Back to Journals » Drug Design, Development and Therapy » Volume 16
Effect of Intravenous Infusion of Lidocaine Compared with Ultrasound-Guided Transverse Abdominal Plane Block on the Quality of Postoperative Recovery in Patients Undergoing Laparoscopic Bariatric Surgery
Authors Sun J, Wang S, Wang J, Gao X, Wang G
Received 3 January 2022
Accepted for publication 11 March 2022
Published 21 March 2022 Volume 2022:16 Pages 739—748
DOI https://doi.org/10.2147/DDDT.S356880
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Tin Wui Wong
Jing Sun,1,2 Shan Wang,1,2 Jun Wang,1,2 Xiuxiu Gao,1,2 Guanglei Wang1,2
1Jiangsu Province Key Laboratory of Anesthesiology, Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China; 2Department of Anesthesiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China
Correspondence: Guanglei Wang, Department of Anesthesiology, The Affiliated Hospital of Xuzhou Medical University, No. 99 Huaihai West Road, Xuzhou, 221000, People’s Republic of China, Tel +8613852087156, Email [email protected]
Purpose: To investigate the effect of intravenous infusion of lidocaine compared with ultrasound-guided transverse abdominal plane (TAP) block on the quality of postoperative recovery and analgesic effect in patients undergoing bariatric surgery.
Patients and Methods: Ninety-nine ASA II–III patients scheduled for elective laparoscopic bariatric surgery were randomized into the lidocaine group (group L), transverse abdominal plane block group (group T), and control group (group C). Group L: a loading dose of 1.5 mg/kg lidocaine was given at induction, followed by 2 mg·kg− 1·h− 1 maintenance until the end of surgery. Group T: ultrasound-guided bilateral administration of 0.25% ropivacaine in the transverse abdominal plane was given after induction of general anesthesia. Group C: no additional treatment was performed. Quality of recovery-40 (QoR-40) was assessed at 24 h after surgery. Consumption of propofol and remifentanil, visual analog scale (VAS) pain scores at rest at 0, 6, 12, and 24 h postoperatively, time to return of intestinal function, use of remedial analgesics within 24 h after surgery, adverse reactions were recorded.
Results: Compared with Group C, Group L and Group T had higher QoR-40 scores at 24 h postoperatively, and the difference was statistically significant (P=0.002 and P=0.003, respectively). However, there was no difference between Group L and Group T (P=0.128). In addition, compared with those of Group T and Group C, VAS scores at 12 h and 24 h postoperatively were lower in Group L (P < 0.0166).
Conclusion: Both intravenous infusion of lidocaine and ultrasound-guided TAP block provided good postoperative recovery and postoperative analgesia for patients with bariatric surgery, and intravenous infusion of lidocaine provided better analgesia at 12 h and 24 h postoperatively compared with TAP block.
Keywords: lidocaine, TAP block, bariatric surgery, postoperative recovery, postoperative pain
A Letter to the Editor has been published for this article.
Introduction
Morbid obesity is often comorbid with metabolic diseases such as diabetes mellitus and hypertension, which reduces patients’ quality of life, increases the risk of death, and reduces their life expectancy.1 Laparoscopic bariatric surgery is currently the main surgical procedure for the treatment of morbid obesity, and intraoperative and postoperative analgesia is still dominated by opioids. However, postoperative complications such as respiratory depression, nausea and vomiting and delayed recovery of gastrointestinal function can easily occur, and nausea and vomiting can lead to increased intragastric pressure, which is one of the causes of anastomotic fistula; moreover they can also lead to increased blood pressure, which can lead to postoperative bleeding.2 Therefore, reducing intraoperative and postoperative opioid use can reduce such complications. It has been reported in the literature that continuous intraoperative infusion of lidocaine or TAP block both have some analgesic effect and can reduce the use of opioids. However, no studies have compared the quality of postoperative recovery, postoperative analgesic effects, and perioperative opioid consumption between intravenous lidocaine and TAP block in morbidly obese patients undergoing laparoscopic bariatric surgery. This study focused on the differences in the quality of postoperative recovery and analgesic effects between the two methods in patients undergoing gastric decompression by intravenous infusion of lidocaine or ultrasound-guided transverse abdominal plane block, providing new ideas for multimodal analgesic strategies and improvements of the quality of postoperative recovery in patients undergoing perioperative gastric decompression.
Patients and Methods
General Information
This is a single-blind, prospective, randomized controlled trial and has been approved by the Ethics Committee of The Affiliated Hospital of Xuzhou Medical University (XYFY2021-KL075-02). The trial was registered prior to patient enrollment at the Chinese Clinical Trial Registry (ChiCTR1900023411). This study complies with the Declaration of Helsinki and adheres to CONSORT guidelines. Written informed consent was obtained from all patients. Patients aged 18–65 years with BMI ≥35 kg/m2 and American Society of Anesthesiologists (ASA) physical status II–III met the inclusion criteria. The exclusion criteria were as follows: Contraindication to nerve block, such as coagulation dysfunction or puncture site infection; history of allergy to local anesthetics; preoperative cardiac arrhythmias; history of postoperative nausea and vomiting or motion sickness; chronic pain, long-term use of opioid analgesics, corticosteroids, and/or pregnancy; history of substance abuse, mental illness, or communication disorders; laparoscopy to open abdomen; and refusal to sign informed consent. Patients were also excluded from the study if surgery lasted more than 3 h; if the patient was allergic to general anesthetic or withdrew halfway; and if they were admitted to the intensive care unit (ICU). Each individual was randomly assigned to the lidocaine group (Group L), transverse abdominal plane block group (Group T), and control group (Group C) in a 1:1:1 ratio by using the tool from www.randomization.com. The details of each patient’s method of anesthesia were stored in an opaque, sealed envelope and opened only by researchers before anesthesia induction. All participants, preoperative and postoperative follow-up assessors and statisticians were blinded to the group allocation.
Anesthesia Protocol
All participants fasted for 8 h and forwent water for 4 h before surgery. After participants entered the operation room, electrocardiogram (ECG) parameters, heart rate (HR), pulse oxygen saturation (SpO2) and upper-limb mean blood pressure (MBP) were monitored. Upper limb venous access was opened, and radial artery puncture was performed under local anesthesia. Anesthesia index (Ai, Pearlcare Anesthesia Depth Monitor, Conview YY-105, Zhe-jiang Yiyang Medical Technology Co., LTD.) was recorded to monitor the depth of anesthesia over a range from 0 to 100. The lower the value, the deeper the anesthesia.
At induction, patients in all three groups were injected with 0.05 mg/kg midazolam, 0.3 mg/kg etomidate, 0.5 µg/kg sufentanil, rocuronium 0.6 mg/kg and 5 mg dexamethasone. A loading dose of 1.5 mg/kg lidocaine was given at induction in Group L. All the medications in the study protocol were dosed based on the dosing body weight [ideal body weight (IBW) + 0.4 × (actual body weight−IBW)]. When the TOF value dropped to zero, a tracheal tube was inserted through the mouth when Ai<60. After tracheal intubation, mechanical ventilation was performed in pressure-controlled–volume-guaranteed ventilation mode (PCV-VG) with a tidal volume of 6–8 mL/kg, an inspiratory-to-expiratory ratio of 1:2, an inspired oxygen concentration of 60%, an oxygen flow rate of 2 L/min, a positive end-expiratory pressure (PEEP) value of 5 cmH2O, and an adjusted respiratory rate to maintain PetCO2 at 35–45 mmHg. Before the start of surgery, the patients in Group L were given lidocaine maintained at a rate of 2.0 mg·kg−1·h−1 after induction until the end of the operation, and the patients in Group T were given 0.25% ropivacaine under ultrasound guidance to perform bilateral transverse abdominal plane blocks (20 mL each side) after induction of general anesthesia. The ultrasound probe was placed on the lateral abdominal wall in the mid-axillary line between the lower costal margin and iliac crest. A spinal needle was advanced using in-plane technique between the aponeurosis of the internal oblique and transversus abdominis muscles (Figure 1). Patients in Group C were not given additional treatment.
 |
Figure 1 Process of TAP block as seen on ultrasound examination. |
Anesthesia was maintained with 2–5 mg·kg−1·h−1 propofol, 0.1–0.3 μg·kg−1·h−1 remifentanil and 1% sevoflurane. The infusion rate of propofol or remifentanil was adjusted during surgery to maintain an Ai value at 40–60, blood pressure and heart rate within 20% of the basal value. After pneumoperitoneum was established, the intra-abdominal pressure fluctuated between 9 and 13 mmHg. Intraoperative blood pressure was maintained within 20% of the baseline value. If the mean blood pressure (MBP) was <30% of the baseline, 3 mg ephedrine (HR < 50 beats/min) or 40 μg phenylephrine (HR≥50 beats/min) was administered. Five milligrams urapidil was injected when the MBP was >30% of the baseline, and 0.5 mg atropine was administered if HR was <40 beats/min. These steps were repeated if necessary. Intraoperative intravenous infusion of sodium, potassium, magnesium, calcium and glucose consisted of a crystalloid solution (multiple electrolytes of sodium, potassium, magnesium, calcium, glucose) and succinyl gelatin in a volume ratio of 2:1. Intraoperative arterial blood was collected intermittently for blood gas analysis to maintain a stable internal environment. All patients were given 12.5 mg dolasetron and 30 mg aminotriol ketorolac intravenously 15 min before the end of surgery. All intravenous and inhaled drugs were stopped after finishing skin closure. The oxygen flow was adjusted to 6 L/min at the end of the operation.
All patients were transferred to the postanesthesia care unit (PACU) for resuscitation and extubation. Residual neuromuscular blockade was antagonized routinely with 1.0 mg neostigmine and 0.5 mg atropine if there were no contraindications. Postoperatively, 60 mg aminotriol ketorolac was routinely administered intravenously by a surgeon unaware of the subgroup, and dezocine was used on an individual basis to maintain VAS below or equal to 4 points, with data collected by study team personnel.
Primary and Secondary Outcomes
Primary Outcome Measure
The primary outcome measure of this study was the Quality of Recovery-40 (QoR-40)3 questionnaire assessed at 24 h after surgery. The QoR-40 questionnaire measures 40 items from 5 dimensions of recovery: physical comfort, physical independence, emotional state, psychological support and pain. Each item is rated from 1 to 5 points, and the aggregate score is 200 points (best quality). 4.8 is the minimal clinically important difference for QoR-40 scale scores.4
Secondary Outcomes Measures
VAS scores at rest were assessed at 0, 6, 12, and 24 h postoperatively. The duration of surgery, extubation time, PACU stay time, intraoperative consumption of propofol and remifentanil, use of vasoactive drugs, blood loss, consumption of diazoxide within 24 h after the operation, adverse reactions, time to first exhaust, number of postoperative bowel movements before discharge and hospital stay durations were also recorded. Pain intensity was evaluated using a VAS score (0 = no pain, 10 = worst imaginable pain). The time to first exhaust was defined as the interval between the end of surgery and the first flatulence.
Sample Size Calculation
The sample size was calculated on the basis of a pilot study in which the mean QoR-40 scores in the three groups were 174, 168, and 152; the standard deviations were 16, 18, and 18, respectively. Assuming an α of 0.05 and β of 0.1 and using PASS version 15.0, accounting for a 20% dropout rate, each group needed 36 patients in this trial.
Statistical Analysis
SPSS statistical software (version 26.0; SPSS Inc., IBM, Chicago, IL, USA) was used for statistical data analysis; The measurement data used the Shapiro–Wilk test to determine the normality of the data distribution, and the Levene method was used to test the homogeneity of variance. Data are expressed as the mean (SD) for parametric continuous variables and as the median (interquartile range [IQR]) for nonparametric distribution of data. Categorical data are expressed as a number (percentage). The chi-square test or Fisher exact test with Yates correction was used to compare differences in categorical variables when appropriate. Analysis of variance was used to compare continuous parametric variables, and the Kruskal–Wallis test was used for continuous nonparametric variables. Post hoc analysis was performed using Bonferroni adjustment in the case of statistically significant differences observed among multiple groups. Two-sided P < 0.05 was considered statistically significant.
Results
A total of 108 patients were screened to participate in the trial, of whom nine were excluded. Two of these patients were excluded because they underwent temporarily canceled surgery, and one had a history of postoperative nausea. After being enrolled, six more patients were excluded; among them, two patients were excluded because surgery took more than 3 h, two patients were converted to open surgery, one patients were admitted to the ICU after the operation and one withdrew halfway. Ultimately, 99 patients were included in the statistical analysis. No significant differences were found among the three groups in terms of baseline characteristics (Table 1, Figure 2).
 |
Table 1 Patient’s Characteristics of Demographic Data |
The median (IQR) QoR-40 scores were 170 (166.00–175.50), 167 (162.50–172.00), and 161 (158.00–163.50) for Group L, Group T, and Group C, respectively. Compared with Group C, the overall QoR-40 scores of Group L and Group T increased significantly at 24 h postoperatively, and the difference was statistically significant (P=<0.001; P=0.001, respectively). When analyzing each questionnaire domain, a significantly higher improvement in Group L was noted for physical comfort, physical independence, and pain at 24 h after surgery compared with Group C (P<0.001; P=0.002; P<0.001, respectively). The scores of these three areas were also significantly higher in Group T than in Group C (P=0.001; P=0.003; P=0.014, respectively). In addition, the pain perception score was higher in Group L than in Group T (P =0.008, Table 2).
 |
Table 2 QoR-40 Score (n=99) |
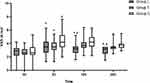
The VAS scores at rest at 6 h postoperatively were significantly lower in Group L and Group T than in Group C (Group L vs Group C, P=0.006; (Group T vs Group C, P=0.011). Compared with Group T and Group C, the VAS scores at 12 h and 24 h postoperatively were lower in Group L (P<0.0166). There was no difference in VAS scores at 0 h among the three groups (P=0.708). When we compared the intragroup values using the repeated measures test, there were significant differences in VAS scores at all time points compared with those at 0 h in all group (Figure 3).
Compared with Group C, intraoperative remifentanil consumption was lower in Group L and Group T (P<0.010, P=0.002, respectively). Consumption of dezocine within 24 h postoperatively was different across groups (Group L vs Group C, P=0.002; (Group T vs Group C, P=0.008). Time to first leave bed and first exhaust were faster in Group L and Group T than in Group C (P=0.006; P=0.011, respectively), and there was no significant difference in intraoperative propofol consumption, use of vasoactive drugs, extubation time, PACU stay time, first postoperative defecation, or hospital stay duration among the three groups (P>0.05, Table 3).
 |
Table 3 Perioperative Data Among the Three Groups |
Five, four and fourteen patients in Group L, Group T and Group C, respectively, had vomiting (P = 0.020), and there was no significant difference in the incidence of postoperative nausea, chills, fever, and hypoxemia among the three groups of patients (P > 0.05, Table 4).
 |
Table 4 Postoperative Incidence of Adverse Reactions |
Discussion
Laparoscopic bariatric surgery is an effective method for the treatment of morbid or complex obesity.5,6 However, there are some serious perioperative challenges and factors that may hinder perioperative outcome or recovery in bariatric surgery patients.7,8 Postoperative pain may have a negative impact on recovery, ambulation, gastrointestinal function, and length of hospital stay. In addition, serious adverse effects such as apnea, hypoxemia, drowsiness, intestinal obstruction, vomiting, delayed activity, and mortality are associated with the use of opioid analgesics in bariatric patients.9,10 Therefore, it is highly desirable to use multimodal strategies to minimize opioid-related side effects and improve postoperative recovery in bariatric patients undergoing surgery. The QoR-40 scale11 not only has a final score but also provides a comprehensive assessment of the quality of recovery in terms of five dimensions: emotional state, physical comfort, psychological support, self-care, and pain perception. The higher the postoperative QoR-40 score is, the better the quality of postoperative recovery. Several studies have shown that the QoR-40 questionnaire can be used for immediate assessment of patients’ recovery quality after surgery.
In recent years, intravenous lidocaine and TAP block have been increasingly used for postoperative analgesia, but their efficacy is still controversial. Studies have found that intravenous infusion of lidocaine and TAP block can relieve postoperative acute pain, reduce the use of opioids, and improve postoperative comfort.12,13 However, G. Dewinter14 found that intravenous lidocaine could not improve the postoperative morphine dosage or postoperative recovery of patients undergoing posterior spinal fusion. Kane15 observed that TAP block did not improve the QOR-40 score after laparoscopic hysterectomy, nor did it reduce the use of narcotic analgesics.
Our study found that intravenous infusion of lidocaine or ultrasound-guided TAP block could both improve postoperative recovery quality scores, but there was no significant difference between the two interventions. The improvement in the total score was mainly reflected in the three aspects of physical comfort, self-care ability and pain perception, which was consistent with Wang’s finding16 that intravenous lidocaine could improve the postoperative recovery quality of patients undergoing thoracoscopic lung cancer.
In addition, we observed a lower prevalence of nausea in the anesthesia care unit in the lidocaine group than in the control group. This observation likely resulted from the lower opioid consumption by the lidocaine group. Several mechanisms have been proposed to explain the opioid-sparing effects of perioperative systemic lidocaine. First, intravenous infusion of lidocaine has anti-inflammatory properties that can minimize the pain caused by surgical inflammation.17,18 Second, systemic lidocaine can also directly block the sodium channel of nerve fibers that transmit pain.19 Last, systemic lidocaine may reduce the need for volatile anesthetic and/or opioid drugs during surgery, which may minimize the development of hyperalgesia after surgery.20–22 This also explains the good analgesia provided by the lidocaine group within 24 h after surgery.
The TAP block group also showed perioperative opioid savings, consistent with previous studies.23 In addition, we observed a reduction in the 6-hour postoperative resting VAS score in the TAP group compared to the control group. However, pain scores at 12 and 24 h were similar in the two groups. This might have been due to the occurrence of rebound pain. This may explain the reported duration of TAP block action between 6 and 8 h after a single injection,24 which is consistent with Emile ’s finding.25 This also explain why the lidocaine group had lower pain scores at 12 and 24 h after surgery than the TAP group and higher scores in the pain perception component of the quality of recovery score. TAP block has been included in the most recent ERAS guidelines for bariatric surgery with strong recommendation and high level of evidence,26 however, compared with the lidocaine group, the TAP group did not show an advantage in our study. Gupta C27 also found that Intravenous lidocaine improved pain score in obese patients undergoing laparoscopic bariatric surgery as compared to TAP Block.
Hanson28 found that a continuous intravenous infusion of lidocaine offers noninferior analgesia compared with ultrasound-guided unilateral, single-injection, TAP block within the first 24 h following kidney transplant surgery. In addition, the Lidocaine group had improved patient satisfaction with analgesia scores in the first 24 h following surgery. However, there was no difference in overall postoperative opioid consumption or opioid-related adverse events between groups. Our study found similar results.
Regarding the safety aspects of the two interventions, the currently recommended protocol for intravenous application of lidocaine is a loading dose of 1.0 to 2.0 mg/kg followed by a continuous intravenous drip of 1.0 to 2.0 mg·kg−1·h−1, when the blood concentration of lidocaine is well below the concentration that produces toxic adverse effects (5.0 μg/mL).29,30 In this study, lidocaine-related adverse reactions did not occur during lidocaine infusion according to dosing body weight. TAP block is a local anesthetic injected between the internal oblique and transverse abdominis muscles to block the abdominal nerve in the plane, thereby blocking the transmission of pain signals in the abdominal wall. Because the needle alignment and depth are clearly visible under ultrasound guidance, with a high success rate of block, in our patients, with the modified technique, the visibility of the muscle layers could be improved by the 15° tilt away from the side in which block had to be performed. An assistant pulled the abdomen toward the opposite side. No adverse reactions related to TAP block occurred during this study.
This study also has some limitations. First, the plasma concentration of lidocaine was not detected in the study, but according to previous studies,31 the dose in this study is safe. Second, although the dose of ropivacaine used for TAP block in our trial has been shown to be effective for postoperative pain relief in other trials,32 the most effective amount of local anesthesia remains controversial, which may be one of the reasons that TAP block has not shown an advantage. Meanwhile, the VAS scores at 12 and 24 hours were higher in the TAP block group may also be related to the duration of action of ropivacaine. Finally, we did not include a joint application of lidocaine and TAP block group. Under the premise of ensuring safety, it may become a more advantageous intervention for multimodal analgesia. However, in a recent intravenous lidocaine international consensus statement on suggestions for postoperative analgesia, intravenous lidocaine should not be used in conjunction with other local anesthetic interventions or while under the effect of other local anesthesia interventions.33
Conclusion
There was no difference between intravenous lidocaine and ultrasound-guided TAP block in improving the quality of postoperative recovery, as both promoted earlier sedation activity and provided good postoperative recovery and postoperative analgesia in patients with laparoscopic bariatric surgery. Intravenous lidocaine provided better analgesia at 12 h and 24 h postoperatively compared with TAP block.
Data Sharing Statement
The individual participant’s data that underlying the results reported in this article would be accessed with approval from the corresponding author after 6 months of publication. The study protocol, statistical analysis plan and clinical study report will also be available.
Acknowledgments
Appreciate for the support from the gynecologists and nursing teams of the Affiliated Hospital of Xuzhou Medical University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Martins Marcelo J, Martins CPMO, Castro-Alves LJ, et al. Pregabalin to improve postoperative recovery in bariatric surgery: a parallel, randomized, double-blinded, placebo-controlled study. J Pain Res. 2018;11:2407–2415. doi:10.2147/JPR.S176468
2. Bamgbade Oluwafemi OA, Oluwole Rong O, Khaw RR. Perioperative analgesia for fast-track laparoscopic bariatric surgery. Obes Surg. 2017;27(7):1828–1834. doi:10.1007/s11695-017-2562-4
3. Myles PS, Weitkamp B, Jones K, et al. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth. 2000;84(1):11–15. doi:10.1093/oxfordjournals.bja.a013366
4. Myles PS. Measuring quality of recovery in perioperative clinical trials. Curr Opin Anaesthesiol. 2018;31(4):396–401. doi:10.1097/ACO.0000000000000612
5. O’Brien PE, Hindle A, Brennan L, et al. Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg. 2019;29(1):3–14. doi:10.1007/s11695-018-3525-0
6. Phillips BT, Shikora SA. The history of metabolic and bariatric surgery: development of standards for patient safety and efficacy. Metabolism. 2018;79:97–107. doi:10.1016/j.metabol.2017.12.010
7. Kassir R, Debs T, Blanc P, et al. Complications of bariatric surgery: presentation and emergency management. Int J Surg. 2016;27:77–81. doi:10.1016/j.ijsu.2016.01.067
8. Trotta M, Ferrari C, D’Alessandro G, et al. Enhanced recovery after bariatric surgery (ERABS) in a high-volume bariatric center. Surg Obes Relat Dis. 2019;15(10):1785–1792. doi:10.1016/j.soard.2019.06.038
9. Sultana A, Torres D, Schumann R. Special indications for Opioid Free Anaesthesia and Analgesia, patient and procedure related: including obesity, sleep apnoea, chronic obstructive pulmonary disease, complex regional pain syndromes, opioid addiction and cancer surgery. Best Pract Res Clin Anaesthesiol. 2017;31(4):547–560. doi:10.1016/j.bpa.2017.11.002
10. Cozowicz C, Poeran J, Zubizarreta N, et al. Non-opioid analgesic modes of pain management are associated with reduced postoperative complications and resource utilisation: retrospective study of obstructive sleep apnoea patients undergoing elective joint arthroplasty. Br J Anaesth. 2019;122(1):131–140. doi:10.1016/j.bja.2018.08.027
11. Chen Y, Wang J, Liu S, et al. Development and validation of the Chinese version of the quality of recovery-40 questionnaire. Ther Clin Risk Manag. 2020;2(16):1165–1173. doi:10.2147/TCRM.S281572
12. Dai Y, Jiang R, Su W, et al. Impact of perioperative intravenous lidocaine infusion on postoperative pain and rapid recovery of patients undergoing gastrointestinal tumor surgery: a randomized, double-blind trial. J Gastrointest Oncol. 2020;11(6):1274–1282. doi:10.21037/jgo-20-505
13. Hamid HK, Emile SH, Saber AA, et al. Laparoscopic-guided transversus abdominis plane block for postoperative pain management in minimally invasive surgery: systematic review and meta-analysis. J Am Coll Surg. 2020;231(3):376–386. doi:10.1016/j.jamcollsurg.2020.05.020
14. Dewinter G, Moens P, Fieuws S, et al. Systemic lidocaine fails to improve postoperative morphine consumption, postoperative recovery and quality of life in patients undergoing posterior spinal arthrodesis. A double-blind, randomized, placebo-controlled trial. Br J Anaesth. 2017;118(4):576–585. doi:10.1093/bja/aex038
15. Kane SM, Garcia-Tomas V, Alejandro-Rodriguez M, et al. Randomized trial of transversus abdominis plane block at total laparoscopic hysterectomy: effect of regional analgesia on quality of recovery. Am J Obstet Gynecol. 2012;207(5):
16. Wang L, Sun J, Zhang X, Wang G. The effect of lidocaine on postoperative quality of recovery and lung protection of patients undergoing thoracoscopic radical resection of lung cancer. Drug Des Devel Ther. 2021;15:1485–1493. doi:10.2147/DDDT.S297642
17. Jeong HJ, Lin D, Li L, Zuo Z. Delayed treatment with lidocaine reduces mouse microglial cell injury and cytokine production after stimulation with lipopolysaccharide and interferonγ. Anesth Anal. 2012;114(4):856–861. doi:10.1213/ANE.0b013e3182460ab5
18. Suto T, Obata H, Tobe M, et al. Long-term effect of epidural injection with sustained-release lidocaine particles in a rat model of postoperative pain. Br J Anaesth. 2012;109(6):957–967. doi:10.1093/bja/aes302
19. Obreja O, Hirth M, Turnquist B, Rukwied R, Ringkamp M, Schmelz M. The differential effects of two sodium channel modulators on the conductive properties of C-fibers in pig skin in vivo. Anesth Analg. 2012;115(3):560–571. doi:10.1213/ANE.0b013e3182542843
20. Hamp T, Krammel M, Weber U, Schmid R, Graf A, Plöchl W. The effect of a bolus dose of intravenous lidocaine on the minimum alveolar concentration of sevoflurane: a prospective, randomized, double-blinded, placebo-controlled trial. Anesth Analg. 2013;117(2):323–328. doi:10.1213/ANE.0b013e318294820f
21. Cho AR, Kwon JY, Kim KH, et al. The effects of anesthetics on chronic pain after breast cancer surgery. Anesth Analg. 2013;116(3):685–693. doi:10.1213/ANE.0b013e31827ee372
22. Petrenko AB, Ishii H, Kohno T, Baba H. When similar is not alike: decreased sensory thresholds after intravenous infusion of remifentanil may not be remifentanil-induced hyperalgesia. Anesth Analg. 2012;115(4):977. doi:10.1213/ANE.0b013e318263ca5c
23. Sinha A, Jayaraman L, Punhani D. Efficacy of ultrasound-guided transversus abdominis plane block after laparoscopic bariatric surgery: a double blind, randomized, controlled study. Obes Surg. 2013;23(4):548–553. doi:10.1007/s11695-012-0819-5
24. Kishore K, Agarwal A. Ultrasound-guided continuous transverse abdominis plane block for abdominal surgery. J Anaesthesiol Clin Pharmacol. 2011;27(3):336–338. doi:10.4103/0970-9185.83677
25. Emile SH, Abdel-Razik MA, Elbahrawy K, et al. Impact of ultrasound-guided transversus abdominis plane block on postoperative pain and early outcome after laparoscopic bariatric surgery: a randomized double-blinded controlled trial. Obes Surg. 2019;29(5):1534–1541. doi:10.1007/s11695-019-03720-y
26. Stenberg E, Dos Reis Falcão LF, O’Kane M, et al. Guidelines for perioperative care in bariatric surgery: Enhanced Recovery After Surgery (ERAS) SocietyRecommendations: a 2021 Update. World J Surg. 2022;40:2065–2083.
27. Gupta C, Valecha UK, Singh SP, et al. Systemic lidocaine versus ultrasound-guided transversus abdominis plane block for postoperative analgesia:A comparative randomised study in bariatric surgical patients. Indian J Anaesth. 2020;64(1):31–36. doi:10.4103/ija.IJA_430_19
28. Hanson NA, Strunk J, Saunders G, et al. Comparison of continuous intravenous lidocaine versus transversus abdominis plane block for kidney transplant surgery: a randomized, non-inferiority trial. Reg Anesth Pain Med. 2021;46(11):955–959. doi:10.1136/rapm-2021-102973
29. Dunn LK, Durieux ME. Perioperative use of intravenous lidocaine. Anesthesiology. 2017;126(4):729–737. doi:10.1097/ALN.0000000000001527
30. Sahmeddini MA, Khosravi MB, Farbood A. Comparison of perioperative systemic lidocaine or systemic ketamine in acute pain management of patients with opioid use disorder after orthopedic surgery. J Addict Med. 2019;13(3):220–226. doi:10.1097/ADM.0000000000000483
31. Yao Y, Jiang J, Lin W, Yu Y, Guo Y, Zheng X. Efficacy of systemic lidocaine on postoperative quality of recovery and analgesia after video-assisted thoracic surgery: a randomized controlled trial. J Clin Anesth. 2021;71:110223. doi:10.1016/j.jclinane.2021.110223
32. De Oliveira GS
33. Foo I, Macfarlane AJR, Srivastava D, et al. The use of intravenous lidocaine for postoperative pain and recovery: international consensus statement on efficacy and safety. Anaesthesia. 2021;76(2):238–250. doi:10.1111/anae.15270
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.