30–50% due to the relationships between breast cancer development and various metabolic disorders caused by overweight/obesity, including insulin resistance and changes in the expression and secretion of adipokines caused by chronic inflammation.6,7 Notably, overweight/obese premenopausal women are at a high risk of breast cancer in China.5 Thus, the main focus of clinical breast cancer research is the identification of effective measures for prevention and treatment.
Multiple studies suggested that adiponectin, the adipokine with the highest concentration in the body, inhibits the occurrence and development of breast cancer,8 but whether adiponectin reduces the breast density and participates in the early prevention of breast cancer is still unclear.9 Metformin, an agonist of adiponectin, was demonstrated to have an anti-breast cancer effect.10 However, verification of this effect in randomized controlled clinical trials is lacking, the effect of metformin on breast density is unclear, and the mechanism of action is unknown. We aimed to investigate whether metformin can prevent breast cancer by increasing adiponectin’s effect on breast density in overweight/obese women and its possible mechanism.
Methods and Material
Study Design
In total, 120 overweight/obese premenopausal female patients were recruited. After informed consent was obtained, the patients were randomly assigned to the control group and metformin group by the “random number” method. The inclusion criteria were as follows: (1) no previous history of diabetes, hypertension, coronary heart disease, stroke, or malignant tumor; (2) a female sex and an age of 21–54 years; (3) no menopause within the first 6 months (clinical menstrual history and FSH, E2); (4) BMI ≥ 24 kg/m2; (5) no abnormality on abreast X-ray within12 months before entering the study; and (6) ability to understand and willingness to sign a written informed consent form. The exclusion criteria were as follows: (1) premenopausal status (including amenorrhea for at least 12 months before entering the study or a history of hysterectomy or bilateral oviduct oophorectomy); (2) severe liver and renal insufficiency or severe malnutrition); (3) diabetes, hypertension, coronary heart disease, stroke or malignant tumor; (4) uncontrolled severe infection; (5) pregnancy and lactation; or (6) mental or neurological diseases resulting in the inability or unwillingness to cooperate. This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Cancer Hospital Affiliated with Chongqing University (Lunshen No. 171, 2019) and registered on the China Clinical Trials website (ChiCTR1900026211).
Intervention Measures
All patients underwent a mammography examination before and after enrollment. The control group received placebo + the basic intervention. The patients were given placebo + diet and exercise interventions without contraindications (the diet and exercise program were formulated, and the implementation was supervised). The metformin group received the basic intervention + metformin, namely, metformin hydrochloride sustained release tablets (provided by Chongqing Kang Kere Pharmaceutical Co., Ltd.).The dose was within the AACE/ACE Obesity Management Guidelines for the safe use of metformin in nondiabetic patients.11 All patients received each intervention measure for 1 year after leaving the hospital.
Lifestyle Interventions
1. Regarding the dietary intervention, an individualized dietary plan was developed, a reasonable proportion of daily total calories and nutrients was calculated, the energy required for three meals was determined, all types of food were reasonably matched, and a personalized nutrition diet was developed. Furthermore, the intake of fresh vegetables and fruits was increased, attention was given to the intake of multiple vitamins, and the allocation was optimized according to the weight of the subject. 2. The exercise guidance recommends performing moderate intensity aerobic exercise, adopting a pulse rate of 60% of the maximum oxygen consumption as the target rate, encouraging the subject to exercise at least 5 times per week 60–90 minutes after a meal for at least 30 minutes per session, and educating the patient to self-assess the pulse rate.
Data Collection and Outcome Measures
The breast feeding history, OCP medication history and family history of breast cancer were collected. Age, height, weight, BMI, BP, parity and age of menarche were measured using the ICSHIB standardized protocol. The concentrations of fasting plasma glucose (FPG), fasting insulin(FIN), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), FSH, E2, and adiponectin were measured by certified laboratories. HOMA-IR was calculated by means of the updated HOMA model (HOMA2), and the coefficients of variation within and between batches of all parameters were <5%.The basic treatment program included a lifestyle intervention as described above; all patients received the basic treatment. The safety variables included adverse events, such as vomiting, vital signs, electrocardiogram, biochemical and hematological measures, and patient-reported hypoglycemic episodes. A serious adverse event was defined as a disability, a life-threatening experience, or an event that required medical or surgical intervention to prevent another outcome, namely, an adverse event that resulted in death or hospitalization. These data were recorded at baseline. The follow-up visits took place during outpatient visits 1 year after leaving the hospital. During the follow-up visits, we collected patient data and information concerning adverse events. Pathological biopsy specimens were obtained from the patients whose BIRADS grade was greater than 4, and malignant tumors were defined as positive.
The primary aim of this trial was to assess the change in the BIRADS grade and pathological biopsy positive rates between patients receiving metformin or placebo intervention. The secondary aims were to assess the changes in metabolic indicators (BMI, HOMA-IR and lipids) and adiponectin after a 1-year treatment period.
Statistical Analyses
The statistical software SPSS 19.0 was employed to perform the statistical analysis. A P-value<0.05 indicates statistical significance. The variables that were continuously and normally distributed are shown as the mean and standard deviation (x±s). Before the statistical analysis, the data were subjected to a normal distribution analysis using a Kolmogorov–Smirnov’s test. When the variance was uniform, the difference between the groups was tested by an independent-samples t-test. The relationships among the parameters at baseline and the changes in the parameters after the treatment were analyzed by simple correlations. The correlations of the variables were determined by a Pearson’s correlation analysis, and a regression analysis was performed to control the effects of covariates and test the independent factors.
Results
Comparison of the Clinical Data of the 2 Groups
There were no significant differences in the breastfeeding history, OCP medication history or family history of breast cancer. No significant difference was found in age, parity, age of menarche, BMI, FPG, FIN, HOMA-IR, LDL-C, HDL-C, TG, TC, SBP, DBP, FSH, E2, AD, or BIRADS grade. There were no significant differences in each parameter between the 2 groups (Table 1).
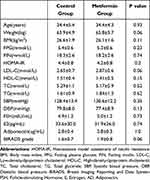 |
Table 1 Baseline Characteristics of Patients in Each Group (X±s) |
Comparison of Each Parameter After the Treatment
Our research suggests that after 1 year of the intervention, some indicators, such as weight, BMI, FPG, FIN, HOMA-IR, AD, BIRADS grade and pathological biopsy positive rate, were significantly improved (P<0.05) (Table 2).
 |
Table 2 The Changes in Parameters After Each Treatment (X±s) |
In addition, there were no differences in LDL-C, HDL-C, TC, TG, SBP, DBP, FSH orE2betweenthe control group and metformin group (P>0.05). Weight, BMI, FPG, FIN, HOMA-IR, LDL-C, TC, BIRADS grade and pathological biopsy positivity were significantly decreased in the metformin group compared with those in the control group (P<0.05), and adiponectin was significantly increased (P<0.05) in the metformin group compared with that in the control group. This result indicates that the metformin intervention may have been a factor in the improvement of each parameter.
Relationships Among the Changes in Each Parameter 1 Year After the Treatment (Table 3)
Our research also examined the correlation between each indicator and the BIRADS grade and pathological biopsy positivity after 1 year of each intervention. Height (R=0.351, P<0.05), weight (R=0.286, P<0.05), BMI (R=0.604, P<0.05), FPG (R=0.197, P<0.05), FIN (R=0.366, P<0.05), HOMA-IR (R=0.384, P<0.05), SBP (R=−0.198, P<0.05), adiponectin (R=−0.728, P<0.05), and pathological biopsy positivity (R=0.543, P<0.05) were associated with the BIRADS grade, and weight (R=0.202, P<0.05), BMI (R=0.290, P<0.05), HOMA-IR (R=0.181, P<0.05), SBP (R=−0.208, P<0.05), adiponectin (R=−0.357, P<0.05), BIDS grade (R=0.05), and SBP (<0.05) were associated with pathological biopsy positivity (R=0.05).
 |
Table 3 Relationships Among Changes in Each Parameter After Treatment |
Regression Analyses of Each Parameter After the Treatment (Tables 4 and 5)
Adiponectin, FBG, and FIN were identified as risk factors for the BIRADS grade; of these variables, adiponectin was the most significant. Adiponectin and HDL-C were identified as major risk factors for pathological biopsy positivity; of these variables, adiponectin was the most significant.
 |
Table 4 Logistic Regression Analyses of the BIRADS Grade After Treatment |
 |
Table 5 Logistic Regression Analyses of Pathologicalbiopsypositivity After Treatment |
Adverse Event
There were no serious adverse events, such as death or coma. Fifteen patients in the metformin group (25%) experienced vomiting and nausea, and the incidence in this group was higher than that in the control group (3 patients, 5%). Ten patients in the metformin group (16.7%) experienced diarrhea, and the incidence was higher than that in the control group (2 patients, 3.3%). Five patients in the control group (8.33%) and one patient in the metformin group (1.67%) developed breast cancer, which was diagnosed by pathological biopsy, and all patients successfully received anticancer treatment.
Discussion
Breast cancer is the most common type of cancer in women and the leading cause of death.12 It is well known that overweight/obesity is a key risk factor for breast cancer.13,14 Overweight/obesity changes insulin sensitivity and regulates estrogen availability and receptor function, thereby increasing the risk and progression of breast cancer, but the mechanism is unclear.7 Some studies have suggested that the risk of breast cancer in women with an increased breast density is 4–6 times higher than that in women without an increased breast density.15 Similar conclusions have been obtained in the Chinese population.5 Moreover, some studies have indicated that the breast density of premenopausal women in China is higher than that in premenopausal women in Western countries.16 Breast density monitoring has become the most important risk predictor of breast cancer, and improving the breast density is of great significance for Chinese premenopausal women.17
Metformin, as the first-line treatment for type 2 diabetes, has been used for many years. Additionally, metformin has been approved for some diseases characterized by metabolic disorders, such as metabolic syndrome, polycystic ovary syndrome (PCOS), and nonalcoholic fatty liver disease. The mechanism mainly depends on the inhibition of hepatic gluconeogenesis and glucose output. In addition, metformin may improve insulin resistance and various metabolic disorders by increasing adiponectin and activating the amp activated protein kinase (AMPK) signaling pathway.10 Some studies suggested that metformin treatment improves metabolic disorders and the tumor microenvironment, reduces the risk of breast cancer, mitigates tumor development and metastasis, enhances the therapeutic sensitivity of chemotherapeutics and improves the prognosis and survival rate of breast cancer patients.18–20 Some studies suggested that metformin directly participates in the regulation of apoptosis in breast cancer cells and plays a direct role in breast tissue physiology via its anti-proliferative effects and induction of apoptosis, which is beneficial for combating breast cancer.21 Therefore, metformin reduces the breast cancer risk in overweight/obese patients regardless of whether the patient has diabetes.10,22 Some studies suggested that obesity induces resistance to anti-VEGF treatment in mice with breast cancer by producing inflammatory and angiogenic factors, while metformin normalizes the expression of FGF-2, thereby reducing the vascular density in mice and restoring tumor sensitivity to anti-VEGF treatment.23
Some studies have found that metformin significantly reduced the insulin levels in non-diabetic breast cancer patients and improved insulin resistance, indicating that metformin may participate in the prevention of breast cancer.24 However, evidence of the role of metformin in breast cancer prevention is still limited.25
Age, gene mutation, breast density, family history, birth history and oral contraceptives were all risk factors for breast cancer; among these factors, age and gene mutations were the strongest risk factors. The breast density is a subjective estimate by the interpreting radiologist of the relative amount of radiopaque breast parenchyma in relation to radiolucent fatty tissue comprising each breast.26 According to the American College of Radiology (ACR), under the Breast Imaging Reporting and Data System (BI-RADS), the breast density should be subjectively classified into one of four categories by interpreting radiologists as follows: nearly total fatty, scattered fibro glandular densities, heterogeneously dense, and extremely dense. Nevertheless, women who fall into the latter two categories (heterogeneously dense and extremely dense) are commonly combined and considered to have “dense breasts”.27 Some researchers believe that the breast density alone explains a significant proportion of cancer risk at the population level.28 This opinion was supported by data from the Canadian Screening Program, which showed that increased breast density accounted for 16% of all breast cancer diagnoses, 40% of interphase cancer diagnoses, and 12% of cancers detected by screening risk factors, including age and genetic mutations.29
A Spanish study suggested that metformin combined with stains reduced the breast density in overweight/obese women.30 Some studies indicated that metformin improves the breast density of postmenopausal women without metabolic syndrome, and the authors speculate that this mechanism is not related to an improvement in insulin resistance but is related to effects on cell proliferation, apoptosis and DNA repair.31 However, some studies demonstrated that metformin has no significant effect on the breast density in postmenopausal patients with type 2 diabetes.32 It has also been reported that the use of metformin in diabetic patients is associated with decreased breast density, but this correlation disappears after adjusting for body weight.33 Therefore, whether metformin improves women’s breast cancer risk by reducing the breast density is unclear, and no study investigated the breast density reduction effect of metformin in overweight/obese premenopausal women without diabetes.
Therefore, a randomized, placebo-controlled trial is needed to evaluate the efficacy of metformin in the prevention of breast cancer. Moreover, there are no reports regarding whether metformin reduces breast density in overweight/obese women or the specific mechanism.
Our research suggests that BMI, FPG, FIN, HOMA-IR, adiponectin, BIRADS grade and pathological biopsy positive rate were significantly improved in the metformin group compared with those in the control group. This result suggests that lifestyle changes and metformin treatment had a greater effect on improvement in each indicator than the intervention with only the lifestyle change.
The current consensus is that overweight and obese postmenopausal women are at an increased risk of breast cancer. However, whether overweight and obesity increase the risk of breast cancer in premenopausal women remains controversial. Amadou et al conducted a meta-analysis of the effect of body weight on breast cancer in premenopausal women based on race, and the results showed that BMI was negatively correlated with breast cancer risk in premenopausal women in Africa but was positively correlated with breast cancer risk in Asian women.34 Therefore, we hypothesized that appropriate weight loss could prevent breast cancer in Chinese premenopausal women. In this study, the patients treated with metformin lost weight and had a reduced risk of breast cancer compared with the controls. It is widely believed that the breast density decreases as BMI increases.5 The regression analysis in this study showed that there was no significant correlation between BMI and breast density or breast biopsy rate after the metformin treatment, suggesting that appropriate weight loss does not increase the risk of breast cancer in premenopausal women treated with metformin.
Insulin resistance (IR) is a complex metabolic disease in which both genetics and the environment are influencing factors.IR has been considered the main mediator of metabolic syndrome. Obesity, especially visceral obesity, is a key factor in the occurrence of IR.35 Studies have shown that insulin resistance can promote the development of breast cancer, and reducing insulin resistance can reduce the risk of postmenopausal breast cancer, but the effect on the risk of premenopausal breast cancer is still controversial.36–38 Eliassen et al conducted a nested case-control study of premenopausal women, and the results showed that high levels of insulin and C-peptide were not associated with breast cancer risk in premenopausal women,39 but the study focused on premenopausal women in the United States and did not conduct an analysis by race. Kachhawa et al studied the risk of breast cancer-related metabolic indicators in pre- and postmenopausal women in India, and the results showed that the insulin level and HOMA-IR were positively correlated with the risk of breast cancer regardless of the menopausal status.40 The research was conducted in an Asian population, and the results were more consistent with the Asian population. Our study suggests that metformin improved insulin resistance and prevented the occurrence of breast cancer. The correlation analysis showed that the insulin level and HOMA-IR were related to the BIRADS grading, suggesting that metformin can reduce the breast density by improving insulin resistance, thereby reducing the risk of breast cancer.
In addition to the above related metabolic indicators, this study further explored the relationship between metformin and adiponectin and between adiponectin and breast cancer risk. Adiponectin is a type of protein or endogenous bioactive polypeptide, and its concentration is the highest among the adipokines. Researchers have suggested that the level of adiponectin in overweight/obese people is significantly decreased compared with that in normal people.41 Many studies have indicated that adiponectin inhibits the occurrence and development of breast cancer and reduces the growth, proliferation and invasion of breast cancer cells regardless of the menopausal status.8 Therefore, the lack of adiponectin may be an important reason for the high incidence of breast cancer in overweight/obese people, and increasing adiponectin may be a good way to reduce the occurrence and development of breast cancer22,42. Previous studies suggested that the adiponectin agonist metformin has an adjuvant treatment effect against breast cancer, but these studies were mostly retrospective analyses and lacked control groups. Most of the study population was diagnosed with breast cancer, received standard adjuvant radiotherapy, endocrine therapy, or biological therapy before or during treatment, or received adjuvant or neoadjuvant therapy before treatment. The interpretation of these research results may be biased.10
A low adiponectin concentration was associated with a poor prognosis in early and/or newly diagnosed breast cancer.43,44 Studies have also indicated that the adiponectin levels are directly associated with high-risk pattern mammograms and a high breast density in obese postmenopausal women.45 High adiponectin levels were significantly associated with a lower breast density in Mexican premenopausal women, but these women were not overweight or obese.46 This result indicates that adiponectin may participate in not only the treatment but also the prevention of breast cancer, but no research investigated the effect of adiponectin on the breast density in overweight/obese premenopausal Chinese women.
Some researchers also reported that adiponectin induced breast cancer cell apoptosis and protected normal cells,47,48 and some studies demonstrated that adiponectin inhibited breast cell fibrosis and participated in reducing the breast density,49,50 suggesting that increasing the adiponectin levels may decrease the breast density. One study indicated that metformin intervention has no significant effect on the breast density in postmenopausal women with diabetes.32 Our target population was premenopausal women without diabetes, and a lifestyle intervention was carried out. On the one hand, the breast density in premenopausal women is higher than that in postmenopausal women; therefore, the improvement in the difference may be more obvious. On the other hand, previous studies suggested that diabetic patients have lower levels of adiponectin than healthy people and that improvement in lifestyle can also improve the adiponectin levels,51 which may contribute to reducing breast density.
Our results show that 7 patients had BIRADS grades greater than 4, including 5 patients diagnosed with breast cancer, in the control group, and 2 patients had BIRADS grades greater than 4, including 1 patient diagnosed with breast cancer, in the metformin group. The positive pathological biopsy rates were 8.33% in the control group and 1.67% in the metformin group. Compared with those in the control group, the BIRADS grade and pathological biopsy positive rate were significantly decreased, and the concentration of adiponectin was significantly increased in the metformin group (P<0.05). Adiponectin and the BIRADS grade did not differ between the 2 groups before the intervention (P>0.05). The above data suggest that the metformin intervention reduced the BIRADS grade and pathological biopsy positive rate and increased adiponectin. The correlation analysis suggested that adiponectin was associated with the BIRADS grade (R=−0.728) and the positive pathological biopsy rate (R=−0.357). The regression analysis showed that the BIRADS grade, adiponectin (OR=−6.182) and pathological biopsy positive rate (OR=5.218) were the most significant risk factors, and for the pathological biopsy positive rate, the BIRADS grade was the most significant risk factor (OR=5.344).Adiponectin plays an important role in many types of malignant tumors, and many researchers have declared that breast cancer is related to the level of adiponectin.8 Increasing adiponectin is a potential way to relieve breast cancer. Our results demonstrate that adiponectin is associated with a decreased breast density, which is an independent risk factor for breast cancer. Therefore, we conclude that adiponectin is more accurate in reflecting the BIRADS grade and the pathological biopsy positive rate and that metformin may reduce the breast density by reducing adiponectin, thereby preventing breast cancer.
Therefore, this randomized controlled trial revealed that metformin and lifestyle interventions may be used as an auxiliary approach to improve the breast density and prevent specific breast cancer outcomes in postmenopausal women, and the main mechanism may be related to improving insulin resistance and blood lipids and increasing the adiponectin concentration. Regarding the BIRADS grade, adiponectin is the most important factor.
There are still some limitations in this study. Specifically, this study is a single-center study with a small sample size, and it lacks long-term follow-up data, especially data regarding the specific stage and outcomes of breast cancer. Furthermore, due to funding reasons, this study used only the molybdenum target for the BIRADS grading and carried out pathological biopsy only in patients with a grade of 4 without MRI detection of the breast density. We look forward to future follow-up research.
Abbreviations
HOMA-IR, homeostasis model assessment of insulin resistance; BMI, body mass index; FPG, fasting plasma glucose; FIN, fasting insulin; LDL-C, low-density-lipoprotein cholesterol; HDL-C, high-density-lipoprotein cholesterol; TC, total cholesterol; TG, total glyceride; SBP, systolic blood pressure; DBP, diastolic blood pressure; BIRADS, Breast Imaging Reporting and Data System; FSH, Follicle-stimulating Hormone; E, estrogen; AD, adiponectin; ELISA, enzyme-linked immunosorbent assay.
Data Sharing Statement
The excel data used to support the findings of this study are available from the corresponding author (email: [email protected]) upon request after the manuscript has been published for 3 months.
Acknowledgments
This work was supported by grants from the Joint Medical Research Project of Chongqing Science and Technology Bureau and Health Commission (2021msxm311);Chongqing Science and Technology Bureau and Health Commission of Chinese Medicine Technology Innovation and Application Development Project (2020ZY013540); General Project of Graduate Education and Teaching Reform of Chongqing University (cquyjg20329); and 2019 Merck Diabetes Research Fund (g-x-2019-056).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global health observatory. Geneva: World Health Organization; 2018. Available from: who.int/gho/database/en/.
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Engmann NJ, Golmakani MK, Miglioretti DL, et al. Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol. 2017;3(9):1228–1236. doi:10.1001/jamaoncol.2016.6326
4. Pettersson A, Graff RE, Ursin G, et al. Mammographic density phenotypes and risk of breast cancer: a meta-analysis. J Natl Cancer Inst. 2014;106(5):dju078. doi:10.1093/jnci/dju078
5. Sung H, Ren J, Li J, et al. Breast cancer risk factors and mammographic density among high-risk women in urban China. NPJ Breast Cancer. 2018;4:3. doi:10.1038/s41523-018-0055-9
6. Shieh Y, Scott CG, Jensen MR, et al. Body mass index, mammographic density, and breast cancer risk by estrogen receptor subtype. Breast Cancer Res. 2019;21(1):48. doi:10.1186/s13058-019-1129-9
7. Dibaba DT, Ogunsina K, Braithwaite D, et al. Metabolic syndrome and risk of breast cancer mortality by menopause, obesity, and subtype. Breast Cancer Res Treat. 2019;174(1):209–218. doi:10.1007/s10549-018-5056-8
8. Gu L, Cao C, Fu J, et al. Serum adiponectin in breast cancer: a meta-analysis. Medicine (Baltimore). 2018;97(29):e11433. doi:10.1097/MD.0000000000011433
9. Macis D, Aristarco V, Johansson H, et al. A novel automated immunoassay platform to evaluate the association of adiponectin and leptin levels with breast cancer risk. Cancers (Basel). 2021;13(13):3303. doi:10.3390/cancers13133303
10. Mallik R, Chowdhury TA. Metformin in cancer. Diabetes Res Clin Pract. 2018;143:409–419. doi:10.1016/j.diabres.2018.05.023
11. Garvey WT, Mechanick JI, Brett EM, et al. American association of clinical endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. EndocrPract. 2016;22(Suppl 3):1–203. doi:10.4158/EP161365.GL
12. Cao W, Chen HD, Yu YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134(7):783–791. doi:10.1097/CM9.0000000000001474
13. Simone V, D’Avenia M, Argentiero A, et al. Obesity and breast cancer: molecular interconnections and potential clinical applications. Oncologist. 2016;21(4):404–417. doi:10.1634/theoncologist.2015-0351
14. Donohoe CL, Lysaght J, O’Sullivan J, et al. Emerging concepts linking obesity with the hallmarks of cancer. Trends Endocrinol Metab. 2017;28(1):46–62. doi:10.1016/j.tem.2016.08.004
15. Atakpa EC, Brentnall AR, Astley S, et al. The relationship between body mass index and mammographic density during a premenopausal weight loss intervention. Study Cancers (Basel). 2021;13(13):3245. doi:10.3390/cancers13133245
16. Dai H, Yan Y, Wang P, et al. Distribution of mammographic density and its influential factors among Chinese women. Int J Epidemiol. 2014;43(4):1240–1251. doi:10.1093/ije/dyu042
17. Yap YS, Lu YS, Tamura K, et al. Insights into breast cancer in the east vs the west: a review. JAMA Oncol. 2019;5(10):1489–1496. doi:10.1001/jamaoncol.2019.0620
18. De A, Kuppusamy G. Metformin in breast cancer: preclinical and clinical evidence. CurrProbl Cancer. 2020;44(1):100488. doi:10.1016/j.currproblcancer.2019.06.003
19. Rennert G, Rennert HS, Gronich N, et al. Use of metformin and risk of breast and colorectal cancer. Diabetes Res Clin Pract. 2020;165:108232. doi:10.1016/j.diabres.2020.108232
20. Samuel SM, Varghese E, Koklesová L, et al. Counteracting chemoresistance with metformin in breast cancers: targeting cancer stem. Cells Cancers (Basel). 2020;12(9):2482. doi:10.3390/cancers12092482
21. Sharma P, Kumar S. Metformin inhibits human breast cancer cell growth by promoting apoptosis via a ROS-independent pathway involving mitochondrial dysfunction: pivotal role of superoxide dismutase (SOD). Cell Oncol (Dordr). 2018;41(6):637–650. doi:10.1007/s13402-018-0398-0
22. Jiralerspong S, Goodwin PJ. Obesity and breast cancer prognosis: evidence, challenges, and opportunities. J Clin Oncol. 2016;34(35):4203–4216. doi:10.1200/JCO.2016.68.4480
23. Incio J, Ligibel JA, McManus DT, et al. Obesity promotes resistance to anti-VEGF therapy in breast cancer by up-regulating IL-6 and potentially FGF-2. Sci Transl Med. 2018;10(432):eaag0945. doi:10.1126/scitranslmed.aag0945
24. Goodwin PJ, Pritchard KI, Ennis M, et al. Insulin-lowering effects of metformin in women with early breast cancer. Clin Breast Cancer. 2008;8(6):501–505. doi:10.3816/CBC.2008.n.060
25. Gronich N, Rennert G. Beyond aspirin-cancer prevention with statins, metformin and bisphosphonates. Nat Rev Clin Oncol. 2013;10(11):625–642. doi:10.1038/nrclinonc.2013.169
26. Lee CI, Chen LE, Elmore JG. Risk-based breast cancer screening: implications of breast density. Med Clin North Am. 2017;101(4):725–741. doi:10.1016/j.mcna.2017.03.005
27. D’Orsi CJ, Sickles EA, Mendelson EB, et al. American College of Radiology Breast Imaging Reporting and Data System BI-RADS.
28. Boyd NF, Martin LJ, Yaffe MJ, et al. Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res. 2011;13(6):223. doi:10.1186/bcr2942
29. Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. doi:10.1056/NEJMoa062790
30. Lee Argov EJ, Acheampong T, Terry MB, et al. Independent and joint cross-sectional associations of statin and metformin use with mammographic breast density. Breast Cancer Res. 2020;22(1):99. doi:10.1186/s13058-020-01336-0
31. Bershteĭn LM, Vasil’ev DA, Kovalenko IG, et al. The influence of metformin and N-acetylcysteine on mammographic density in postmenopausal women. VoprOnkol. 2012;58(1):45–49.
32. Ozturk MA, Ozturk S, Eryilmaz M, et al. Does metformin affect mammographic breast density in postmenopausal women with type 2 diabetes. Gynecol Endocrinol. 2020;36(9):800–802. doi:10.1080/09513590.2020.1725972
33. Oskar S, Engmann NJ, Azus AR, et al. Gestational diabetes, type II diabetes, and mammographic breast density in a U.S. racially diverse population screened for breast cancer. Cancer Causes Control. 2018;29(8):731–736. doi:10.1007/s10552-018-1048-6
34. Amadou A, Ferrari P, Muwonge R, et al. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose-response meta-analysis. Obes Rev. 2013;14(8):665–678. doi:10.1111/obr.12028
35. Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol. 2013;3(1):1–58. doi:10.1002/cphy.c110062
36. Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121(4):856–862. doi:10.1002/ijc.22717
37. García-Estévez L, Cortés J, Pérez S, et al. Obesity and breast cancer: a paradoxical and controversial relationship influenced by menopausal status. Front Oncol. 2021;11:705911. doi:10.3389/fonc.2021.705911
38. Jung SY, Mancuso N, Papp J, et al. Post genome-wide gene-environment interaction study: the effect of genetically driven insulin resistance on breast cancer risk using Mendelian randomization. PLoS One. 2019;14(6):e0218917. doi:10.1371/journal.pone.0218917
39. Eliassen AH, Tworoger SS, Mantzoros CS, et al. Circulating insulin and C-peptide levels and risk of breast cancer among predominately premenopausal women. Cancer Epidemiol Biomarkers Prev. 2007;16(1):161–164. doi:10.1158/1055-9965.EPI-06-0693
40. Kachhawa P, Kachhawa K, Agrawal D, et al. Association of dyslipidemia, increased insulin resistance, and serum CA 15-3 with increased risk of breast cancer in urban areas of North and Central India. J Midlife Health. 2018;9(2):85–91. doi:10.4103/jmh.JMH_77_17
41. Parida S, Siddharth S, Sharma D. Adiponectin, obesity, and cancer: clash of the bigwigs in health and disease. Int J Mol Sci. 2019;20(10):2519. doi:10.3390/ijms20102519
42. Cha YJ, Koo JS. Adipokines as therapeutic targets in breast cancer treatment. Expert OpinTher Targets. 2018;22(11):941–953. doi:10.1080/14728222.2018.1538356
43. Iyengar NM, Zhou XK, Gucalp A, et al. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin Cancer Res. 2016;22(9):2283–2289. doi:10.1158/1078-0432.CCR-15-2239
44. Lira LG, Justa RM, Carioca AAF, et al. Plasma and erythrocyte ω-3 and ω-6 fatty acids are associated with multiple inflammatory and oxidative stress biomarkers in breast cancer. Nutrition. 2019;58:194–200. doi:10.1016/j.nut.2018.07.115
45. Vega S, Basurto L, Saucedo R, et al. Similar to adiponectin, serum levels of osteocalcin are associated with mammographic breast density in postmenopausal women. J ObstetGynaecol Can. 2018;40(2):186–192. doi:10.1016/j.jogc.2017.06.036
46. Dossus L, Rinaldi S, Biessy C, et al. Circulating leptin and adiponectin, and breast density in premenopausal Mexican women: the Mexican Teachers’ Cohort. Cancer Causes Control. 2017;28(9):939–946. doi:10.1007/s10552-017-0917-8
47. Pham DV, Raut PK, Pandit M, et al. Globular adiponectin inhibits breast cancer cell growth through modulation of inflammasome activation: critical role of sestrin2 and AMPK signaling. Cancers (Basel). 2020;12(3):613. doi:10.3390/cancers12030613
48. Katira A, Tan PH. Adiponectin and its receptor signaling: an anti-cancer therapeutic target and its implications for anti-tumor immunity. Expert OpinTher Targets. 2015;19(8):1105–1125. doi:10.1517/14728222.2015.1035710
49. Khan S, Shukla S, Sinha S, et al. Role of adipokines and cytokines in obesity-associated breast cancer: therapeutic targets. Cytokine Growth Factor Rev. 2013;24(6):503–513. doi:10.1016/j.cytogfr.2013.10.001
50. Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev. 2007;8(5):395–408. doi:10.1111/j.1467-789X.2007.00396.x
51. Lei X, Wu Q, Leng W, et al. Exenatide reduces cardiomyocyte apoptosis by stimulating adiponectin secretion and activating APPL1-AMPK-PPARα axis. Ann Transl Med. 2019;7(14):326. doi:10.21037/atm.2019.06.17
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
