Back to Journals » International Journal of Women's Health » Volume 14
Effect of Low-Power Visible-Light-Activated Photodynamic Therapy (PDT) on Primary Dysmenorrhea: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial
Authors Gu B, Zhu S, Ding X, Deng Y, Ma X, Gan J, Wang Y, Sun A
Received 18 March 2022
Accepted for publication 7 July 2022
Published 4 August 2022 Volume 2022:14 Pages 1029—1036
DOI https://doi.org/10.2147/IJWH.S367051
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Bei Gu,1 Shiyang Zhu,2 Xuesong Ding,2 Yan Deng,2 Xiao Ma,2 Jingwen Gan,2 Yanfang Wang,2 Aijun Sun2
1Department of Obstetrics and Gynecology, Beijing Shijitan Hospital, Capital Medical University, the Ninth Clinical Medical College of Peking University, Beijing, People’s Republic of China; 2National Clinical Research Center for Obstetric & Gynecologic Diseases, Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Aijun Sun, Tel +86 18600045466, Email [email protected]
Background: Primary dysmenorrhea (PD) is one of the most common complaints in women of childbearing age. Therefore, this trial aimed to assess the efficacy and safety of low-power visible-light-activated photodynamic therapy (PDT) in the treatment of primary dysmenorrhea (PD), and to further investigate their possible mechanisms of action.
Methods: This study was conducted by using a multicenter, randomized, open, parallel control design. Qualified subjects are randomly assigned to two groups: Group A (low-power visible-light-activated PDT group), Group B (placebo group) and are treated with corresponding protocols for three consecutive menstrual cycles. Baseline data are collected during the trial period. Changes in the scores of VAS scales and the fluctuation of pain factors (PGE2, PGF2α) are recorded before and after the treatment for each group. A comparison of effectiveness in pain control and symptom control is made among the two groups.
Results: After treatment, for the PDT group, the scores of VAS scales decline compared with the scores before treatment. The level of pain factors including PGE2 and PGF2α also drops significantly (P < 0.05). There are no serious adverse events during the study.
Conclusion: Low-power visible-light-activated PDT is a new type of treatment for primary dysmenorrhea which is safe, effective and does not affect normal pregnancy preparation. It may exert its therapeutic effect by adjusting downward the level of PGE2, PGF2α in the body. These factors can be used not only to study the treatment mechanism for primary dysmenorrhea, but also to serve as quantitative indicators for objective assessment of whether dysmenorrhea is relieved.
Keywords: primary dysmenorrhea, low-power visible-light-activated photodynamic therapeutic device, prostaglandins E2, prostaglandins F2alpha
Background
Primary dysmenorrhea (PD) is one of the common gynecological problems that tortures many women. Normally, it is a clinical syndrome without any organic pathology but usually manifests itself in periodic pain in the lower abdomen before, during or after menstruation. In some cases, waist pain, heaviness and distension in the lower abdomen, coldness of limbs, nausea, emesis, dizziness and weakness may also occur besides abdominal pain. In severe cases, diarrhea, paleness may occur and even syncope due to severe pain. According to statistics, the incidence of PD is as high as 45%-95%,1 which seriously affects women’s physical and mental health. The female students in high schools, colleges and universities may suffer from absenteeism or loss of concentration in the lessons and fatigue.1
At present, NSAIDs and oral contraceptives are commonly used as the first and second-line treatment for PD. However, not all patients have a tolerance to the adverse effects of NSAIDs and oral contraceptives.2 Sometimes the application of these two types of medicines is also restricted by cultural factors. The pathogenesis of PD mainly involves the release of PGs, in which PGF2α, PGE2, AVP, OT, ET, NO, and β-EP directly or indirectly affect the synthesis and secretion of prostaglandins, which causes spastic contractions of the uterus.2 This is the essence of dysmenorrhea. Therefore, reducing the synthesis and secretion of prostaglandins and suppressing uterine contractions have become important thoughts to treat the disease. According to conventional Chinese medicine, PD is derived from Qi-stagnation and blood stasis syndrome.2 In contrast to western pain killers which usually act on a single pathway, TCM has a significant effect on pain perception through a holistic regulation of the human body with multiple components and targets.3 Low-power visible-light-activated photodynamic therapeutic devices (referred to as PDT devices), which combine low-power visible-light therapy and TCM acupoint stimulation, are a new technology to treat PD. It can reduce the pain and effectively relieve dysmenorrhea symptoms. Another reason for the need for new PD treatments is the lack of relief from NSAIDs and hormonal therapies. However, no relevant studies have so far explored its possible mechanism of action. This study, in addition to VAS, assesses the effectiveness and safety of PDT devices in PD treatment. Furthermore, by comparing the level of PGE2 and PGF2α in the peripheral blood of the subjects before and after treatment, the study also explores the possible mechanisms of action of PDT devices in treating PD.
Data and Methods
Study Design and Participants
The study was a multicenter, prospective, randomized, double-blind, placebo-controlled trial aiming at evaluating the efficacy and safety of PDT on PD management. The study protocol was reviewed and approved by the Institutional Review Board of eight participating hospitals (No.ZS-1913) and it has been registered on ClinicalTrials.gov (NCT03953716). Participants were recruited from seven provinces in China from March 2019 to August 2020 and written informed consent was obtained from each participant. This study complies with the Declaration of Helsinki.1
Patients aged 18 to 35 years old with informed consent, diagnosed with PD, and with regular menstrual cycles (21–35 days), were eligible for the current study. The exclusion criteria are as follows: (1) had known pelvic pathologies that potentially caused secondary dysmenorrhea including endometriosis, pelvic inflammatory disease, adenomyosis, uterine fibromatosis, intrauterine devices, chronic abdominal pain, and ovarian cysts; (2) use of any analgesic or hormonal agents that could interfere with the outcomes within 12 weeks preceding enrollment; (3) had a concurrent neuropathologic disease, anemia, immunodeficiency, or dysfunction in liver and kidney; (4) had an addiction for alcohol, drugs or cigarette.
A power analysis (two-sample t-test, performed in PASS software) showed that a sample size of 137 women would detect a difference in pain reduction of 2 points weighed by the visual analog scale (α = 0.05 and 1−β = 0.90). Thus, with a 15% of dropout rate, a total of 156 participants were needed in this trial (78 patients in each group).
Interventions
The study adopts a multicenter, randomized, open, parallel control design. The subjects are randomly assigned to two groups in the ratio of 1:1, according to their randomly selected numbers, namely Group A (low-power visible-light-activated PDT group, referred to as PDT group) and Group B (placebo group). Then the corresponding treatments were given to different groups for the assessment and comparison of clinical efficacy and safety of PDT devices. Further studies on the possible mechanism of action of PDT devices were carried out.
Primarily, the usage of PDT devices could be described as: Download the Eospal Yuban mobile APP. Ensure that the device is fully charged. Make the medical gel patches ready (wipe them with alcohol wipes after each use so that they can be reused next time). Locate the right acupoint according to the acupoint map. Secondly, the steps of PDT device usage could be described as: Stand straight or lie down on the back. Put the medical gel patches on the acupoints of “Qihai” and “Guanyuan”. Connect the main unit and the light sensor. Attach the light sensor to the patches. Power the main unit up. Start the “Eospal Youban” APP on the mobile phone and enter the treatment module. Turn on the Bluetooth of the mobile phone. Search and pair the phone with the PDT device. Click “Start” on the APP interface to start the treatment. Thirdly, the back-stage monitoring could be accomplished in the following way: The APP automatically displays the treatment time. If the connection between the APP and the PDT device is broken, the APP, after reconnecting, directly displays the remaining treatment time. The device automatically turns off at the end of the 20 min treatment and the APP returns to the start page. The use of the PDT device by the subjects in Group A is monitored by Shenzhen Guangpu Medical Co., Ltd and all data is collected for the researcher to track the use closely and urge the subjects to use the device as required.
Treatment Protocols for Group A are stated as: Start to use the PDT device after the end of previous menstruation. Once a day for 20 minutes for 5 days (one treatment cycle). Start the next treatment cycle at a 2-days interval. Repeat it until the next menstruation. Use it for 3 menstrual cycles. Treatment Protocols for Group B are given here: Placebo 3.5 g BID×10 d (taken for 10 days starting from 3–5 days before menstruation) × 3 menstrual cycles. Take the medicine with warm water.
Measurement and Endpoints
As the primary outcome, the pain intensity of dysmenorrhea was assessed on the 1st or 2nd day of menstruation using a 10-point visual analog scale (VAS) ranging from 0 (no pain) to 10 (worst pain) at baseline and every 4 weeks for three consecutive menstrual cycles. The pain intensity was ranked by VAS ratings, in which mild pain scored 1–3, moderate pain 4–6, and severe pain 7–10.
Secondary Evaluation Indexes, ie the level of PGE2 and PGF2α in the peripheral blood before and after treatment were quantified by enzyme-linked immunosorbent assay (ELISA) according to the manufacturers’ instructions (RENJIE Biotech Inc., Shanghai, China). Blood samples were collected on the 2nd day of the menstrual cycle at baseline and week 12 of the treatment. The samples were centrifuged at 3000 revolutions per minute for 20 min, after which the supernatants were stored at −80 °C. Serums were thawed at room temperature.
Statistical Analysis
Patients who completed the entire treatment and all study assessments were included in the statistical analysis. Data are presented as mean ± standard error of the mean (SEM). Repeated measures were used to analyse the VAS scores between the groups. Basic characteristics, changes in biochemical factors, and uterine artery flow indices between groups were compared using an independent sample t-test. A paired t-test was applied to determine the intra-group differences in longitude comparison between basal values and endpoint results. Statistical analysis was conducted using SPSS version 26.0 (IBM, Armonk, NY, USA). Statistical significance was set at P<0.05.
Results
Demographic Characteristics
Under the supervision of the health professionals, a total of 201 patients were screened for eligibility, of whom 156 eligible patients were enrolled and randomized to either the placebo (n=78) or PDT group (n=78). Of these, 19 (12.0%) participants were lost to follow-up, 137 participants (72 in PDT and 65 in placebo) completed the 12-week study and post-treatment evaluation and were included in the full analysis. In the study entry, the subject characteristics of the two groups matched well. The subject characteristics are demonstrated in Table 1.
 |
Table 1 Characteristics at Baseline Between PDT Group and Placebo Group |
Pain Scores
The absolute changes in VAS scores of all participants are shown in Table 2 and Figure 1. The PDT group showed a significantly more pronounced reduction in VAS scores (p<0.001) compared with the placebo group. PDT’s effect on pain alleviation occurred during the first cycle of treatment and persisted for the whole treatment course. Scores are shown in 6 sets as VAS 0, VAS 1, VAS 2, VAS 3, VAS 4 and VAS 5. VAS 0 is for before treatment; VAS 1, 2 and 3 are for treatment after 1, 2 and 3 cycles respectively; VAS 4 and 5 are for 1 and 2 cycles respectively after treatment stops. The results are presented in Table 2.
 |
Table 2 Changes in VAS Scores Before and After Treatment of the Two Groups |
 |
Figure 1 Changes in VAS scores before and after treatment of the two groups. VAS: **p<0.01. |
The results show a marked change in VAS scores after treatment (P<0.05): VAS scores of the treatment group are markedly lower than the control group (P<0.05). VAS scores after treatment stops are also markedly lower than those before treatment.
Biochemical Parameters
The absolute changes in the concentration of pain mediators are summarized in Figure 2. Compared with baseline values, both groups demonstrated a significant decline in PGE2 and PGF2α, but the degree is more significant in the PDT group than in the placebo group.
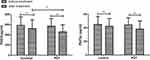 |
Figure 2 Changes in PGE2 and PGF2α levels before and after treatment of the two groups. (n=65 for control group and n=72 for PDT group). **p < 0.01, *p < 0.05. |
The results show that PGE2 and PGF2α levels lowered significantly after treatment in the PDT group (P<0.05); PGE2 and PGF2α levels also lowered after treatment in the control group but they were not as significant as those in the treatment group.
Discussions
The incidence of PD is as high as 45–95%.1 Due to its high incidence and periodical recurrence, PD has become one of the major factors that interrupt and affect the physical, and mental health of adolescent and young reproductive-age women, their family lives and social roles.4 Therefore, how to treat PD safely and effectively has become an important issue for PD patients and clinicians.
The pathogenesis of PD mainly involves the release of PGs, in which PGF2α, PGE2, AVP, OT, ET, NO, and β-EP directly or indirectly affect the synthesis and secretion of prostaglandins, which cause spastic contractions of the uterus. This is the essence of dysmenorrhea. Therefore, reducing the synthesis and secretion of prostaglandins and suppressing uterine contractions have become important thoughts to treat the disease.
The study adopts a multicenter, randomized, open and parallel control design. Pain scales and pain-factors-related biochemical indexes are used to assess and compare the clinical relief effects and biochemical index changes of PDT devices. The study also compares the clinical efficacy and safety of different treatments and explores the possible mechanisms of action of photodynamic therapy for PD. VAS is used for assessing different aspects. VAS, which directly reflects the degree of pain is the most used method for quantitative pain assessment. Meanwhile, the result of pain-factors-related biochemical indicators also shows the clinical effect of different treatment methods, in particular the PDT devices for treating PD. By comparing the level of PGE2, PGF2α in peripheral blood before and after treatment, the study further explores the possible mechanism of action of the PDT device.
There is no statistically significant difference among the groups before treatment. Three months after the treatment, the pain relief efficiency of the PDT group becomes significantly higher than the placebo group. During the follow-up investigation period, VAS scores of the PDT group continued to decrease before starting to increase. Meanwhile, the score of the placebo group shows a constantly rising trend. PDT devices not only reduce the severity of PD-associated symptoms but also shorten the duration of symptoms. In terms of safety, no adverse reactions are reported in the PDT group throughout the study. PDT devices also demonstrate some long-term efficacy, which lasts for at least 1 to 2 menstrual cycles after the end of the treatment. Compared with oral drugs, the advantages of PDT include no allergic reaction to drugs and no hepatic first-pass effect, good patient compliance, and no impact on patients’ normal preparation for pregnancy and pregnancy itself. Its disadvantage is that it takes some time to complete the treatment, which is not as convenient as oral medication. Regarding the mechanism of action, it is clear that clinical application of oral contraceptives inhibits endometrial hyperplasia, reduces the quantity of menstrual blood and the release of PGs, and inhibits ovulation, which helps relieve dysmenorrhea symptoms. There are relatively few studies on the mechanism of action of TCMs. Different TCMs may exert their therapeutic effects through the same components and the same action pathways, such as changing the level of PGF2α,5,6 ET6,7 NO8 and β-EP,7,8 adjusting platelet-activating factors,6 and inhibiting cyclooxygenase and lipoxygenase9 in the body. However, if searching for studies on PDT devices for PD treatment, the only result is the study carried out by Zhang Jie’s team which is also the first of its kind. They compare the PDT group with a blank control group and find that PDT devices not only reduce the pain level but also relieve dysmenorrhea symptoms, which is consistent with the result of this study. The researchers of Zhang Jie’s team believe that the irradiation of visible light with a specific wavelength on the acupoints of Qihai and Guanyuan located below the navel facilitates the synthesis and release of NO in the uterine, thereby relieving dysmenorrhea. However, it’s unfortunate that the study does not further explore the mechanism of action of PDT devices.
So far, the pathogenesis of PD is thought to be mainly related to PGs. The lower abdominal pain and discomfort, nausea, vomiting, lumbosacral and thigh pain and other associated symptoms in PD patients are similar to the side effects caused by the clinical use of PGs. It shows that the occurrence of PD is closely related to PGs, PGF2α in particular, which not only causes effective contraction of uterine blood vessels and muscle layers but also enhances the sensitivity of nerve endings to pain in PD patients by lowering the threshold for pain perception.10,11 During menstruation, the endometrium falls off as a result of the decline of estrogen level, which releases a large amount of PGs into the blood causing uterine contraction that increases intrauterine pressure and a decrease in uterine blood supply. Ischemia and hypoxia of the myometrium and endometrium12 are thus triggered, releasing even more PGs and aggravating painful spasms. In this process, the interaction of OT, AVP and PGs determines the occurrence of dysmenorrhea. AVP, which has similar effects as estrogen, not only enhances the contraction of the uterus and small blood vessels but also increases the sensitivity of the uterus to OT and facilitates the synthesis and release of PGs.13 The increase of PGs not only aggravates uterine contraction but also amplifies the expression of epinephrine and increases the sensitivity of the uterus to OT,13 which enables OT to connect with its receptor and play its role. The connection of OT with the receptor of contraction hormone directly induces contraction of uterine myocytes and indirectly activates the phosphatidylinositol cycle and stimulates the synthesis and release of PGF2α.14 On the other hand, NO, ET, and β-EP also have an impact on the occurrence of PD through other pathways. NO has double effects.15 The increase of NO helps soothe the pain while its decrease facilitates the transmitting of the pain messages. During menstruation, the oxidative stress response and the increase of inflammatory mediators in the body cause damage to vascular endothelial cells, triggering a decline of vasodilators such as NO and an increase of ET with vasoconstrictor effect and thromboxane A2, which prompt vasospasm.14 However, the occurrence of dysmenorrhea is not only attributed to internal secretion, but also the psychological and social conditions of the subjects.16 During menstruation, due to the fluctuation of hormone levels and the increase of inflammatory mediators, some women may experience negative moods such as anxiety and tension, which in turn increase their pain sensitivity. The fact that β-EP stimulates the release of opioids, inhibits the transmission of pain factors and helps adjust the function of the uterus13 makes it a key factor in improving the patients’ mood and soothing their pain. In conclusion, these factors are closely related to the occurrence of primary dysmenorrhea. It is also speculated that dysmenorrhea-associated discomfort may be relieved by decreasing the level of PGE2, PGF2α, ET, OT, AVP and increasing the level of NO and β-EP.
By comparing the level of PGE2, PGF2α in the peripheral blood before and after treatment, the study further explores the possible mechanism of action of the PDT device. It, to some extent, confirms the conclusion that PDT devices do work as described by Zhang Jie’s team. Chinese researchers believe that acupoints are complexes of skin, muscle and nerves that are densely innervated and prone to be easily excited. The two acupoints of “guanyuan” and “qihai” are the principal ones for treating PD using acupuncture, moxibustion and acupoints massage. A large number of studies have shown that stimulating these acupoints with acupuncture or moxibustion has a positive therapeutic effect on relieving PD-related discomfort. The exposure of the two acupoints of “qihai” and “guanyuan” located below navel to the low-power visible light produced by PDT devices stimulates uterine tissue cells, soothes uterine smooth muscles, restores uterine tissue blood circulation and enhances the metabolism of damaged uterine muscle cells,20 thus relieving the pains of dysmenorrhea. It is noteworthy that the study also finds significant changes in PGE2, PGF2α levels in the peripheral blood of the subjects in the PDT group, which indicates that the PDT device can relieve dysmenorrhea by adjusting downward the level of these factors. Besides stimulating acupoints by visible light, the PDT device can also exert its efficacy through a mechanism of action similar to that of partial heat therapy. When visible light irradiates the acupoints in the lower abdomen, light energy is converted to heat energy after a certain period. The heat thus produced dilates part of the blood vessels in the lower abdomen, increases blood supply,17,18 produces a sense of thermal comfort19 and relieves part of the muscle tension and the pain induced by uterine spasm, thus relieving dysmenorrhea in ways similar to that of partial heat therapy.
The result of the placebo group in the study also shows some degree of improvement and long-term efficacy. The subjects in this group believe that they have received active treatment and are psychologically comforted and supported. Their tension and anxiety during menstruation are thus relieved, which helps relieve their subjective feeling of pain. The placebo effect fully shows that psychological factors are one of the important factors triggering dysmenorrhea-related discomfort during the menstrual period.17
Conclusions
In summary, patients with PD can benefit from the administration of PDT by a more pronounced reduction in pain intensity. With inhibition of PGE2 and PGF2α, its therapeutic effect seems to be associated with a synergistic combination of vasoconstrictors and neurological stress hormone.
Limitations of the Study
Further investigation into the molecular mechanism of PDT behind pain attenuation must be investigated and it may provide a profound foundation for its clinical use as an alternative therapy for PD. In addition, it is needed to justify the discrepancies between the active and control conditions. Creating a control condition for this type of intervention is very difficult, but the designers of the study must attempt to standardize most elements of the two treatments, including the timing of treatment events across the cycles, and modality (ie, a large difference between taking a pill and having electrodes placed on the abdomen and activated (or not in the case of a control condition) by an App). Finally, how were these two treatment groups explained to participants?
Abbreviations
PD, primary dysmenorrhea; PDT, low-power visible-light-activated photodynamic therapeutic; VAS, visual analog scale; PGE2, prostaglandin E2; PG, prostaglandins; TCM, traditional Chinese medicine; PGF2α, prostaglandin F2alpha; ELISA, enzyme-linked immunosorbent assay.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Compliance with Ethics Guidelines
This trial was approved by the Ethics Committee of Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Science (No.ZS-1913). All participants provided written informed consent.
Acknowledgments
We are grateful to all the women who participated in our study, and appreciate the arduous work performed by the clinicians and nurses in cooperating hospitals, namely Beijing Obstetrics and Gynecology Hospital, First People’s Hospital of Hangzhou affiliated with Zhejiang University, the Second Affiliated Hospital of Nanchang University, the Hubei Maternal and Child Health Hospital, the First Affiliated Hospital of Chongqing Medical University, Fujian Maternal and Child Health Hospital, and Liuzhou Maternal and Child Health Hospital. We would like to thank Editage (www.editage.cn) for English language editing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study received funding from the Capital’s Funds for Health Improvement and Research (CFH: 2020-2-40113). This study received funding from the Natural Science Foundation of China 82074143.
Disclosure
The authors declare that they have no competing interests.
References
1. Michelle P, Cynthia F. Diagnosis and management of dysmenorrhea. BMJ. 2006;332(7550):1134–1138. doi:10.1136/bmj.332.7550.1134
2. Jung J, Ko MM, Lee MS, Lee SM, Lee JA. Diagnostic indicators for blood stasis syndrome patients with gynaecological diseases. Chin J Integr Med. 2018;24(10):752–757. doi:10.1007/s11655-017-2813-1
3. Luo Y, Wang CZ, Sawadogo R, Tan T, Yuan CS. Effects of herbal medicines on pain management. Am J Chin Med. 2020;48(1):1–16. doi:10.1142/S0192415X20500019
4. Rencz F, Péntek M, Stalmeier PFM, et al. Bleeding out the quality- adjusted life years: evaluating the burden of primary dysmenorrhea using time trade-off and willingness-to-pay methods. Pain. 2017;158(11):2259–2267. doi:10.1097/j.pain.0000000000001028
5. Pan Q, Zhang ZQ, Tian CY, Yu T, Yang R, Chai XL. Effect and signaling pathways of Nelumbinis folium in the treatment of hyperlipidemia assessed by network pharmacology. World J Tradit Chin Med. 2021;7(4):445–455. doi:10.4103/2311-8571.328619
6. Burnett N, Lemyre M. No.345-primary dysmenorrhea consensus guideline. J Obstet Gynaecol Can. 2017;39(7):585–595.
7. Pegado R, Silva LK, da Silva Dantas H, et al. Effects of transcranial direct current stimulation for treatment of primary dysmenorrhea: preliminary results of a randomized sham-controlled trial. Pain Med. 2019;20(1):1–9. doi:10.1093/pm/pny251
8. Lee CH, Roh JW, Lim CY, et al. A multicenter, randomized, double- blind, placebo-controlled trial evaluating the efficacy and safety of a far infrared-emitting sericite belt in patients with primary dysmenorrhea. Complement Ther Med. 2011;19(4):187–193. doi:10.1016/j.ctim.2011.06.004
9. Kannan P, Claydon LS, Lim C-Y, Hong JH, Lee JK, Min EG. Some physiotherapy treatments may relieve menstrual pain in women with primary dysmenorrhea: a systematic review. J Physiother, 2014, 60(1):13–21mary dysmenorrhea. Complement Ther Med. 2011;19(4):187–193.
10. Gebeyehu MB, Mekuria AB, Tefera YG, et al. Prevalence, impact, and management practice of dysmenorrhea among University of Gondar students, northwestern Ethiopia: a cross-sectional study. Int J Reprod Med. 2017;2017:1–8. doi:10.1155/2017/3208276
11. Al-Kindi R, Al-Bulushi A. Prevalence and impact of dysmenorrhoea among Omani high school students. Sultan Qaboos Univ Med J. 2011;11(4):485–491.
12. De Sanctis V, Soliman AT, Elsedfy H, et al. Dysmenorrhea in adolescents and young adults: a review in different countries. Acta Biomed. 2017;87(3):233–246.
13. Ma L, Lei QL, Su Q. Network pharmacology approach to determine active compounds and potential targets associated with the anti-abortion effects of scutellariae radix. World J Tradit Chin Med. 2020;6(3):341–352. doi:10.4103/wjtcm.wjtcm_35_20
14. Agarwal AK, Agarwal A. A study of dysmenorrhea during menstruation in adolescent girls. Indian J Commun Med. 2010;35:159–164. doi:10.4103/0970-0218.62586
15. Hailemeskel S, Demissie A, Assefa N. Primary dysmenorrhea magnitude, associated risk factors, and its effect on academic performance: evidence from female university students in Ethiopia. Int J Women's Health. 2016;8:489–496. doi:10.2147/IJWH.S112768
16. Ylikorkala O, Dawood MY. New concepts in dysmenorrhea. Am J Obstet Gynecol. 1978;130(7):833–847. doi:10.1016/0002-9378(78)90019-4
17. Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update. 2015;21(6):762–778. doi:10.1093/humupd/dmv039
18. Brinckmann JA, Cunningham AB, V. Harter D. Reviewing threats to wild Rhodiola sachalinensis, a medicinally valuable yet vulnerable species. World J Tradit Chin Med. 2021;7(3):299–306. doi:10.4103/wjtcm.wjtcm_47_21
19. Dawood MY. Primary dysmenorrhea: advances in pathogenesis and management. Obstet Gynecol. 2006;108(2):428–441. doi:10.1097/01.AOG.0000230214.26638.0c
20. Davis AR, Westhoff CL. Primary dysmenorrhea in adolescent girls and treatment with oral contraceptives. J Pediatr Adolesc Gynecol. 2001;14(1):3–8. doi:10.1016/S1083-3188(00)00076-0
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
